Introduction
Melanoma, a malignancy arising from melanocytes,
demonstrates a rising global incidence, although a concurrent
decline in mortality has been observed in recent years (1). Histopathologically, melanomas have
been classically categorized into three main subtypes based on the
presence of a radial growth phase: Nodular melanoma, superficial
spreading melanoma and lentigo maligna melanoma (2).
Melanomas were divided into those etiologically
related to sun exposure and those that are not, as determined by
their mutational signatures, anatomic site and epidemiology.
Melanomas on the sun-exposed skin were further divided by the
histopathologic degree of cumulative solar damage (CSD) of the
surrounding skin, into low and high CSD, on the basis of degree of
associated solar elastosis. Low-CSD melanomas include superficial
spreading melanomas and high-CSD melanomas incorporate lentigo
maligna and desmoplastic melanomas. The ‘nonsolar’ category
includes acral melanomas, some melanomas in congenital nevi,
melanomas in blue nevi, Spitz melanomas, mucosal melanomas and
uveal melanomas (3).
Furthermore, an increased number of melanocytic nevi
and the presence of atypical nevi are established risk factors for
melanoma development. Notably, the presence of multiple atypical
nevi is associated with a six-fold increase in the risk of melanoma
formation, a stronger association compared to the presence of a
single atypical nevus (4).
Surgical resection remains the primary treatment for
early-stage disease. However, melanoma is highly aggressive and
prone to distant metastasis. For patients with stage IV melanoma,
where the disease is generally no longer amenable to curative
surgical resection, systemic therapy thus becomes particularly
crucial. Currently approved first-line regimens for advanced
melanoma include programmed cell death protein-1 (PD-1) inhibitors
as monotherapy, nivolumab plus relatlimab, and ipilimumab combined
with nivolumab, and for patients with BRAF mutation, combination
BRAF/mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitor
therapy (5). Nevertheless, the
efficacy of targeted therapy is often compromised by the emergence
of drug resistance. For instance, resistance to BRAF inhibitors,
such as PLX4720 (an analog of vemurafenib) and dabrafenib, can
reduce their efficacy (6).
Additionally, in the context of combined BRAF and MEK inhibition,
studies using the well-characterized mutant V600E BRAF model have
demonstrated that acquired resistance can develop. Specifically,
continuous treatment with the combination of PLX4720 and PD0325901
(Mirdametinib) for >77 days led to the development of acquired
resistance, evidenced by the regrowth of three
combination-resistant tumors (7).
Furthermore, several novel treatment modalities under
investigation, such as oncolytic viruses (OVs) and neoadjuvant
therapy, hold notable promise. The present study describes the case
of a young male patient with early-stage cutaneous melanoma who
developed widespread systemic metastases and acquired resistance to
targeted therapy 3 years after surgery. The present case highlights
the key need for rigorous postoperative follow-up and emphasizes
the urgency of developing strategies to potentially overcome
resistance to targeted agents in the future.
Case report
In November 2024, a 24-year-old man was admitted to
Binzhou People's Hospital (Binzhou, China), due to epigastric pain
and discomfort lasting for 10 days. In January 2021, approximately
3 years prior to this, the patient had presented with black papules
on the anterior chest that had been present for over a decade,
which were initially excised in an outpatient setting at Binzhou
People's Hospital (Binzhou, China) under the diagnosis of a dermal
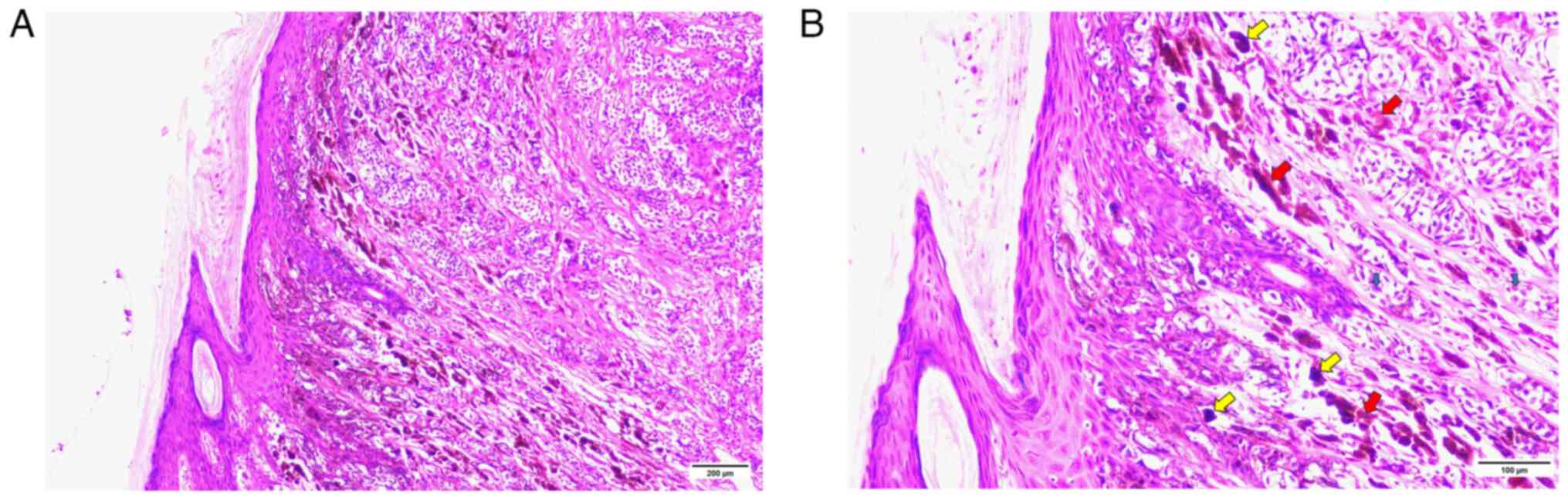
melanocytic nevus. Postoperative pathology revealed cutaneous
epithelioid malignant melanoma with a Breslow thickness of 0.75 mm
and no ulceration (Fig. 1). In
January 2021, the patient underwent wide local excision of the
anterior thoracic lesion, along with sentinel lymph node biopsy of
the left supraclavicular region under general anesthesia. Pathology
from this procedure revealed acute and chronic inflammation of the
skin and subcutaneous tissues without evidence of tumor cells
(according to pathology report; data not shown). The
histopathological analysis was performed according to standard
procedures. None of the nine lymph nodes examined contained
metastatic tumor. Based on these findings and the American Joint
Committee on Cancer (AJCC) Cancer Staging Manual, Eighth Edition
(2017), the patient was classified as having T1aN0M0, stage IA
disease (8). No adjuvant therapy
was administered postoperatively. Follow-up imaging, including
chest (Fig. S1A and B) and
abdominal (Fig. S1C and D) CT, and
ultrasounds of the submandibular (Fig.
S2A and B), cervical supraclavicular (Fig. S2C and D), axillary (Fig. S2E and F) and inguinal (Fig. S2G and H) regions, conducted 6
months and 1 year after surgery, demonstrated no abnormalities. In
January 2022, the patient underwent a laparoscopic appendectomy for
acute appendicitis; intraoperative exploration at that time
revealed no notable visceral abnormalities.
