Introduction
Colorectal cancer (CRC) is one of the most prevalent
malignancies of the digestive system worldwide. In 2022, it ranked
third among all cancers worldwide with >1.9 million new cases
reported (1). Although various
therapies, including surgery, have markedly improved patient
outcomes, the postpertrative survival rat for CRC patients ranges
from 30 to 80%, indicating considerable heterogeneity in outcomes
among this population. Thus, further clinical research remains
necerrssary. Stage II–III CRC represents the initial phase of tumor
invasion and lymph node metastasis, for which surgical resection
combined with chemotherapy serves as the primary treatment strategy
(2). Due to the complex
pathological mechanisms, tumor cells exhibit significant
heterogeneity. Different subclones show marked variations in
treatment sensitivity and microenvironmental adaptability. New
blood vessels can supply oxygen and nutrients to cancer cells while
secreting factors that promote matrix degradation, assisting cancer
cells in breaking through physical barriers. Consequently, CRC
patients face high rates of postoperative recurrence (30–50%),
metastasis (approximately 40%), and mortality (approximately 10.5%)
(3). While radical surgery can
remove the bulk of tumor, residual cancer cells (undetectable by
imaging) retain proliferative capacity and exhibit marked
heterogeneity, enabling adaptation to diverse microenvironments,
such as inflammatory or hypoxic microenvironments. Furthermore,
tumor-induced angiogenesis provides nutrients and oxygen while
degrading the extracellular matrix, facilitating vascular and
lymphatic invasion, which are key processes driving CRC recurrence
and metastasis (4,5). Furthermore, adjuvant chemotherapy
often leads to treatment resistance through upregulation of immune
checkpoint proteins such as programmed death-ligand 1 (PD-L1) and
cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), increased
drug efflux pumps and epigenetic modifications in cancer cells,
diminishing therapeutic efficacy (6). Investigating prognostic factors
following radical resection in patients with stage II–III CRC is
essential to prevent adverse outcomes, with particular emphasis on
evaluating predictive value.
Tumor biomarkers serve as key indicators for cancer
detection and diagnosis. Serum microRNA-497-5p (miR-497-5p) acts as
a negative regulator, suppressing tumor cell proliferation and
promoting apoptosis via protein tyrosine phosphatase 3 inhibition
(7). Postoperative nutritional
status in patients with stage II–III CRC markedly influences
immune-metabolic function and physiological recovery. Albumin (ALB)
and hemoglobin (Hb), which are widely used clinical nutrition
markers, serve key roles in prognosis (8). A retrospective analysis of the
hematological profiles of patients with ovarian cancer identified
that ALB levels are associated with tumor matastasis. Furthermore,
a validation study in an esophageal cancer cohort demonstrated that
ALB could serve as a potential predictor for distant metastasis,
highlighting its value in cross-cancer predictive modeling
(9). Similarly, a prognostic study
in esophageal squamous cell carcinoma demonstrated an association
between low pre-treatment Hb levels with poor outcomes (10). The ALB-Hb score, integrating both
nutritional markers, provides a comprehensive assessment of patient
prognosis, anemia status, inflammatory burden and malignant
progression. The Kallikrein family, particularly Kallikrein-related
peptidase 5 (KLK5), contributes to CRC cell proliferation, invasion
and metastasis by cleaving proteins into bioactive peptides and
degrading extracellular matrix (11). A retrospective cohort study of
gastric adenocarcinoma biomarkers demonstrated a notable
association between elevated KLK5 levels and poor prognosis in
patients with esophageal adenocarcinoma (12). Angiopoeitin-2 (Ang-2), a key
regulator of angiogenesis, promotes endothelial cell migration and
proliferation (13). The levels of
Ang-2 fluctuate with tumor aggressiveness and neovascularization. A
retrospective cohort study of 52 patients with hepatocellular
carcinoma demonstrated an association between high Ang-2 expression
and adverse outcomes (14). While
miR-497-5p, ALB-Hb score, KLK5 and Ang-2 are mechanistically
associated with cancer progression, their combined prognostic
potential remains understudied in CRC (15).
A study on factors influencing lymph node metastasis
and recurrence in 801 patients with CRC revealed that the lymph
node metastasis rate is ~12.5% following surgical resection and CRC
lymphadenectomy (16). Existing
clinical studies have demonstrated that patients with CRC face risk
of local recurrence after surgery and alternative treatment
strategies should be considered if pathological specimens indicate
lymph node metastasis (17,18). In summary, patients with stage
II–III CRC experience slow recovery of physiological function
following radical resection, high risk of recurrence and
metastasis, and have a postoperative 5-year survival rate ranging
from 50 to 70%, with a progression-free survival rate of 45–65%
(19). Therefore, clinical
attention should be directed toward investigating postoperative
prognosis factors and their predictive value. The present study
aimed to construct a prognostic nomogram model for patients with
stage II–III CRC following radical resection by analyzing
retrospective baseline data. Furthermore, the present study aimed
to explore the predictive value of biochemical marker levels for
poor prognosis. By evaluating preoperative biochemical indicators,
the present study aimed to advance research on tumor biomarkers,
further refine the precision of individualized postoperative
treatment and improve quality of life.
Materials and methods
Study design
The sample size (20) was calculated using the formula
n=(Z1-α/2+Z1-β)2x(σ12+σ22)/δ2,
where n is the sample size, Zα/2 denotes the Z value
corresponding to the significance level, Z1-β is the key
value of the standard normal distribution for a power of 1-β, β is
the probability of a type II error, σ12 and
σ22 represent the variances of each group and
δ2 is the square of the expected effect size. With
α=0.05, β=0.2, σ1=20, σ2=25 and δ=7.23, the
required sample size was determined to be 154 cases. A
retrospective study was conducted using baseline data from 154
patients with stage II–III CRC treated at Tianjin Haihe Hospital
(Tianjin, China) or Guangzhou First People's Hospital (Guangzhou,
China) between December 2021 and December 2024. Among them, 83 were
male and 71 were female, with ages ranging from 41 to 79 years
(median, 58 years). The present study was approved by the Ethics
Committee of Tianjin Haihe Hospital [approval no. 2024HHWZ(A)-003;
Tianjin, China] and all procedures were performed in accordance
with the Declaration of Helsinki.