Upon the current admission in November 2024, the
patient presented with a cachectic appearance but was otherwise
without notable comorbidities. Hematological tests were within
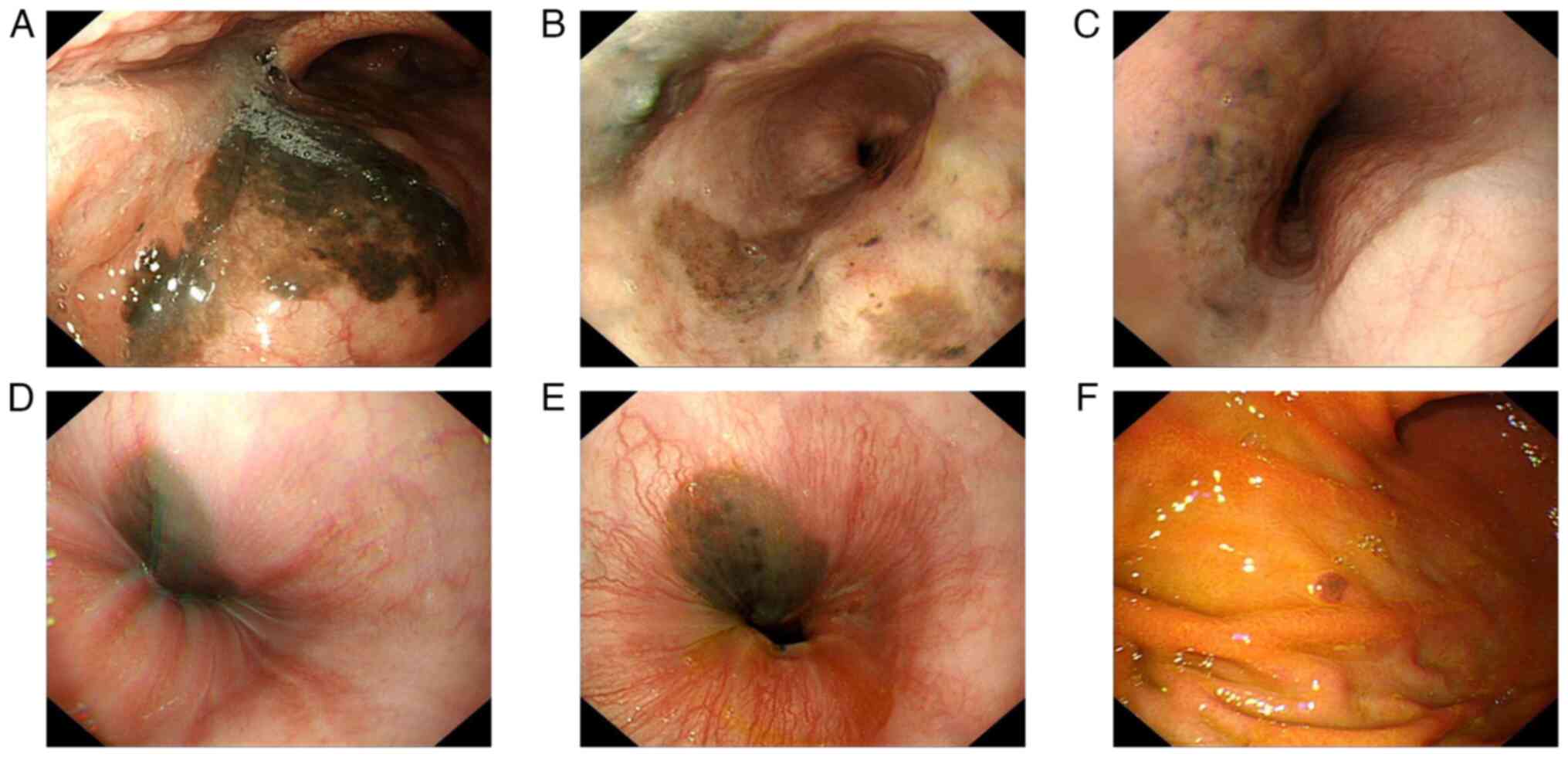
normal limits. Gastroscopy, however, revealed extensive melanosis
throughout the upper gastrointestinal tract, involving the pharynx
(Fig. 2A) and the entire esophagus
(Fig. 2B-E). This diffuse black
mucosal appearance is consistent with the endoscopic presentation
of melanoma metastasis (9,10). A pigmented protruding lesion, ~0.5
cm in diameter, in the gastric body (Fig. 2F) was biopsied and confirmed as
metastatic melanoma. These findings not only explained the
abdominal pain of the patient but also indicated advanced disease.
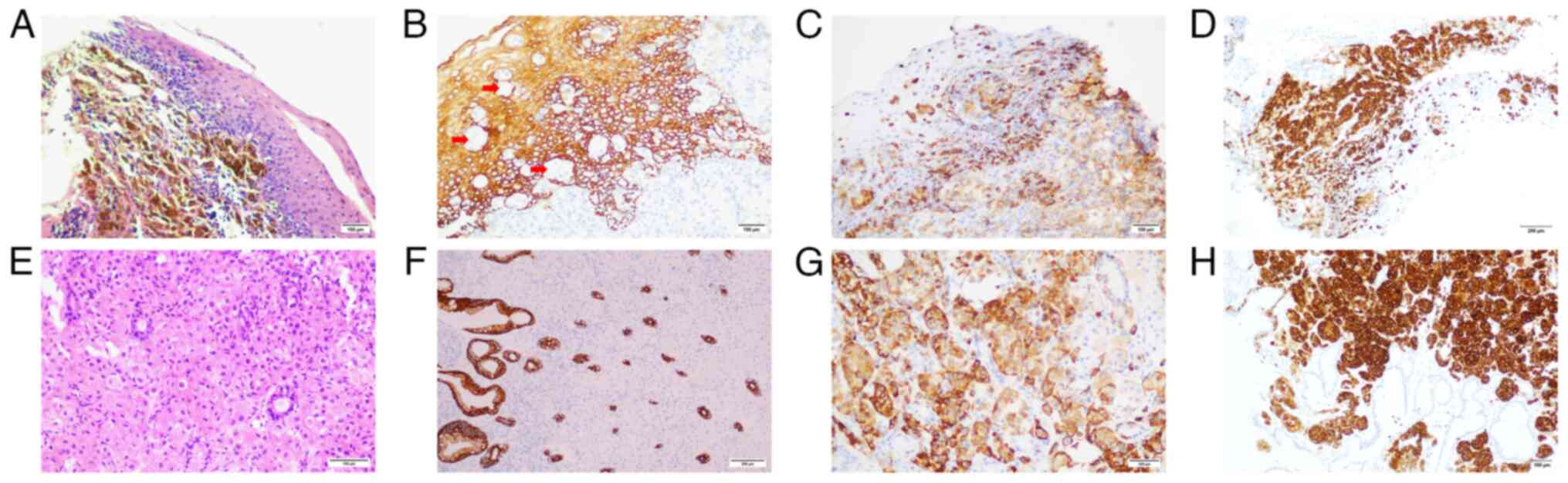
Immunohistochemistry was key in confirming the diagnosis: Lesional
cells from both esophageal (Fig.
3A-D) and gastric (Fig. 3E-H)
sites were negative for the epithelial marker pan-cytokeratin
(CKpan) (Fig. 3B and F), but
positive for the melanocytic markers human melanoma black 45
(HMB45) (Fig. 3C and G) and
melanoma antigen A (Melan-A) (Fig. 3D
and H), confirming metastatic melanoma. Tissue specimens were
fixed in 3.7% neutral buffered formalin at room temperature for 12
h and embedded in paraffin. Sections were cut to a thickness of 4
µm. The blocking reagent used was 3% H2O2,
applied at room temperature for 10 min. The primary antibodies, all
ready-to-use, were obtained from Fuzhou Maixin Biotechnology
Development Co., Ltd., and incubated at 37°C for 30 min (CKpan:
Rabbit polyclonal, cat. no. RAB-0050; HMB45: Mouse monoclonal, cat.
no. MAB-0360; Melan-A: Mouse monoclonal, cat. no. MAB-0275). The
MaxVision™ Detection Kit (HRP-conjugated, ready-to-use;
cat. no. KIT-5010) from Fuzhou Maixin was used as the secondary
reagent, with incubation at 37°C for 15 min. A BRAF mutation was
also identified, providing a molecular basis for targeted therapy.
The BRAF mutation was identified using the Human Braf Gene Mutation
Detection Kit (PCR fluorescent probe method) from DoGene Inc., with
amplification and detection performed on an ABI 7500 real-time PCR
instrument, strictly following the manufacturer's instructions.