Inclusion criteria
Inclusion criteria were as follows: i) Patients who
met CRC diagnostic standards per Localized colon cancer: European
Society for Medical Oncology Clinical Practice Guidelines for
diagnosis, treatment and follow-up (21); ii) patients who fulfilled radical
resection criteria outlined in Treatment of Metastatic Colorectal
Cancer: American Society of Clinical Oncology Guideline (22); iii) patients who underwent radical
CRC surgery at our hospital with postoperative survival >1 year;
iv) patients who had complete medical records in our hospital
system, including demographic information, diagnosis reports,
surgical details and histopathologically confirmed CRC; v) CRC
classified as stage II [no lymph node or distant metastasis, but
tumor penetration beyond the intestinal wall (potential invasion
into pericolic fat, peritoneum or adjacent organs)] or III
(metastasis in ≥1 regional lymph node without distant spread) by
the TNM staging system (23) and
vi) no history of preoperative chemotherapy.
Exclusion criteria
Exclusion criteria were as follows: i) Previously
diagnosed CRC; ii) non-primary CRC; iii) previous treatment with
radical CRC surgery resection; iv) concurrent malignancies in other
organs/sites; v) history of invasive surgical procedures within 1
year prior to enrollment and vi) hematological disease.
Baseline data collection
Baseline data from 154 patients with stage II–III
CRC were retrieved from the medical system at Tianjin Haihe
Hospital and Guangzhou First People's Hospital, including basic
demographics [sex, age, weekly exercise frequency (≥5 or <5
times), BMI and type of surgical treatment (intestinal segment or
total rectal resection)], tumor disease status [tumor diameter,
pathological type (adenocarcinoma and mucinous adenocarcinoma
combined with imprinted cell carcinoma), tumor site (rectum, right
or left half colon), tumor classification (ulcer and bulge type),
invasion depth (T1-2, T3 and T4) and histological differentiation],
biochemical indices (ALB, Hb, miR-497-5p, KLK5 and Ang-2) and
nutritional score (ALB-Hb score).
Assessment of poor prognosis in
patients with stage II–III CRC
Based on follow-up medical records of patients with
stage II–III CRC, recurrence, metastasis (cancer metastasized to
tissue and organs such as liver and lungs through blood or
lymphatic fluid) or death within 1-year post-radical resection were
considered adverse prognostic events for patients with stage II–III
CRC.
Preoperative assessment of
histological differentiation
Histological differentiation was performed through
standardized processing of surgically resected specimens. This
included fixation in 10% neutral buffered formalin at room
temperature for 24–48 h, paraffin embedding, and sectioning (4 µm),
followed by hematoxylin-eosin staining. The hematoxylin staining
time was 5–8 min and the eosin staining time was 1–3 min, with all
staining steps performed under room temperature conditions.
Morphology and arrangement characteristics of glandular structures
were observed under laser scanning confocal optical microscope to
calculate the proportion of gland formation. Histological
differentiation in patients with stage II–III CRC was classified
as: Well-(>95% gland formation, low malignancy), medium (50–95%
gland formation, intermediate malignancy with malignant potential)
or low differentiated (5–50% gland formation, rapid growth rate and
high malignancy) (24).
Preoperative assessment of tumor
classification
The gross tumor morphology was classified based on
preoperative malignant features and invasion patterns as follows:
Ulcerative (ulcers penetrating the muscular propria with potential
transmural invasion) and protruding/polypoid type
(luminal-protruding masses with limited peripheral infiltration)
(25).
Preoperative assessment of
infiltration depth
The depth of tumor invasion in patients with stage
II–III CRC was classified as follows: T1-2, tumor invaded submucosa
or muscularis propria; T3, tumor penetrated through muscularis
propria into subserosa or non-peritonealized pericolic tissue and
T4, tumor invaded beyond visceral peritoneum or adjacent
organs/structures (26).
Preoperative assay of miR-497-5p
Morning fasting venous blood samples (3 ml) were
collected using anticoagulant tubes [cat. no. RT-cxkng;
Bosengtian'ai (Beijing) Technology Co., Ltd.]. The samples were
stored at 4°C for ≤24 h before processing. After centrifugation
(483 × g, radius, 13.5 cm; duration, 15 min; temperature, 4°C), the
supernatant was separated and stored at −80°C. All samples were
analyzed within 1 week. Total RNA was extracted using the miRNeasy
Mini kit (cat. no. 74104; Qiagen GmbH). RT was performed using the
PrimeScript RT Master Mix (Perfect Real Time) kit (cat. No. RR036A;
TaKaRa Bio Inc.), follow the manufacturer's instructions. Reverse
transcription-quantiative PCR was performed under the following
thermocycling conditions: Initial denaturation at 95°C for 2 min,
followed by 35 cycles of denaturation (95°C; 5 sec), annealing
(56°C; 5 sec) and extension (72°C; 35 sec). The primers were as
follows: miR-497-5p forward (F), 5′-CAGCAGCACACTGTGGTTTGT-3′ and
reverse (R), 5′-TAGCCTGCAGCACACTGTGGT-3′ and U6 (internal control)
F, 5′-ATTGGAACGATACAGAGAAGATT-3′ and R, 5′-GGAACGCTTCACGAATTTG-3′.
Quantitative detection was performed using a fluorescent dye
(Product No. 4913850001; Roche). The relative expression of
miR-497-5p calculated using the 2−ΔΔCq method (27).
Preoperative analysis of ALB and
Hb
Plasma was separated by centrifugation (11,180 × g;
radius, 10 cm; duration, 10 min; temperature, 4°C). ALB levels were
measured colorimetrically using the bromocresol green method at a
wavelength of 628 nm (reaction time 10 min) at room temperature,
while Hb levels were determined using the cyanmethemoglobin method
at a wavelength of 540 nm (reaction time 5 min). PBS) was used as a
blank control. All experiments involving samples and standards were
independently repeated three times.
Preoperative analysis of KLK5
Morning fasting venous blood samples (5 ml per
patient) were transferred to anticoagulant tubes. The samples were
stored at 4°C for ≤24 h prior to processing. The samples were
centrifuged (11,180 × g; radius, 10 cm; duration, 10 min;
temperature, 4°C) to separate the supernatant, which was stored at
−80°C. All analyses were completed within 1 week. KLK5 protein
expression in patients with stage II–III CRC was detected using an
ELISA kit (cat. no. KLK5; JINGMEI) in strict accordance with the
manufacturer's instructions.
Preoperative analysis of Ang-2
Fasting antecubital venous blood samples (5 ml) were
transferred to anticoagulant tubes. Following anticoagulation
treatment, samples were stored at 4°C for ≤24 h. The samples were
centrifuged (11,180 × g; radius, 10 cm; duration, 10 min;
temperature, 4°C) to separate the supernatant, which was stored at
−80°C. All measurements were completed within 1 week using a
commercial Ang-2 ELISA kit (cat. no. EK1215; MultiSciences)
accordance with the manufacturer's instructions to determine Ang-2
levels.
Preoperative score of ALB-Hb
The ALB-Hb scoring system was used to assess
nutritional status and anemia severity. ALB was scored as follows:
0, ≥35 g/l; 1, 30–35 g/l; 2, 25–30 g/l; 3, 20–24 and 4, <20 g/l.
Hb was scored as follows: 0, ≥12 g/dl; 1, 10–12 g/dl; 2, 7–9 g/dl
and 3, <7 g/dl. A higher composite total score indicated poorer
preoperative nutritional health status, suggesting a negative
association between the composite score and health status (28).
Observation indicators
Patients with stage II–III CRC were stratified into
poor [experiencing recurrence, metastasis (hematogenous/lymphatic
spread to liver/lungs) or death within 1-year post-radical
resection] and good prognosis group (no recurrence, metastasis, or
death events within 1 year after radical resection, with both the
1-year overall survival rate and progression-free survival rate
being 100%). Statistical analysis was performed using 2025 SPSSAU
(spssau.com/indexs.html). Significant variables were identified
through univariate analysis of baseline characteristics. After
multicollinearity testing, a Cox regression model was empoloyed to
analyze the hazard ratio (HR), 95% CI and concordance (C)-index.
The nomogram function was used to transform the model into a
nomogram, and calibration curves were validated using the Bootstrap
resampling method with 1,000 repetitions to evaluate the
consistency between the predicted probabilities of the nomogram and
the actual observed probabilities).
Statistical analysis
Statistical analysis was performed using SPSS
(version 27.0; IBM Corp.). This study is based on data from three
independent experimental replicates. Categorical data were analyzed
by χ2 test. Measurement data were assessed for normal
distribution using the Shapiro-Wilk test, with normally distributed
data expressed as mean ± SD and compared using unpaired t-test.
Non-normally distributed data were presented as median (25 and 75th
percentile) and analyzed using non-parametric Mann Whitney U test
(Z-score). Variables demonstrating significant differences were
incorporated into a Cox proportional hazards regression model
(variables were initially screened using univariate Cox
proportional hazards regression, and those with P<0.05 were
included in the multivariate Cox proportional hazards regression
model), with poor postoperative prognosis following radical
resection as the dependent variable and baseline characteristics as
independent variables. A stepwise selection method (entry criteria,
P=0.05) was used to identify significant prognostic factors. The
model evaluated the predictive value of each factor by calculating
HRs with corresponding 95% CIs. P<0.05 was considered to
indicate a statistically significant difference.
Results
Prognosis of patients with stage
II–III CRC following radical surgery
Among 154 patients, 63 patients (40.91%) were
classified into the poor prognosis group, while 91 (59.09%) were
classified as good prognosis group (data not shown). Within the
poor prognosis group, 49 patients (77.78%) demonstrated
recurrences, eight (12.70%) demonstrated metastases and six (3.90%)
died. These results indicated that ~60% of patients with stage
II–III CRC achieved favorable postoperative recovery following
radical resection, although a notable proportion experienced a poor
prognosis.
Univariate analysis of factors
affecting the prognosis of patients with stage II–III CRC
Preoperative levels of ALB (27.26±3.58 vs.
30.61±3.74 g/l), Hb [5.94 (4.80, 7.60) vs. 9.23 (8.20, 10.40) g/dl]
and miR-497-5p (0.35±0.04 vs. 0.41±0.02; all P<0.001) were
significantly lower in the poor compared with the good prognosis
group (Table I). By contrast, KLK5
(3.82±1.41 vs. 3.28±1.33 ng/ml), Ang-2 [3.32 (3.20, 3.60) vs. 3.19
(3.00, 3.40) g/l] and ALB-Hb score [5.00 (4.00, 5.00) vs. 3.00
(2.00, 4.00); all P<0.001] were significantly higher in the poor
prognosis group. All comparisons were significant (ALB, t=5.561;
Hb, Z=−7.742; miR-497-5p, t=12.275; KLK5, t=2.417; Ang-2, Z=−4.236;
ALB-Hb, Z=−7.727), which suggested these biomarkers may influence
the prognosis in patients with stage II–III CRC.
 | Table I.Univariate analysis of factors
affecting prognosis of patients with stage II–III CRC. |
Table I.
Univariate analysis of factors
affecting prognosis of patients with stage II–III CRC.