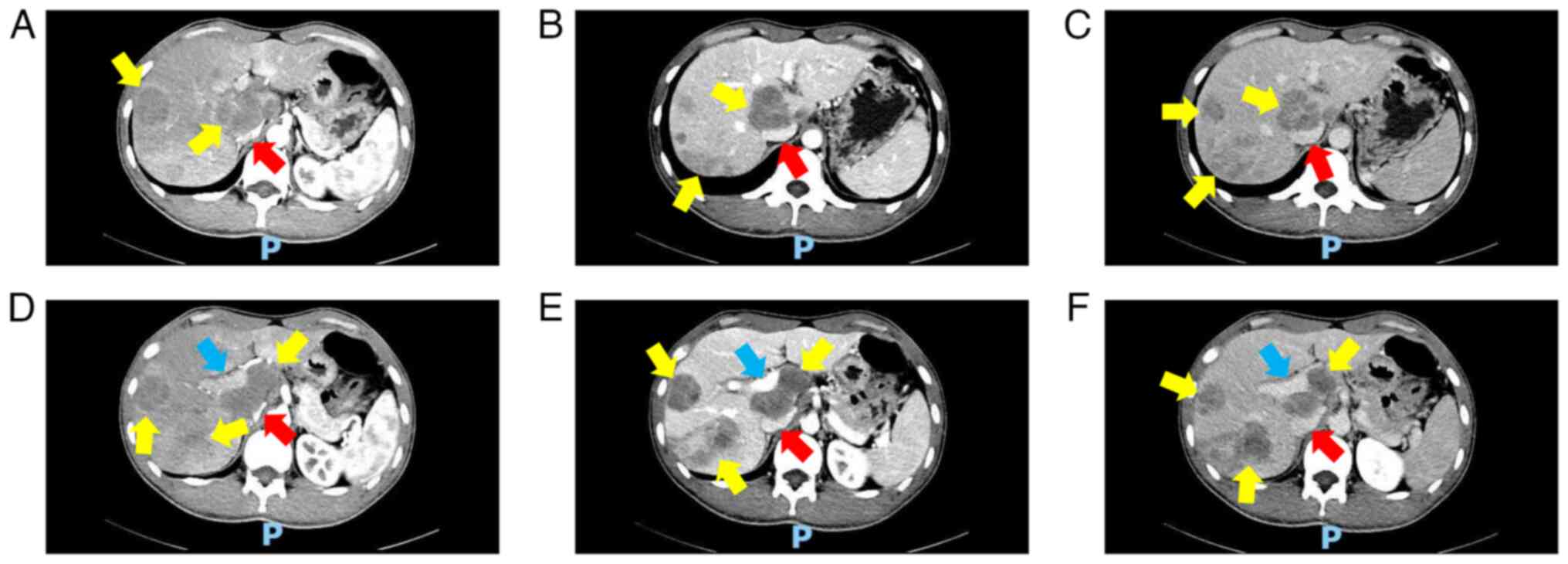
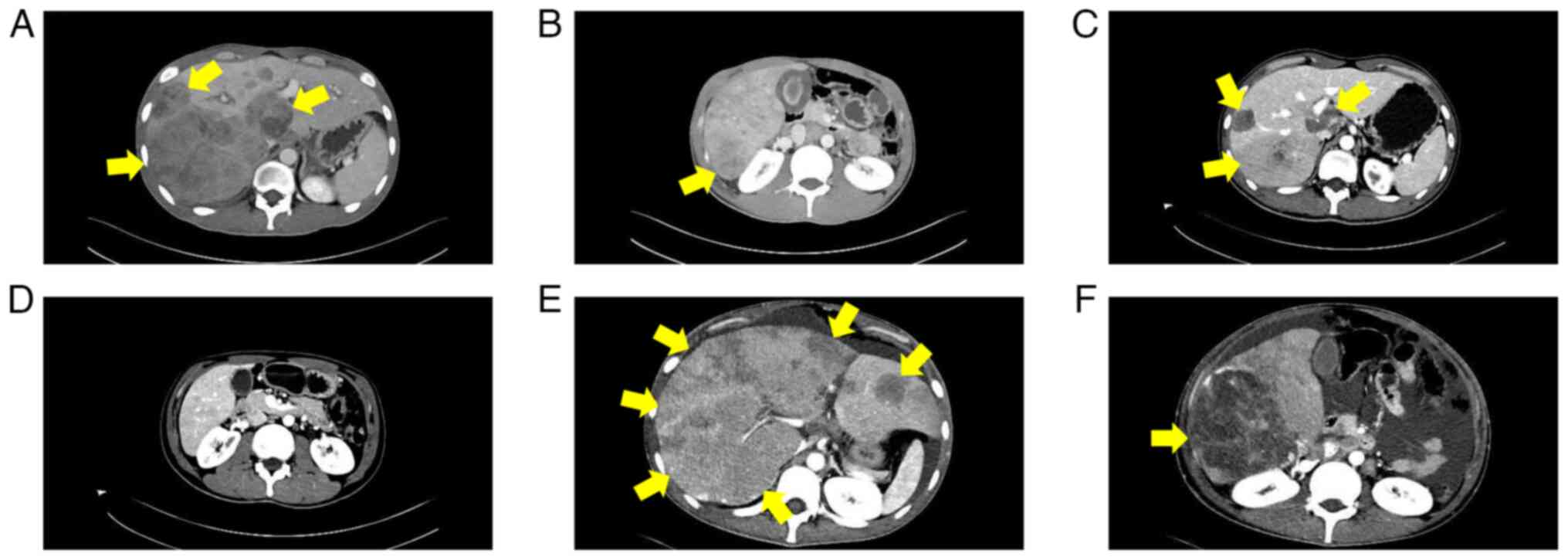
Abdominal CT (Fig. 4) exhibited
numerous metastatic lesions throughout the liver, including a large
irregular mass in the porta hepatis compressing the portal vein and
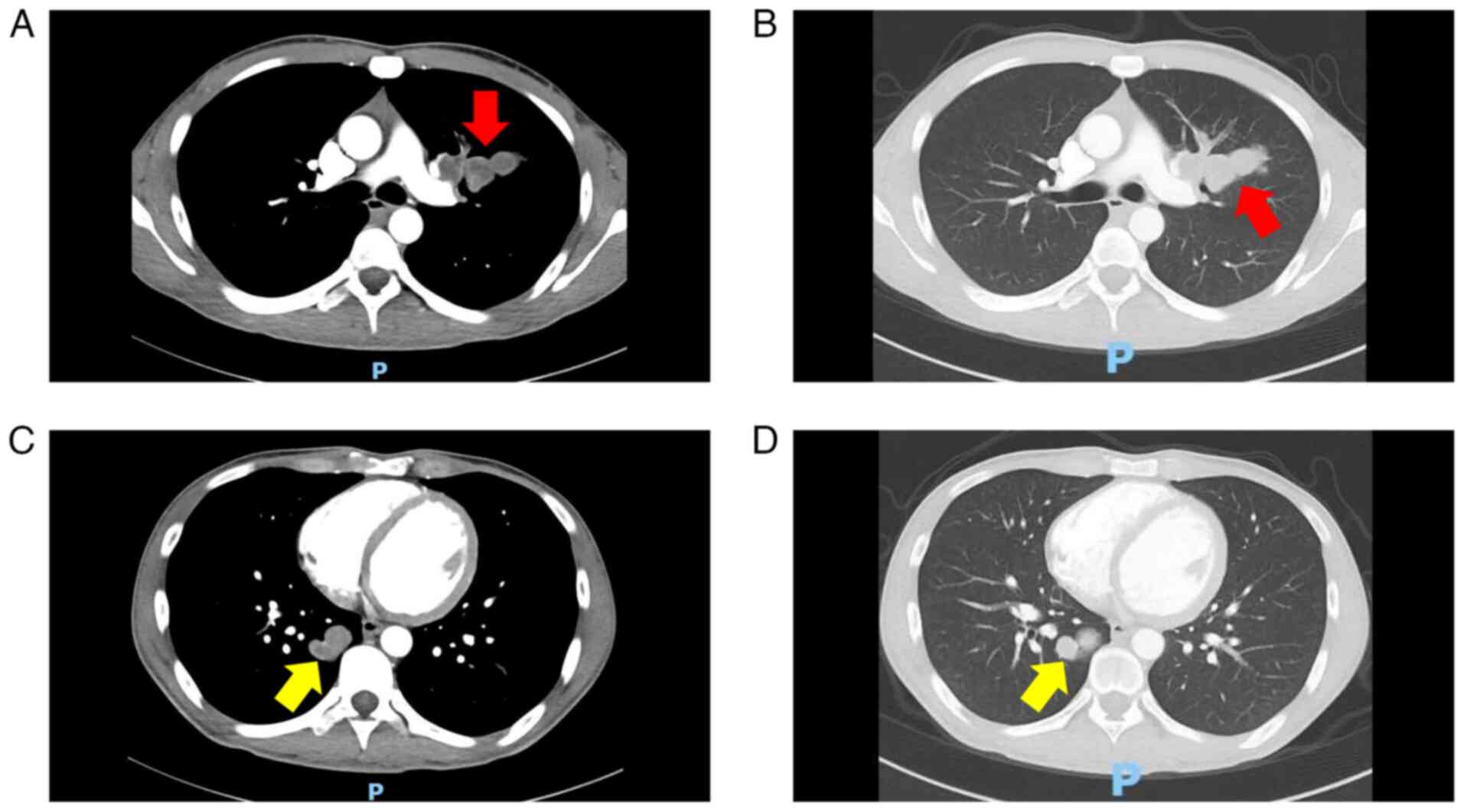
inferior vena cava. Chest CT (Fig.