| A, Demography |
|---|
|
|---|
| Characteristic | n | Poor prognosis
group (n=63) | Good prognosis
group (n=91) |
χ2/t/Z-score | P-value |
|---|
| Sex |
|
|
| 0.117 | 0.732 |
|
Male | 83 | 35 | 48 |
|
|
|
Female | 71 | 28 | 43 |
|
|
| Mean age,
years |
| 59.25±6.32 | 57.96±7.93 | 1.076 | 0.284 |
| Weekly exercise
frequency |
|
|
| 1.922 | 0.166 |
| ≥5 | 54 | 19 | 35 |
|
|
|
<5 | 100 | 44 | 56 |
|
|
| Median BMI,
kg/m2 | - | 18.51 (17.00,
19.50) | 18.05 (17.30,
19.60) | −0.404 | 0.686 |
| Type of
resection |
|
|
| 0.154 | 0.695 |
|
Intestinal segment | 64 | 25 | 39 |
|
|
| Total
rectal | 90 | 38 | 52 |
|
|
|
| B, Tumor disease
status |
|
|
Characteristic | n | Poor prognosis
group (n=63) | Good prognosis
group (n=91) |
χ2/t/Z-score | P-value |
|
| Median tumor
diameter, cm |
| 5.54 (4.20,
6.80) | 4.96 (4.20,
6.90) | −0.382 | 0.702 |
| Pathological
type |
|
|
| 0.059 | 0.808 |
|
Adenocarcinoma | 138 | 56 | 82 |
|
|
| Mucinous
adenocarcinoma + signet ring cell carcinoma | 16 | 7 | 9 |
|
|
| Tumor site |
|
|
| 0.192 | 0.908 |
|
Rectum | 63 | 27 | 36 |
|
|
| Right
half colon | 37 | 15 | 22 |
|
|
| Left
half colon | 54 | 21 | 33 |
|
|
| Tumor
classification |
|
|
| 0.283 | 0.595 |
|
Ulcer | 24 | 11 | 13 |
|
|
|
Bulge | 130 | 52 | 78 |
|
|
| Invasion depth |
|
|
| 3.616 | 0.164 |
|
T1-2 | 33 | 12 | 21 |
|
|
|
T3 | 82 | 30 | 52 |
|
|
|
T4 | 39 | 21 | 18 |
|
|
| Histological
differentiation |
|
|
| 1.467 | 0.226 |
|
Well-medium | 92 | 34 | 58 |
|
|
|
Low | 62 | 29 | 33 |
|
|
|
| C, Biochemical
indices |
|
|
Characteristic | n | Poor prognosis
group (n=63) | Good prognosis
group (n=91) |
χ2/t/Z-score | P-value |
|
| Mean ALB, g/l |
| 27.26±3.58 | 30.61±3.74 | 5.561 | <0.001 |
| Median Hb,
g/dl |
| 5.94
(4.80,7.60) | 9.23
(8.20,10.40) | −7.742 | <0.001 |
| Mean
miR-497-5p |
| 0.35±0.04 | 0.41±0.02 | 12.275 | <0.001 |
| Mean KLK5,
ng/ml |
| 3.82±1.41 | 3.28±1.33 | 2.417 | 0.017 |
| Median Ang-2,
g/l |
| 3.32
(3.20,3.60) | 3.19
(3.00,3.40) | −4.236 | <0.001 |
|
| D, Nutritional
scores |
|
|
Characteristic | n | Poor prognosis
group (n=63) | Good prognosis
group (n=91) |
χ2/t/Z-score | P-value |
|
| Median ALB-Hb
score |
| 5.00
(4.00,5.00) | 3.00
(2.00,4.00) | −7.727 | <0.001 |
Dependent and independent variable
assignment and multicollinearity analysis
Postoperative prognosis in patients with stage
II–III CRC was set as the dependent variable (0 for good prognosis
and 1 for poor prognosis; Table
II). Preoperative ALB, Hb, miR-497-5p, KLK5 and Ang-2 levels
and ALB-Hb score were assigned as independent variables (actual
measured values). The collinear analysis revealed that there was no
multicollinearity between levels of preoperative ALB, Hb,
miR-497-5p, KLK5 and Ang-2 and ALB-Hb scores (variation inflation
factor ≤10 and tolerance ≥0.1), which indicated their suitability
for inclusion in the Cox proportional hazards regression model.
 | Table II.Dependent and independent variable
assignment and multicollinearity analysis. |
Table II.
Dependent and independent variable
assignment and multicollinearity analysis.
| Factor | VIF value | Tolerance |
|---|
| ALB levels | 4.059 | 0.246 |
| Hb levels | 4.005 | 0.250 |
| ALB-Hb score | 8.753 | 8.753 |
| miR-497-5p
levels | 1.069 | 0.936 |
| KLK5 levels | 1.040 | 0.962 |
| Ang-2 levels | 1.167 | 0.857 |
Cox proportional risk regression
analysis of factors affecting the prognosis of patients with stage
II–III CRC
Multivariate Cox proportional hazards regression
analysis of significant univariate variables revealed that
preoperative ALB, Hb, miR-497-5p, KLK5 and Ang-2 levels and ALB-Hb
score were factors influencing the prognosis for patients with
stage II–III CRC following radical resection (all P<0.05;
Table III). These parameters were
incorporated into the prognosis model development for patients with
stage II–III CRC.
 | Table III.Multivariate Cox proportional risk
regression analysis of factors affecting the prognosis of patients
with stage II–III CRC. |
Table III.
Multivariate Cox proportional risk
regression analysis of factors affecting the prognosis of patients
with stage II–III CRC.
| Factors | β | SE | Z-score | P-value | OR | HR 95%CI |
|---|
| ALB levels | −0.271 | 0.039 | −6.892 | <0.001 | 0.763 | 0.706–0.824 |
| Hb levels | −0.161 | 0.059 | −2.703 | 0.007 | 0.851 | 0.758–0.957 |
| ALB-Hb score | 1.353 | 0.147 | 9.215 | <0.001 | 3.868 | 2.901–5.157 |
| miR-497-5p
levels | −6.518 | 1.414 | −4.610 | <0.001 | 0.001 | 0.000–0.024 |
| KLK5 levels | 0.214 | 0.089 | 2.403 | 0.016 | 1.238 | 1.040–1.474 |
| Ang-2 levels | 1.353 | 0.147 | 9.215 | <0.001 | 3.868 | 2.901–5.157 |
Nomogram, calibration curves for
predicting the prognosis of patients with stage II–III CRC
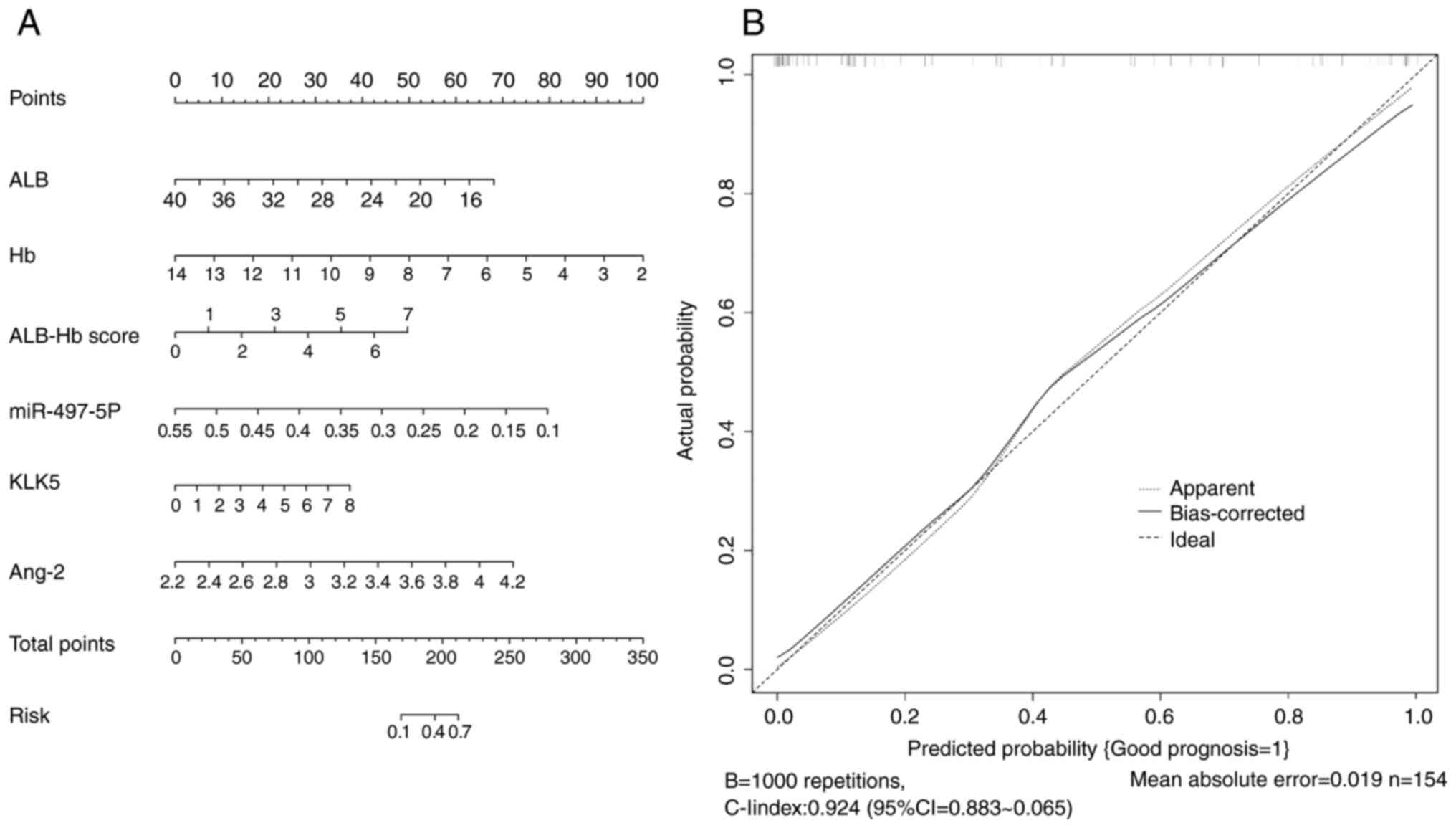
A nomogram and calibration curve were constructed
based on the aforementioned analysis (Fig. 1). The calibration curve closely
approximated the ideal curve, with a C-index of 0.803 (95% CI,
0.802–0.804). The nomogram demonstrated good accuracy and
predictive performance, indicating high accuracy of the Cox
proportional hazards prediction model. These results suggested that
preoperative ALB, Hb, miR-497-5p, KLK5 and Ang-2 levels and ALB-Hb
score have significant predictive value for poor prognosis in
patients with stage II–III CRC.
Discussion
For patients with stage I CRC, the 5-year survival
rate after surgery is >90%, indicating a high surgical success
rate, while patients with stage IV CRC (with distant metastasis)
who undergo palliative surgery, the 5-year survival rate is less
than 10%. However, stage II–III CRC constitutes the predominant
clinical cohort, which makes their postoperative outcomes relevant
for clinical practice. Investigating prognostic factors and their
predictive value in these patients may directly guide
post-resection intervention strategies and prolong survival
(29). In the present study, 154
patients with stage II–III CRC were stratified by postoperative
outcomes (63 in the poor and 91 in the good prognosis group).
Preoperative ALB, Hb, miR-497-5p, KLK5 and Ang-2 levels and ALB-Hb
score were significant prognostic factors with high predictive
value for adverse outcomes.
miR-497-5p, a recognized tumor suppressor, is
frequently downregulated in patients with CRC (30). Long non-coding RNA (lncRNA)
AC009022.1 exacerbates CRC progression by inhibiting miR-497-5p
expression, thereby enhancing cancer cell proliferation, migration
and invasion (31). Elevated
AC009022.1 levels are associated with poor patient prognosis, a
finding consistent with existing research on lncRNAs in CRC
advancement (27). A previous study
confirmed that the B cell lymphoma-2/miR-497 values are associated
with cancer metastasis and shorter survival in patients with CRC
(32). The poor prognosis group
exhibited significantly lower preoperative miR-497-5p levels
compared with the good prognosis group. Located on human chromosome
17p13.1, miR-497-5p serves as a molecular sponge to upregulate
solute carrier family 7 member 5, a transporter of large neutral
amino acids associated with tumor proliferation and invasiveness.
This mechanism modulates cell cycle progression, apoptosis and
other oncogenic processes (33).
Furthermore, miR-497-5p targets multiple oncogenes (for example,
26S proteasome non-ATPase regulatory subunit 7, a putative CRC
target) by binding their 3′-untranslated regions, suppressing gene
expression and promoting cancer cell apoptosis (34,35).
In patients with stage II–III CRC with limited lymph
node/metastasis spread, post-resection residual cancer cells may
disseminate via hematogenous/lymphatic routes. In this process,
miR-497-5p slows disease progression by decreasing the resistance
to chemotherapeutic drugs such as oxaliplatin, irinotecan or
5-fluorouracil and affecting cell function. By contrast, patients
with lower miR-497-5p levels demonstrate no inhibition of malignant
biological behaviors such as invasion, metastasis, and
proliferation in CRC cells. Therefore, patients with stage II–III
CRC with low preoperative miR-497-5p levels demonstrate more severe
malignant disease after surgery, resulting in a higher incidence of
adverse prognosis.
There is an association between metastasis and
recurrence of cancer following radical CRC surgery with the
weakening of the immune response and delayed wound healing
(36). Proteins are key for
synthesis and secretion of immune cells; insufficient protein
levels may impair the production and function of immune cells,
leading to a decrease in immune cell count and further weakening
the immune response capacity (37).