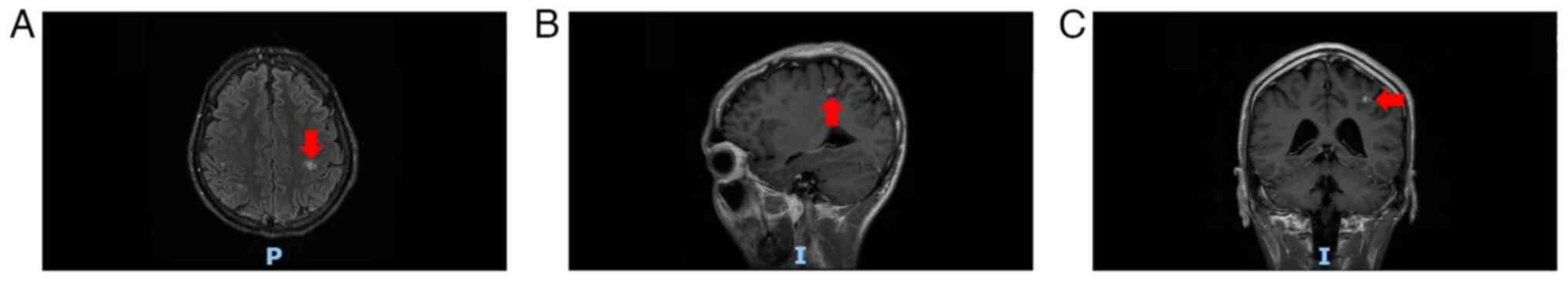
5) and cranial MRI (Fig. 6)
further revealed multiple metastases in the lungs and intracranial
cavity, respectively. A final diagnosis of stage IV melanoma was
established. Due to the extensive metastatic burden, the patient
was not a candidate for surgery and the prognosis was considered to
be poor. Following a multidisciplinary team discussion in November
2024, combined targeted therapy with dabrafenib (150 mg orally
twice daily, administered as two 75-mg tablets per dose) and
trametinib (2 mg orally once daily, administered as one 2-mg
tablet) was initiated.
Serial abdominal CT scans documented the clinical
course, illustrating both initial treatment response and subsequent
resistance (Fig. 7). Abdominal CT
scans in January 2025 (Fig. 7B and
E) revealed marked reduction in liver metastases compared with
the first follow-up scan obtained 1 week after treatment initiation
(November 2024; Fig. 7A and D),
indicating a partial response. However, in early February 2025, the
patient suffered a rupture of a liver metastasis, which was managed
with emergency transcatheter arterial chemoembolization at Binzhou
People's Hospital. Subsequent follow-up imaging in late February
2025 (Fig. 7C and F) revealed
notable regrowth of the liver lesions, demonstrating acquired
resistance to targeted therapy. Laboratory tests from the same date
indicated acute liver failure, with elevated transaminases [alanine
transaminase, 523 U/l (reference range, 0–50 U/l); aspartate
aminotransferase, 1,541 U/l (reference range, 17–59 U/l)],
hyperbilirubinemia [total bilirubin, 115.7 µmol/l (reference range,
3–22 µmol/l)] and coagulopathy [prothrombin time, 29.9 sec
(reference range 8.8–13.8 sec)].
The patient succumbed to the disease in March 2025,
due to liver failure and multiple organ failure. The overall
survival from stage IV diagnosis to mortality was 4 months.
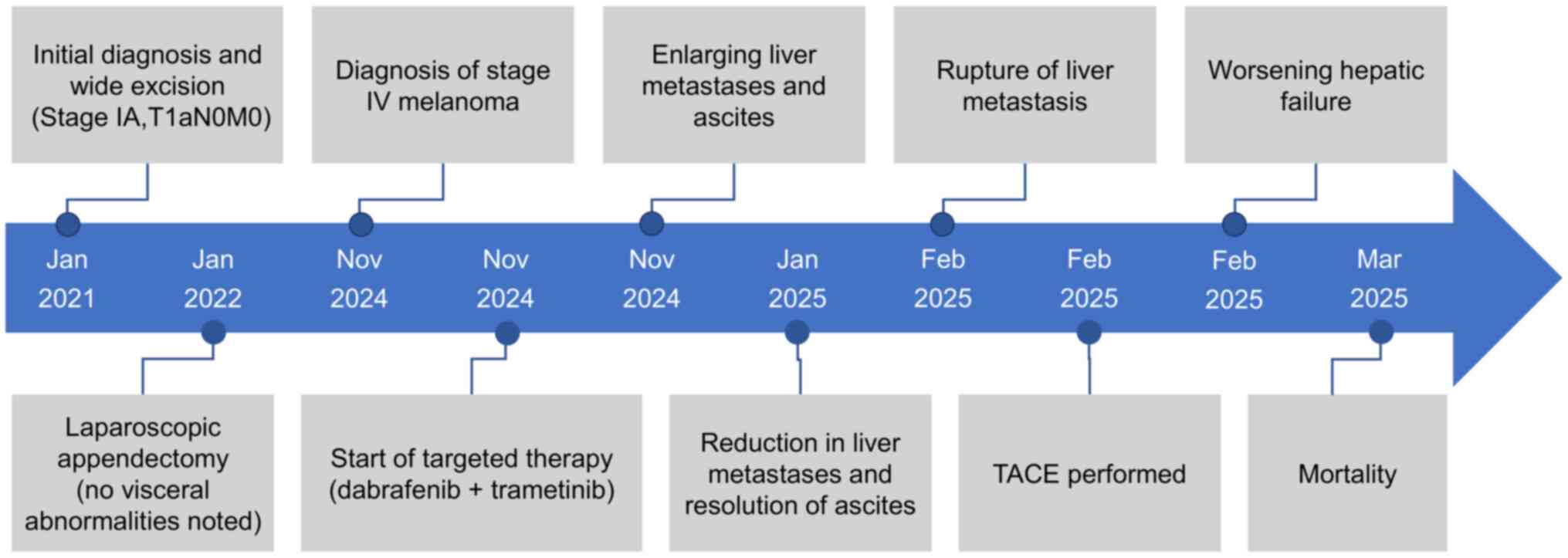
Fig. 8 provides a visual timeline
summarizing this rapid and complex clinical course, from diagnosis
and initial treatment response to disease progression and
mortality.
Discussion
Melanoma ranks among the most serious forms of skin
cancer. According to GLOBOCAN 2022 estimates, there were ~331,722
new melanoma cases globally, accounting for ~21% of all skin cancer
diagnoses (11). Despite this
comparatively lower incidence, melanoma was responsible for ~58,667
annual deaths, resulting in a case-fatality rate of ~17.7%. For
context, non-melanoma skin cancers, which comprise ~79% of all skin
cancer cases, resulted in an estimated 69,416 deaths in the same
period (12). This disparity
underscores the disproportionate lethality of melanoma relative to
its incidence. In the United States, an estimated 104,960 novel
cases of cutaneous melanoma and 8,430 associated mortalities are
projected for 2025. Between 2015 and 2021, the 5-year relative
survival rate was 94.7% (13).
Furthermore, the 5-year relative survival rate for cutaneous
melanoma across all ethnic groups in the United States has
demonstrated a consistent upward trend from 1975 to 2019 (1). Survival outcomes are strongly
influenced by the stage at diagnosis. Specifically, patients with
localized melanoma exhibit a 5-year survival rate of ~100%, whereas
those with regional lymph node involvement or distant metastases
exhibit 5-year relative survival rates of 75.7 and 34.6%,
respectively (13).
Malignant cutaneous melanoma occurs more frequently
in Caucasian populations (14). In
Europe, North America and Oceania, the trunk is the most common
site of occurrence in men (31–58%), whereas women more commonly
develop melanoma on the lower limbs and hips (26–40%). In
South-East Asia, both men (41–58%) and women (37–60%) demonstrate a
higher incidence of melanoma on the lower limbs and hips (15). Epidemiological studies report that
primary melanomas of the head, neck and trunk demonstrate a male
predominance (16,17), whereas melanomas of the lower limbs
show a female predominance (18).
Data from a study of 1,177 patients with stage I–II melanoma
identified the head and neck as a high-risk primary site. Head and
neck melanomas were overrepresented in metastatic cohorts,
accounting for 35 of 108 (32.4%) lymphatic metastases and 37 of 108
(34.3%) hematogenous metastases. Survival analysis further
confirmed this elevated risk, demonstrating a 5-year hematogenous
metastasis-free survival of 78.9%-the lowest of all sites- and a
5-year lymphatic metastasis-free survival of 84.7%, which was only
marginally higher than that for acral melanomas. Consequently, head
and neck melanomas feature high aggressiveness, frequent recurrence
and a significantly worse overall survival profile (19). Melanoma incidence is generally
higher in men, with a rate ~1.6 times higher compared with that in
women (20). Nevertheless, melanoma
remains one of the most common cancer types in young adults aged
25–29 years, particularly among young women (21,22).