The tissue wound healing process requires a large amount of
protein, iron and other nutrients to support the synthesis of
collagen for repair. Furthermore, a regression cohort study of
patients with hepatocellular carcinoma reported that nutritional
status-associated scores helped predict the prognosis of patients
treated with anti-hepatocellular carcinoma therapy (38). The present study revealed that the
poor prognosis group had significantly lower ALB and Hb levels but
higher ALB-Hb scores compared with the good prognosis group. These
results suggested that nutritional status notably impacted
postoperative recovery in patients with stage II–III CRC after
radical resection, which aligns with previous research (39). ALB and Hb are key elements in the
assessment of nutritional status and immune function. ALB is
synthesized by liver with a normal range of 40–55 g/l; low
expression is common in malnourished individuals (40). Hb is a key indicator for the
assessment of anemia, with a normal range of 120–160 g/l in male
and 110–150 g/l in female patients; low expression commonly
presents in anemic individuals with insufficient oxygen supply
(41). Patients with cancer exhibit
a high metabolic state because the consumption of large amounts of
nutrient energy required for growth of malignant tumors weakens the
immune system and recovery ability of body (42). Certain patients with stage II–III
CRC demonstrate metastasis to neighboring tissue and organs before
surgery and incomplete surgical removal leads to continuous
consumption of energy, which triggers malnutrition. Furthermore,
postoperative chemotherapeutic drugs damage healthy cells, leading
to gastrointestinal dysfunction and affecting nutrient absorption,
thus aggravating malnutrition (43). Therefore, nutrition-associated
indicators such as preoperative levels of ALB, Hb and ALB-Hb scores
boast predictive value for prognostic outcomes in patients
undergoing radical surgery for stage II–III CRC.
Inflammation is a key factor in the development,
metastasis and drug resistance formation of various types of cancer
(44), including colorectal, liver,
pancreatic cancer, gastric cancer, and non-small cell lung cancer.
The inflammatory response activates KLK5, which exacerbates the
systemic inflammatory response via the secretion of
pro-inflammatory factors such as Tumor Necrosis Factor-α,
Interleukin-1β (IL-1β), and Interleukin-6 (IL-6), providing a
chronic inflammatory environment for tumor growth and accelerating
tumor progression (45). KLK5
participates in tumor neovascularization by cleaving extracellular
matrix components such as laminin, fibronectin, and type IV
collagen, facilitating tumor cell penetration through the basement
membrane. Furthermore, KLK5 increases intratumoral vascular
permeability, enhancing tumor cell transmigration across vascular
walls and lymphatic vessels (46).
Ang-2 is associated with intratumoral angiogenesis, tumor
invasiveness and metastatic potential (47). Ang-2 is also involved in tumor
angiogenesis, growth and metastasis by increasing vascular
permeability and reducing the ability of the immune system to
identify and remove tumor cells (48). Thus, the incidence of poor prognosis
in patients with cancer increases with Ang-2 levels (49). The present study demonstrated that
preoperative Ang-2 levels in the poor prognosis group were higher
compared with those in the good prognosis group, indicating that
aberrantly high Ang-2 may predict adverse outcomes following
radical resection in stage II–III CRC. Furthermore, patients with
stage II–III CRC with elevated preoperative KLK5/Ang-2 levels
demonstrated significantly higher postoperative inflammation risks.
Within the chronic inflammatory microenvironment, sustained KLK5
activation promotes tumor angiogenesis and vascular permeability,
facilitating nutrient supply for cancer progression. This cascade
further elevates Ang-2 levels, exacerbating vascular leakage and
causing uneven chemotherapeutic distribution while enhancing
circulating tumor cell invasiveness and distant metastasis
potential, collectively contributing to treatment resistance and
poor prognosis, thereby establishing preoperative Ang-2 as a
predictive biomarker for adverse outcomes post-radical resection
(50,51).
CRC is characterized by high postoperative
recurrence rates, which makes long-term prognostic monitoring
clinically key. However, the present study had limitations. The
present study only investigated 1-year postoperative outcomes in
patients with stage II–III CRC, representing a relatively short
follow-up period. As a two-center retrospective analysis, the
present study did not directly predict the long-term prognosis.
Furthermore, the results may be influenced by the accuracy of
original data. Future research should focus on long-term outcomes
post-radical resection by integrationg more clinicopathological
features (such as perineural invasion, lymphovascular invasion, and
number of lymph nodes examined), imaging characteristics, and
nutritional indicators (weight change, dietary habits, physical
activity levels) to develop a more comprehensive predictive
model.
In summary, ~60% of patients with stage II–III CRC
achieved favorable recovery within 1 year of radical tumor
resection, while a subset experienced poor postoperative outcomes.
Preoperative levels of ALB, Hb, miR-497-5p, KLK5 and Ang-2, along
with ALB-Hb score, were identified as notable prognostic factors
with high predictive value and clinical relevance. These biomarkers
should be incorporated into clinical screening protocols to guide
targeted interventions to potentially improve patient prognosis in
future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QW, HW and LL conceived and designed the present
study. QW and HW confirm the authenticity of all the data in the
present study. ZW contributed to data collection and analysis. HW
wrote the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Haihe Hospital (Tianjin, China) [approval no.
2024HHWZ(A)-003] and all procedures were performed in accordance
with the Declaration of Helsinki. The requirement for informed
consent was waived due to the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023.PubMed/NCBI
|
|
2
|
Li X, Jonnagaddala J, Yang S, Zhang H and
Xu XS: A retrospective analysis using deep-learning models for
prediction of survival outcome and benefit of adjuvant chemotherapy
in stage II/III colorectal cancer. J Cancer Res Clin Oncol.
148:1955–1963. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamura Y, Yamaura T, Kinjo Y, Harada K,
Kawase M, Kawabata Y, Kanto S, Ogo Y and Kuroda N: Level of
inferior mesenteric artery ligation in sigmoid colon and rectal
cancer surgery: analysis of apical lymph node metastasis and
recurrence. Dig Surg. 40:167–177. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geng S, Yu X and Yu S: Efficacy and safety
of natural killer cells injection combined with XELOX chemotherapy
in postoperative patients with stage III colorectal cancer in
China: A prospective randomised controlled clinical trial study
protocol. BMJ Open. 14:e0803772024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi K, Ono Y, Kitano Y, Oba A, Sato
T, Ito H, Mise Y, Shinozaki E, Inoue Y, Yamaguchi K, et al:
Prognostic impact of tumor markers (CEA and CA19-9) on patients
with resectable colorectal liver metastases stratified by tumor
number and size: Potentially valuable biologic markers for
preoperative treatment. Ann Surg Oncol. 30:7338–7347. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grange R, Rousset P, Williet N, Guesnon M,
Milot L, Passot G, Phelip JM, Le Roy B, Glehen O and Kepenekian V:
Metastatic colorectal cancer treated with combined liver resection,
cytoreductive surgery, and hyperthermic intraperitoneal
chemotherapy (HIPEC): Predictive factors for early recurrence. Ann
Surg Oncol. 31:2378–2390. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elfiky AM, Eid MM, El-Manawaty M, Elshahid
ZA, Youssef EM and Mahmoud K: Production of novel theranostic
nano-vector based on superparamagnetic iron oxide
nanoparticles/miR-497 targeting colorectal cancer. Sci Rep.
15:42472025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han S, Chen Y, Wang Y and Xu H:
Application of problem-oriented nursing model combined with early
enteral nutrition support in the perioperative period of stage
II/III gastric cancer patients. Nutr Cancer. 77:1028–1034. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Q, Li Y and Wang T: Development and
validation of prediction model for early warning of ovarian
metastasis risk of endometrial carcinoma. Medicine (Baltimore).
102:e354392023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J, Du L, Lei X and Zhang Z: Prognostic
value of esophageal cancer immune prognostic index in advanced
esophageal squamous cell carcinoma patients with anti-programmed
cell death-1 therapy. Cancer Med. 12:11334–11343. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng Q, Shen Y, Zhao P, Cheng M, Wu Y and
Zhu Y: Biomarker implication of kallikrein-related peptidases as
prognostic tissue substrates of poor survival in colorectal cancer.
Cancer Cell Int. 20:2602020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abuduhadeer X, Xu X, Aihesan K, Yilihamu
M, Zhao Y and Zhang W: Clinical significance of kallikrein 5 as a
novel prognostic biomarker in gastric adenocarcinoma. J Clin Lab
Anal. 35:e239582021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang X, Zheng S, Li S, Huang Y, Zhang W,
Liu F and Cao Q: Machine learning-based pathomics model predicts
angiopoietin-2 expression and prognosis in hepatocellular
carcinoma. Am J Pathol. 195:561–574. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng J, Du PZ, Yang C, Tao YY, Li L, Li
ZM and Yang L: DCE-MRI-based radiomics in predicting angiopoietin-2
expression in hepatocellular carcinoma. Abdom Radiol (NY).
48:3343–3352. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saeed A, Park R, Pathak H, Al-Bzour AN,
Dai J, Phadnis M, Al-Rajabi R, Kasi A, Baranda J, Sun W, et al:
Clinical and biomarker results from a phase II trial of combined
cabozantinib and durvalumab in patients with
chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC
cohort. Nat Commun. 15:15332024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishikawa S, Hirano Y, Deguchi K, Ishii T,
Ishiyama Y, Okazaki N, Fujii T, Kataoka A, Sasaki M, Shimamura S
and Yonezawa H: Risk factors for lymph node metastasis and
recurrence in T1 colorectal cancer: analysis of 801 patients in a
single institute. Am Surg. 89:5312–5317. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishimaru K, Kawai K, Nozawa H, Sasaki K,
Murono K, Emoto S, Ishii H, Anzai H, Sonoda H, Yamauchi S, et al:
Hazard function analysis of metastatic recurrence after colorectal
cancer surgery-A nationwide retrospective study. J Surg Oncol.
123:1015–1022. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koedam TWA, Bootsma BT, Deijen CL, van de
Brug T, Kazemier G, Cuesta MA, Fürst A, Lacy AM, Haglind E, Tuynman
JB, et al: Oncological outcomes after anastomotic leakage after
surgery for colon or rectal cancer: Increased risk of local
recurrence. Ann Surg. 275:e420–e427. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alkader MS, Al-Majthoub MZ, Al-Qerem WA,
Alkhader DM, Alhusban AM, Abdulkareem MA, Abweny B, Hamawi AT,
Muslem HF, Omeish RA, et al: Prognostic factors influencing
survival in stage II and stage III colorectal cancer patients.
Cureus. 15:e465752023.PubMed/NCBI
|
|
20
|
Dinart D, Bellera C and Rondeau V: Sample
size estimation for cancer randomized trials in the presence of
heterogeneous populations. Biometrics. 78:1662–1673. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Argilés G, Tabernero J, Labianca R,
Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P,
Yoshino T, Taieb J, et al: Localised colon cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 31:1291–1305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morris VK, Kennedy EB, Baxter NN, Benson
AB III, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming
DA, et al: Treatment of metastatic colorectal cancer: ASCO
guideline. J Clin Oncol. 41:678–700. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Yang Z, Lyu Z, Ouyang K, Wang J,
Wu D and Li Y: Pathological-features-modified TNM staging system
improves prognostic accuracy for rectal cancer. Dis Colon Rectum.
67:645–654. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiina O, Kudo SE, Ichimasa K, Takashina
Y, Kouyama Y, Mochizuki K, Morita Y, Kuroki T, Kato S, Nakamura H,
et al: Differentiation grade as a risk factor for lymph node
metastasis in T1 colorectal cancer. DEN Open. 4:e3242023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joanito I, Wirapati P, Zhao N, Nawaz Z,
Yeo G, Lee F, Eng CLP, Macalinao DC, Kahraman M, Srinivasan H, et
al: Single-cell and bulk transcriptome sequencing identifies two
epithelial tumor cell states and refines the consensus molecular
classification of colorectal cancer. Nat Genet. 54:963–975. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan Y, Liu R, Xue JW and Feng Z:
Construction and validation of artificial intelligence pathomics
models for predicting pathological staging in colorectal cancer:
Using multimodal data and clinical variables. Cancer Med.
13:e69472024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu C and Zhang F: LncRNA AC009022.1
enhances colorectal cancer cells proliferation, migration, and
invasion by promoting ACTR3B expression via suppressing miR-497-5p.