In European populations, among adolescents and young adults (aged
15–39 years), the incidence is higher in women (8.99%) compared
with that in men (4.86%) (23).
According to Surveillance, Epidemiology and End Results data from
2018–2022, the highest proportion of primary cutaneous melanoma
cases occurred in individuals aged 65–74 years (27.4%), followed by
those aged 55–64 years (21.5%). By contrast, only 4.4% of cases
were diagnosed in patients <34 years of age (13). Overall, the risk of developing
melanoma increases with age (22).
Melanomas have a pronounced tendency to metastasize
to distant sites, with trunk melanomas exhibiting a particularly
high risk of distant spread. The most common first site of distant
metastasis is the skin (38%), followed by the lungs (36%), liver
(20%) and brain (20%) (24). Median
survival time varies according to the number of metastatic sites,
with 7 months recorded for patients with one distant metastasis
site, 4 months for those with two sites and only 2 months for those
with three or more sites (24).
However, the clinical features of the present case
are markedly divergent from the aforementioned epidemiological
statistics, highlighting its distinctive value as a cautionary
case. While epidemiological data indicate that primary cutaneous
melanoma most frequently occurs in the age group of 65–74 years
(13), the present patient was 21
years old at initial diagnosis, representing a considerably younger
demographic for this disease. More notably, the primary lesion was
located on the chest, with a Breslow thickness of only 0.75 mm,
absence of ulceration, no evidence of metastasis and pathological
characteristics generally associated with a favorable prognosis.
According to the AJCC 8th edition staging system, the patient was
classified as stage IA (T1aN0M0) (8). Based on current epidemiological
statistics, such patients would be expected to have a 5-year
survival rate of 100% (12).
However, the patient in the present case developed widespread
systemic metastases within 3 years of surgery, with merely 4 years
elapsing from the initial stage IA diagnosis to mortality from
stage IV disease. This rapid and fatal progression in a patient
with initially favorable pathological staging revealed the
limitations of relying solely on conventional pathological staging
for prognosis prediction. Therefore, for young patients with
melanoma, even those with lesions suggesting a favorable prognosis,
enhanced post-operative health education and improved follow-up
compliance should be implemented in the future. Furthermore,
individualized surveillance strategies should be considered,
potentially including shortened follow-up intervals or increased
imaging frequency, to achieve early detection and timely
intervention of tumor recurrence.
Risk factors for cutaneous melanoma are broadly
classified as exogenous or endogenous. Exogenous factors include
solar and artificial ultraviolet (UV) radiation, specific lifestyle
habits and immunosuppression. Endogenous factors comprise skin
color, hair color, eye color, the number of melanocytic nevi, the
presence of atypical nevi, sex, medical history, hereditary factors
and genetic susceptibility (25–27).
Among these, UV radiation represents the most notable environmental
risk factor for all types of skin cancer (28). The number of common and atypical
nevi is also a key independent predictor of melanoma risk. A
previous study indicated that patients with a high number of nevi
(n=101-120) have a risk ~7 times greater compared with those with
few nevi (n=0-15) (4).
Surgical resection remains the primary treatment for
early-stage melanoma. However, high-risk patients treated with
surgery alone have only a 40–60% probability of remaining
disease-free at 5 years and numerous patients may eventually
experience locoregional or distant recurrence (29). According to the National
Comprehensive Cancer Network (NCCN) guidelines, preferred adjuvant
therapy options for patients with stage IIB-IV cutaneous melanoma
include anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)
agents and anti-PD-1 immune checkpoint inhibitors (ICIs), or for
patients with BRAF mutations, targeted therapy. In addition,
several emerging immunotherapies and neoadjuvant regimens are
becoming viable options for patients with melanoma. Systemic
therapies, particularly ICIs (such as ipilimumab, pembrolizumab,
atezolizumab and relatlimab) and BRAF-targeted treatments (such as
dabrafenib and trametinib), have markedly improved the prognosis of
patients with metastatic (stage III and IV) cutaneous melanoma and
have also demonstrated notable benefit in early-stage (IIB/C)
primary melanoma (30). Although
melanoma incidence continues to rise, mortality has been declining
by an average of ~4% annually since 2015. This trend reflects major
advances in the treatment of advanced and metastatic disease,
largely attributable to progress in immunotherapy and targeted
agents (31).
ICIs comprise CTLA-4 inhibitors (such as
ipilimumab), PD-1 inhibitors (such as pembrolizumab and nivolumab),
programmed cell death-ligand 1 (PD-L1) inhibitors (such as
atezolizumab) and lymphocyte-activation gene 3 (LAG-3) inhibitors
(such as relatlimab). CTLA-4 and PD-1 inhibitors enhance the
ability of T cells to eliminate cancer cells by blocking CTLA-4
immune checkpoint molecules and PD-1/PD-L1 interactions,
respectively (32). By contrast,
LAG-3 inhibitors bind to the LAG-3 receptor and disrupt its
interaction with ligands, thereby alleviating LAG-3
pathway-mediated immunosuppression and promoting T-cell
proliferation. Checkpoint immunotherapy is indicated for patients
with both BRAF mutant-type and wild-type melanoma (33). A previous study reported that 15.5%
of patients with unresectable stage IV melanoma achieved a complete
response after first-line ICI treatment, with 5-year
progression-free survival and overall survival rates of 79 and 83%,
respectively, among those achieving a complete response (34).
Ipilimumab, the first ICI approved for advanced
melanoma, demonstrated a significant survival benefit in stage IV
disease by increasing the median overall survival to 10.1 months,
compared to 6.4 months with the glycoprotein 100 (gp100) peptide
vaccine alone (35). Due to the
high immunogenicity of melanoma, anti-PD-1 inhibitors are
particularly effective in this malignancy (36). Although randomized clinical trials
[CheckMate 238 with nivolumab (37,38);
KEYNOTE-054 (39)/716 (40,41)
with pembrolizumab] have demonstrated that adjuvant PD-1 inhibitors
improve recurrence-free survival in patients with resectable stage
IIB-IV melanoma, an overall survival benefit has not been observed
to date (42). Ongoing first-line
clinical trials are evaluating PD-1 inhibitors in combination with
multiple classes of agents, including histone deacetylase
inhibitors (HDAC6i Nexturastat A) (43), novel immunotherapies (NT-I7)
(44), vaccines (IDO/PD-L1
targeting peptide vaccine) (45)
and toll-like receptor 9 agonist (Nelitolimod) (46). For patients with metastatic melanoma
harboring BRAF V600E/K mutations, the combination of ipilimumab and
nivolumab is considered a preferred first-line treatment regardless
of mutation status, establishing ICI as a primary therapeutic
option (5). In a previous study by
Hodi et al (47), 945
patients with unresectable stage III or IV melanoma received either
ipilimumab plus nivolumab, nivolumab monotherapy or ipilimumab
alone. The 4-year overall survival rate was 53% in the combination
group, compared with 46% in the nivolumab group and 30% in the
ipilimumab group. Among patients with mutant-type BRAF, the 4-year
overall survival rates were 62, 50 and 33% for the combination,
nivolumab and ipilimumab groups, respectively; corresponding rates
for patients with wild-type BRAF were 49, 45 and 28%. These results
suggested that combination therapy may offer enhanced benefit
compared with nivolumab monotherapy, particularly in patients with
BRAF-mutations.
LAG-3 inhibitors represent the first class of LAG-3
protein inhibitors worldwide. On March 18, 2022, the Food and Drug
Administration (FDA) approved the fixed-dose combination of
relatlimab and nivolumab for the treatment of adult and pediatric
patients aged ≥12 years with unresectable or metastatic melanoma.
This combination acts through simultaneous inhibition of LAG-3 and
PD-1, and has demonstrated efficacy in previously untreated
metastatic or unresectable melanoma (48). For most patients with metastatic
melanoma, anti-PD-1-based therapy combined with either ipilimumab
or relatlimab offers the potential for long-term survival and
possibly a cure (49).
Targeted therapies are designed to specifically
address molecular abnormalities or target key pathways essential
for melanoma cell proliferation and survival (32). BRAF inhibitors (vemurafenib,
dabrafenib and encorafenib) and MEK inhibitors (trametinib and
cobimetinib) represent the main classes of agents used in this
approach. The BRAF serine/threonine kinase, an isoform of the Raf
family, functions within the MAPK pathway, which serves a key role
in regulating cell proliferation (50). BRAF mutations occur most frequently
in malignant melanoma (51), with
the V600E variant accounting for >80% of all BRAF mutations
(50). It has been reported that
~50% of patients with metastatic melanoma harbor a BRAF V600
mutation. MEK also serves a key function within the MAPK cascade.
In patients with metastatic melanoma (stage IV or unresectable
stage III), dabrafenib monotherapy resulted in a median
progression-free survival (PFS) time of 5.1 months (52). A previous study by Long et al
(53) reported that combination
therapy with dabrafenib and trametinib in treatment-naive patients
with BRAF V600 mutation-positive metastatic melanoma yielded a
median overall survival time of >2 years, with ~20% of patients
remaining progression-free at 3 years. These findings indicated
that the combination was associated with prolonged survival and
durable responses in a subset of individuals. In addition, compared
with vemurafenib monotherapy, dabrafenib combined with trametinib
significantly improved overall survival in previously untreated
patients with BRAF V600E or V600K metastatic melanoma, without
increasing overall toxicity (54).
Due to its generally acceptable safety profile, adjuvant dabrafenib
plus trametinib is now considered a reasonable option for patients
with advanced resectable BRAF-mutated melanoma (55). Overall, concurrent BRAF and MEK
inhibition has been reported to yield notably improved clinical
outcomes compared with BRAF inhibitor monotherapy.
In 2022, Corrie et al (56) demonstrated that combination
therapies outperform monotherapy in BRAF-mutated unresectable or
metastatic melanoma. Among these, the
atezolizumab/vemurafenib/cobimetinib triple therapy and the
encorafenib/binimetinib combination exhibited the most favorable
efficacy profiles. Furthermore, encorafenib/binimetinib
demonstrated an enhanced safety profile compared with all other
combination regimens, including triple therapy. In 2023, results
from a phase III trial reported by Haist et al (57) indicated that triple combination
therapy, comprising an ICI, a BRAF inhibitor and a MEK inhibitor,
prolonged response duration and enabled long-term PFS. However,
most of these trials did not meet their primary endpoints and
therefore do not support the routine first-line use of triple
therapy in patients with BRAF V600-mutant melanoma. That said,
subgroup analyses and preliminary data in patients with melanoma
brain metastases suggested that patients with higher disease burden
or rapidly progressing disease may derive greater benefit from such
regimens. The addition of atezolizumab to the targeted therapy
combination of vemurafenib and cobimetinib was reported to be safe
and tolerable, and significantly improved PFS in patients with
advanced melanoma harboring BRAF V600 mutations (58). For most patients with BRAF
V600-mutant metastatic melanoma, the preferred treatment sequence
involves initial therapy with nivolumab plus ipilimumab, followed
by BRAF and MEK inhibitors if required (59).
Beyond the established approaches of ICI and
targeted therapy, the potential benefits of several emerging
immunotherapies and neoadjuvant treatments for patients with
melanoma warrant further research in the future.
OVs represent one of the most novel and promising
forms of intratumoral immunotherapy. The first OV approved for
melanoma treatment was talimogene laherparepvec (T-VEC), a modified
herpes simplex virus type 1, indicated for recurrent melanoma after
initial surgery (60). Previous
studies indicated that T-VEC combined with ipilimumab had a
manageable safety profile and appeared to yield notably increased
efficacy compared with either agent alone (61). For patients with regional or distant
metastatic melanoma, including those with visceral involvement,
T-VEC in combination with ICIs may represent a promising novel
therapeutic alternative (61).
Neoadjuvant therapy refers to induction treatment
administered to reduce tumor burden before primary treatment,
typically surgery (62). This
approach includes both neoadjuvant immunotherapy and neoadjuvant
targeted therapy; it serves a particularly key role in locally
advanced, resectable stage III–IV or borderline resectable
melanoma. In such settings, neoadjuvant therapy may facilitate
early eradication of micrometastases within a more immunologically
responsive microenvironment, potentially offering enhanced curative
potential compared with adjuvant therapy, while also reducing the
need for extensive surgery, achieving microscopic R0 resection,
decreasing the risk of distant metastasis and providing key
biomarker information to potentially guide subsequent adjuvant
treatment in the future (29).
Patel et al (63) reported
that, in patients with high-risk, resectable stage III–IV melanoma,
the 2-year event-free survival rate in the neoadjuvant-adjuvant
group was ~23% higher compared with that in the adjuvant-only
group. Patients receiving pembrolizumab both before and after
surgery exhibited significantly longer event-free survival times
compared with those receiving adjuvant pembrolizumab alone. Amaria
et al (64) demonstrated
that neoadjuvant treatment with dabrafenib and trametinib in
patients with resectable stage IIIB-IV BRAF-mutant cutaneous
melanoma significantly improved progression-free survival rate at a
median follow-up time of 18.6 months. However, neoadjuvant therapy
carries the risk of disease progression during treatment, which may
render initially resectable tumors unresectable or lead to distant
spread (65).
According to the NCCN guidelines, first-line
systemic therapy for advanced BRAF-mutant melanoma includes either
combined immunotherapy or combined targeted therapy (66). However, the choice of optimal
first-line treatment remains a subject of debate. BRAF/MEK
inhibitors typically induce a strong and rapid initial response,
yet disease progression remains almost inevitable. By contrast,
ICIs may exhibit a lower initial response rate, possibly due to the
time required for immune activation, but can provide more durable
long-term clinical benefits (67,68).
For patients with BRAF-mutant melanoma with active brain
metastases, several BRAF/MEK inhibitors have demonstrated notable
activity with manageable safety profiles and can lead to marked
tumor regression (69–71). By contrast, ipilimumab tends to be
more effective when brain metastases are small and asymptomatic
(72). Furthermore, a previous
study indicated that, although dabrafenib plus trametinib resulted
in PFS and overall response rates similar to those of nivolumab
plus ipilimumab, dabrafenib plus trametinib was associated with
significantly shorter treatment duration and lower healthcare costs
(73). In the present case, the
poor performance status of the patient and rapid progressive
disease necessitated swift disease control to minimize early risks.
After multidisciplinary discussion and detailed communication with
the patient and family, combined targeted therapy was selected as
the first-line regimen, based on its faster onset of action and
higher rates of tumor regression. The present case underscores that
the optimal first-line strategy for advanced melanoma should be
individualized based on a comprehensive evaluation of multiple
clinical and patient-specific factors.
Although BRAF/MEK inhibitors demonstrate high
efficacy in BRAF-mutant melanoma, their clinical benefits are
frequently constrained by intrinsic, adaptive and acquired
resistance mechanisms. Intrinsic resistance may result from
loss-of-function mutations in PTEN (74,75)
and neurofibromatosis type 1 (76,77),
leading to activation of the PI3K-AKT-mTOR pathway. Adaptive
resistance often involves upregulation of receptor tyrosine kinases
(78), which can reactivate ERK
signaling and thereby drive treatment resistance. The most
prevalent form, acquired resistance, primarily involves MAPK
pathway reactivation through various molecular alterations,
including MEK mutations (79), BRAF
alternative splicing (80) and BRAF
amplification (81). These
mechanisms collectively contribute to the development of treatment
resistance and diminished therapeutic efficacy. To address these
challenges, various combination regimens involving inhibitors have
been developed. Among these, the combination of BRAF and MEK
inhibitors has been reported to yield notably increased overall
survival compared with BRAF inhibitor monotherapy (54). Based on this evidence, the
dabrafenib plus trametinib combination received FDA approval for
patients with unresectable or metastatic melanoma harboring BRAF
V600E/K mutations. In the present case, the observed pattern of
initial tumor regression followed by rapid progression strongly
suggests the emergence of acquired resistance, possibly driven by
one or more of the aforementioned molecular events. Thus, the
present case highlights the current limitations of combination
targeted therapy and supports the need for future research into
strategies that can potentially overcome or reverse resistance.
Melanoma exhibits a notable tendency for distant
metastasis. Standardized postoperative follow-up is essential for
patients with early-stage disease who have undergone surgical
resection. According to NCCN guidelines, patients with stage IA
melanoma should undergo history and physical examination (H&P)
(with emphasis on nodes and skin) every 6–12 months for the first 5
years after surgery, while routine laboratory tests and imaging
studies are not recommended (66).