J Cell Biochem. 121:1934–1944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou J and Yang D: Prognostic significance
of hemoglobin, albumin, lymphocyte and platelet (HALP) score in
hepatocellular carcinoma. J Hepatocell Carcinoma. 10:821–831. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Zhang X, Zhao D, Hu M, Ge X and
Xia L: An individualized EMT-related gene signature to predict
recurrence-free survival in stage II/III colorectal cancer
patients. Dig Dis Sci. 67:5116–5126. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gattuso G, Longo F, Spoto G, Ricci D,
Lavoro A, Candido S, Di Cataldo A, Broggi G, Salvatorelli L, Magro
G, et al: Diagnostic and prognostic significance of a four-miRNA
signature in colorectal cancer. Int J Mol Sci. 26:12192025.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Lei C, Chen B and Zhu Q: LncRNA
FGD5-AS1 facilitates the radioresistance of breast cancer cells by
enhancing MACC1 expression through competitively sponging
miR-497-5p. Front Oncol. 11:6718532021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kattan SW, Hobani YH, Abubakr Babteen N,
Alghamdi SA, Toraih EA, Ibrahiem AT, Fawzy MS and Faisal S:
Association of B-cell lymphoma 2/microRNA-497 gene expression ratio
score with metastasis in patients with colorectal cancer: A
propensity-matched cohort analysis. J Clin Lab Anal. 36:e242272022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song M and Liu J: Circ_0067717 promotes
colorectal cancer cell growth, invasion and glutamine metabolism by
serving as a miR-497-5p sponge to upregulate SLC7A5. Histol
Histopathol. 38:53–64. 2023.PubMed/NCBI
|
|
34
|
Bai J, Xu J, Zhao J and Zhang R: lncRNA
SNHG1 cooperated with miR-497/miR-195-5p to modify
epithelial-mesenchymal transition underlying colorectal cancer
exacerbation. J Cell Physiol. 235:1453–1468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen K, Wang Z, Zong QB, Zhou MY and Chen
QF: miR-497-5p-RSPO2 axis inhibits cell growth and metastasis in
glioblastoma. J Cancer. 13:1241–1251. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao X, Dou LZ, Zhang YM, Liu Y, He S, Ke
Y, Liu XD, Liu YM, Wu HR, Li ZQ, et al: Risk factors for residual
cancer or lymph node metastasis after endoscopic noncurable
resection of early colorectal cancer. Zhonghua Zhong Liu Za Zhi.
45:335–339. 2023.(In Chinese). PubMed/NCBI
|
|
37
|
Wang L, Chen X, Zhang H, Hong L, Wang J,
Shao L, Chen G and Wu J: Comprehensive analysis of transient
receptor potential channels-related signature for prognosis, tumor
immune microenvironment, and treatment response of colorectal
cancer. Front Immunol. 13:10148342022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Z, Liang Y, Zhong D, Dai Z, Shang J,
Lai C, Zou H, Yao Y, Feng T and Huang X: Prognostic value of
inflammation-immunity-nutrition score in patients with
hepatocellular carcinoma treated with anti-PD-1 therapy. J Clin Lab
Anal. 36:e243362022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lv Q, Rao SQ and Xiang Z: Preoperative
hemoglobin to albumin ratio as a prognostic predictor for patients
with colorectal cancer surgery. Updates Surg. 77:761–769. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Záhorec R, Marek V, Waczulikova I,
Veselovský T, Palaj J, Kečkéš Š and Durdík Š: Predictive model
using hemoglobin, albumin, fibrinogen, and neutrophil-to-lymphocyte
ratio to distinguish patients with colorectal cancer from those
with benign adenoma. Neoplasma. 68:1292–1300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li K, Yan J, Zhang H, Lu C, Wang W, Guo M,
Zhang X and Zhang Z: Prognostic value of preoperative white blood
cell to hemoglobin ratio and fibrinogen to albumin ratio in
patients with colorectal cancer. Medicine (Baltimore).
103:e370312024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ambrosio MR, Spagnoli L, Perotti B,
Petrelli F, Caini S, Saieva C, Usai S, Bianchini M, Cavazzana A,
Arganini M and Amorosi A: Paving the path for immune enhancing
nutrition in colon cancer: Modulation of tumor microenvironment and
optimization of outcomes and costs. Cancers (Basel). 15:4372023.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fang Y, Lin L, Ruan H and Qin X: Natural
orifice specimen extraction surgery yields superior long-term
oncological outcomes compared to traditional laparoscopic surgery
in stage II–III rectal cancer. Am J Cancer Res. 15:3286–3298. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pei J, Gao Y and Wu A: An
inflammation-related subtype classification for analyzing tumor
microenvironment and clinical prognosis in colorectal cancer. Front
Immunol. 15:13697262024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang JS, Kim N, Kim JY, Do SI, Cho Y, Kim
HS and Kim YB: Kallikrein 5 overexpression is associated with poor
prognosis in uterine cervical cancer. J Gynecol Oncol. 31:e782020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alves MG, Kodama MH, da Silva EZM, Gomes
BBM, da Silva RAA, Vieira GV, Alves VM, da Fonseca CK, Santana AC,
Cecílio NT, et al: Relative expression of KLK5 to LEKTI is
associated with aggressiveness of oral squamous cell carcinoma.
Transl Oncol. 14:1009702021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ao J, Chiba T, Kanzaki H, Kanayama K,
Shibata S, Kurosugi A, Iwanaga T, Kan M, Sakuma T, Qiang N, et al:
Serum angiopoietin 2 acts as a diagnostic and prognostic biomarker
in hepatocellular carcinoma. J Cancer. 12:2694–2701. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song Y, Bai G, Li X, Zhou L, Si Y, Liu X,
Deng Y and Shi Y: Bioinformatics analysis of human kallikrein 5
(KLK5) expression in metaplastic triple-negative breast cancer.
Cancer Innov. 2:376–390. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kraljević M, Marijanović I, Barbarić M,
Sokolović E, Bukva M, Cerić T and Buhovac T: Prognostic and
predictive significance of VEGF, CD31, and Ang-1 in patients with
metastatic clear cell renal cell carcinoma treated with first-line
sunitinib. Biomol Biomed. 23:161–169. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Papachristopoulou G, Malachias A, Devetzi
M, Kamouza E, Scorilas A, Xynopoulos D and Talieri M: Uncovering
the clinical impact of kallikrein-related peptidase 5 (KLK5) mRNA
expression in the colorectal adenoma-carcinoma sequence. Clin Chem
Lab Med. 57:1251–1260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Antoniotti C, Marmorino F, Boccaccino A,
Martini S, Antista M, Rossini D, Zuco V, Prisciandaro M, Conca V,
Zucchelli G, et al: Early modulation of Angiopoietin-2 plasma
levels predicts benefit from regorafenib in patients with
metastatic colorectal cancer. Eur J Cancer. 165:116–124. 2022.
View Article : Google Scholar : PubMed/NCBI
|