However, in the present case, suboptimal adherence to scheduled
follow-up visits was noted. Furthermore, clinical experience
indicates that disease progression can be markedly rapid in certain
young patients following recurrence. Therefore, we propose a dual
approach: i) Establishing a structured patient education and
follow-up system to ensure consistent implementation of
guideline-recommended evaluations through regular reminders; and
ii) formulating personalized, risk-adapted surveillance strategies
for young patients with high-risk features. This strategy aims to
avoid excessive medical intervention while enabling early detection
and timely management of recurrent disease.
Dacarbazine served as the key metastatic melanoma
treatment for decades; however, its use has declined with the
emergence of more effective options such as targeted therapy and
immunotherapy (32). Current
systemic treatments for advanced melanoma primarily include ICIs
and targeted therapies. Anti-PD-1 therapy is now a standard option
for resected stage IIB-IV disease, while BRAF-targeted therapy is
the standard for resected stage III–IV melanoma. Both modalities
have been associated with a 35–50% relative improvement in
recurrence-free survival and distant metastasis-free survival
rates, although neither has demonstrated a notable overall survival
benefit (82). Although combination
therapies generally outperform monotherapies, the optimal
combination strategy for maximizing patient benefit requires
further investigation. In addition, emerging treatments such as OVs
and neoadjuvant regimens demonstrate notable promise and may
represent future advances in the management of advanced
melanoma.
In conclusion, the present report describes the case
of a 24-year-old male patient with early-stage cutaneous melanoma
who developed multiple systemic metastases 3 years after radical
resection and who succumbed 4 months after the diagnosis of
metastatic disease. The present case underscores the importance of
establishing a strict post-operative education and follow-up system
for patients with early-stage melanoma, and developing a
personalized risk-monitoring strategy. Treatment selection for
advanced melanoma should not be restricted to ICIs or targeted
therapy alone; however, a judicious integration of multiple
modalities may yield improved outcomes. Novel approaches such as
oncolytic virotherapy and neoadjuvant strategies also represent
promising treatment options in the future. The field of advanced
melanoma treatment continues to present numerous opportunities,
innovations and challenges. Novel optimized treatment strategies
may become available that potentially prolong patient survival in
the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DX and PW conceptualized and designed the present
case report and provided overall supervision of the research. XH
performed a comprehensive literature search, conducted analysis and
interpretation of the clinical course, and drafted the initial
manuscript. LZ and DX collected and assembled all clinical data,
including imaging studies and laboratory results, and confirm the
authenticity of all raw data. LZ prepared the figures. DX
critically reviewed and revised the manuscript for important
intellectual content. All authors read and approved the final
manuscript and are accountable for all aspects of the work.
Ethics approval and consent to
participate
The present case report was approved by the Binzhou
People's Hospital Clinical Trial Ethics Committee (Binzhou, China;
approval no. YXKYLL-20251101). Written informed consent was
obtained from the patient. The present case report complied with
the Declaration of Helsinki.
Patient consent for publication
Written consent for the publication of data and any
related images was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
2
|
International Agency for Research on
Cancer (IARC), . WHO Classification of Skin Tumours. Elder DE,
Massi D, Scolyer RA and Willemze R: 11. 4th Edition. IARC; Lyon:
2018
|
|
3
|
Elder DE, Bastian BC, Cree IA, Massi D and
Scolyer RA: The 2018 World Health Organization classification of
cutaneous, mucosal, and uveal melanoma: Detailed analysis of 9
distinct subtypes defined by their evolutionary pathway. Arch
Pathol Lab Med. 144:500–522. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gandini S, Sera F, Cattaruzza MS, Pasquini
P, Abeni D, Boyle P and Melchi CF: Meta-analysis of risk factors
for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer.
41:28–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Switzer B, Piperno-Neumann S, Lyon J,
Buchbinder E and Puzanov I: Evolving management of stage IV
melanoma. Am Soc Clin Oncol Educ Book. 43:e3974782023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang T, Xiao M, Ge Y, Krepler C, Belser E,
Lopez-Coral A, Xu X, Zhang G, Azuma R, Liu Q, et al: BRAF
inhibition stimulates melanoma-associated macrophages to drive
tumor growth. Clin Cancer Res. 21:1652–1664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanchez IM, Purwin TJ, Chervoneva I, Erkes
DA, Nguyen MQ, Davies MA, Nathanson KL, Kemper K, Peeper DS and
Aplin AE: In vivo ERK1/2 reporter predictively models response and
resistance to combined BRAF and MEK inhibitors in melanoma. Mol
Cancer Ther. 18:1637–1648. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
American Joint Committee on Cancer, . AJCC
Cancer Staging Manual. 6th Edition. Springer New York; NY: 2002
|
|
9
|
Taal BG, Westerman H, Boot H and Rankin
EM: Clinical and endoscopic features of melanoma metastases in the
upper GI tract. Gastrointest Endosc. 50:261–263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Yan Y and Jiang CM: Primary
malignant melanoma of the esophagus with unusual endoscopic
findings: A case report and literature review. Medicine
(Baltimore). 95:e34792016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
International Agency for Research on
Cancer and Global Cancer Observatory, Cancer Today Globocan 2022, .
Melanoma of Skin. https://gco.iarc.who.int/media/globocan/factsheets/cancers/16-melanoma-of-skin-fact-sheet.pdfApril
29–2025
|
|
12
|
International Agency for Research on
Cancer, Global Cancer Observatory and Cancer Today Globocan 2022, .
Non-Melanoma Skin Cancer. https://gco.iarc.who.int/media/globocan/factsheets/cancers/17-non-melanoma-skin-cancer-fact-sheet.pdfApril
29–2025
|
|
13
|
National Cancer Institute Surveillance and
Epidemiology, End Results Program, . Cancer Stat Facts: Melanoma of
the Skin. https://seer.cancer.gov/statfacts/html/melan.htmlMay
7–2025
|
|
14
|
Karimkhani C, Dellavalle RP, Coffeng LE,
Flohr C, Hay RJ, Langan SM, Nsoesie EO, Ferrari AJ, Erskine HE,
Silverberg JI, et al: Global skin disease morbidity and mortality:
An update from the global burden of disease study 2013. JAMA
Dermatol. 153:406–412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Carlo V, Eberle A, Stiller C, Bennett
D, Katalinic A, Marcos-Gragera R, Girardi F, Larønningen S, Schultz
A, Lima CA, et al: Sex differences in survival from melanoma of the
skin: The role of age, anatomic location and stage at diagnosis: A
CONCORD-3 study in 59 countries. Eur J Cancer. 217:1152132025.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jayasinghe D, Nufer KL, Betz-Stablein B,
Soyer HP and Janda M: Body site distribution of acquired
melanocytic naevi and associated characteristics in the general
population of caucasian adults: A scoping review. Dermatol Ther
(Heidelb). 12:2453–2488. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lallas K, Kyrgidis A, Chrysostomidis A,
Vakirlis E, Apalla Z and Lallas A: Clinical, dermatoscopic,
histological and molecular predictive factors of distant melanoma
metastasis: A systematic review and meta-analysis. Crit Rev Oncol
Hematol. 202:1044582024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olsen CM, Thompson JF, Pandeya N and
Whiteman DC: Evaluation of sex-specific incidence of melanoma. JAMA
Dermatol. 156:553–560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calomarde-Rees L, García-Calatayud R,
Requena Caballero C, Manrique-Silva E, Traves V, García-Casado Z,
Soriano V, Kumar R and Nagore E: Risk factors for lymphatic and
hematogenous dissemination in patients with stages I to II
cutaneous melanoma. JAMA Dermatol. 155:679–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waseh S and Lee JB: Advances in melanoma:
Epidemiology, diagnosis, and prognosis. Front Med (Lausanne).
10:12684792023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
PDQ Adult Treatment Editorial Board, .
Melanoma Treatment (PDQ®): Health Professional Version.
PDQ Cancer Information Summaries. National Cancer Institute (US);
Bethesda, MD, USA: 2025
|
|
22
|
American Cancer Society, . Key Statistics
for Melanoma Skin Cancer. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.htmlMay
7–2025
|
|
23
|
Indini A, Didoné F, Massi D, Puig S,
Casadevall JR, Bennett D, Katalinic A, Sanvisens A, Ferrari A,
Lasalvia P, et al: Incidence and prognosis of cutaneous melanoma in
European adolescents and young adults (AYAs): EUROCARE-6
retrospective cohort results. Eur J Cancer 1990.
213:1150792024.PubMed/NCBI
|
|
24
|
Balch CM, Soong SJ, Murad TM, Smith JW,
Maddox WA and Durant JR: A multifactorial analysis of melanoma. IV.
Prognostic factors in 200 melanoma patients with distant metastases
(stage III). J Clin Oncol. 1:126–134. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordon R: Skin cancer: An overview of
epidemiology and risk factors. Semin Oncol Nurs. 29:160–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wunderlich K, Suppa M, Gandini S, Lipski
J, White JM and Del Marmol V: Risk factors and innovations in risk
assessment for melanoma, basal cell carcinoma, and squamous cell
carcinoma. Cancers (Basel). 16:10162024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Conforti C and Zalaudek I: Epidemiology
and risk factors of melanoma: A review. Dermatol Pract Concept. 11
(Suppl 1):e2021161S2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raimondi S, Suppa M and Gandini S:
Melanoma Epidemiology and Sun Exposure. Acta Derm Venereol.
100:57462020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rutkowski P and Mandala M: Perioperative
therapy of melanoma: Adjuvant or neoadjuvant treatment. Eur J Surg
Oncol. 50:1079692024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maher NG, Vergara IA, Long GV and Scolyer
RA: Prognostic and predictive biomarkers in melanoma. Pathology.
56:259–273. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ernst M and Giubellino A: The current
state of treatment and future directions in cutaneous malignant
melanoma. Biomedicines. 10:8222022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Russano F, Rastrelli M, Dall'Olmo L, Del
Fiore P, Gianesini C, Vecchiato A, Mazza M, Tropea S and Mocellin
S: Therapeutic treatment options for in-transit metastases from
melanoma. Cancers (Basel). 16:30652024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Long GV, Swetter SM, Menzies AM,
Gershenwald JE and Scolyer RA: Cutaneous melanoma. Lancet.
402:485–502. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chatziioannou E, Leiter U, Thomas I, Keim
U, Seeber O, Meiwes A, Boessenecker I, Gonzalez SS, Torres FM,
Niessner H, et al: Features and long-term outcomes of stage IV
melanoma patients achieving complete response under anti-PD-1-based
immunotherapy. Am J Clin Dermatol. 24:453–467. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brandlmaier M, Hoellwerth M, Koelblinger
P, Lang R and Harrer A: Adjuvant PD-1 checkpoint inhibition in
early cutaneous melanoma: Immunological mode of action and the role
of ultraviolet radiation. Cancers (Basel). 16:14612024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ascierto PA, Del Vecchio M, Mandalá M,
Gogas H, Arance AM, Dalle S, Cowey CL, Schenker M, Grob JJ,
Chiarion-Sileni V, et al: Adjuvant nivolumab versus ipilimumab in
resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-Year
results from a multicentre, double-blind, randomised, controlled,
phase 3 trial. Lancet Oncol. 21:1465–1477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Larkin J, Del Vecchio M, Mandalá M, Gogas
H, Arance Fernandez AM, Dalle S, Cowey CL, Schenker M, Grob JJ,
Chiarion-Sileni V, et al: Adjuvant Nivolumab versus Ipilimumab in
resected stage III/IV melanoma: 5-year efficacy and biomarker
results from CheckMate 238. Clin Cancer Res. 29:3352–3361. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bührer E, Kicinski M, Mandala M, Pe M,
Long GV, Atkinson V, Blank CU, Haydon A, Dalle S, Khattak A, et al:
Adjuvant pembrolizumab versus placebo in resected stage III
melanoma (EORTC 1325-MG/KEYNOTE-054): Long-term, health-related
quality-of-life results from a double-blind, randomised,
controlled, phase 3 trial. Lancet Oncol. 25:1202–1212. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Long GV, Luke JJ, Khattak MA, de la Cruz
Merino L, Del Vecchio M, Rutkowski P, Spagnolo F, Mackiewicz J,
Chiarion-Sileni V, Kirkwood JM, et al: Pembrolizumab versus placebo
as adjuvant therapy in resected stage IIB or IIC melanoma
(KEYNOTE-716): Distant metastasis-free survival results of a
multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol.
23:1378–1388. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luke JJ, Rutkowski P, Queirolo P, Del
Vecchio M, Mackiewicz J, Chiarion-Sileni V, de la Cruz Merino L,
Khattak MA, Schadendorf D, Long GV, et al: Pembrolizumab versus
placebo as adjuvant therapy in completely resected stage IIB or IIC
melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial.
Lancet. 399:1718–1729. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ochenduszko S, Puskulluoglu M,
Pacholczak-Madej R and Ruiz-Millo O: Adjuvant anti-PD1
immunotherapy of resected skin melanoma: An example of
non-personalized medicine with no overall survival benefit. Crit
Rev Oncol Hematol. 202:1044432024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Knox T, Sahakian E, Banik D, Hadley M,
Palmer E, Noonepalle S, Kim J, Powers J, Gracia-Hernandez M,
Oliveira V, et al: Selective HDAC6 inhibitors improve anti-PD-1
immune checkpoint blockade therapy by decreasing the
anti-inflammatory phenotype of macrophages and down-regulation of
immunosuppressive proteins in tumor cells. Sci Rep. 9:61362019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Phoon YP, Wolfarth AA, Funchain P, Ko JS,
Choi D, Dedkova E, Bateman DD, Corvin J, Melenhorst JJ and Gastman
BR: NT-I7, a novel long-acting interleukin-7, promotes anti-PD-1
efficacy in an autologous humanized melanoma model. Sci Rep.
15:379502025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lorentzen CL, Kjeldsen JW, Ehrnrooth E,
Andersen MH and Marie Svane I: Long-term follow-up of anti-PD-1
naïve patients with metastatic melanoma treated with IDO/PD-L1
targeting peptide vaccine and nivolumab. J Immunother Cancer.
11:e0067552023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ribas A, Milhem MM, Hoimes CJ, Amin A, Lao
CD, Conry RM, Hunt JP, Daniels GA, Almubarak M, Shaheen M, et al:
Phase 2, open-label, multicenter study of nelitolimod in
combination with pembrolizumab in anti-PD-1 treatment-Naïve
advanced melanoma. Clin Cancer Res. 31:4070–4078. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hodi FS, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J,
Dummer R, et al: Nivolumab plus ipilimumab or nivolumab alone
versus ipilimumab alone in advanced melanoma (CheckMate 067):
4-Year outcomes of a multicentre, randomised, phase 3 trial. Lancet
Oncol. 19:1480–1492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mullick N and Nambudiri VE:
Relatlimab-nivolumab: A practical overview for dermatologists. J Am
Acad Dermatol. 89:1031–1037. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chan PY and Corrie PG: Curing stage IV
melanoma: Where Have we been and where are we? Am Soc Clin Oncol
Educ Book. 44:e4386542024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Smalley KSM: Understanding melanoma
signaling networks as the basis for molecular targeted therapy. J
Invest Dermatol. 130:28–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Garnett MJ and Marais R: Guilty as
charged: B-RAF is a human oncogene. Cancer Cell. 6:313–319. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hauschild A, Grob JJ, Demidov LV, Jouary
T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr,
Kaempgen E, et al: Dabrafenib in BRAF-mutated metastatic melanoma:
A multicentre, open-label, phase 3 randomised controlled trial.
Lancet. 380:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Long GV, Weber JS, Infante JR, Kim KB,
Daud A, Gonzalez R, Sosman JA, Hamid O, Schuchter L, Cebon J, et
al: Overall survival and durable responses in patients with BRAF
V600-mutant metastatic melanoma receiving dabrafenib combined with
trametinib. J Clin Oncol. 34:871–878. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Robert C, Karaszewska B, Schachter J,
Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R,
Grange F, Mortier L, et al: Improved overall survival in melanoma
with combined dabrafenib and trametinib. N Engl J Med. 372:30–39.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sierra-Davidson K and Boland GM: Advances
in adjuvant and neoadjuvant therapy for melanoma. Hematol Oncol
Clin North Am. 38:953–971. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Corrie P, Meyer N, Berardi R, Guidoboni M,
Schlueter M, Kolovos S, Macabeo B, Trouiller JB and Laramée P:
Comparative efficacy and safety of targeted therapies for
BRAF-mutant unresectable or metastatic melanoma: Results from a
systematic literature review and a network meta-analysis. Cancer
Treat Rev. 110:1024632022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Haist M, Stege H, Kuske M, Bauer J, Klumpp
A, Grabbe S and Bros M: Combination of immune-checkpoint inhibitors
and targeted therapies for melanoma therapy: The more, the better?
Cancer Metastasis Rev. 42:481–505. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gutzmer R, Stroyakovskiy D, Gogas H,
Robert C, Lewis K, Protsenko S, Pereira RP, Eigentler T, Rutkowski
P, Demidov L, et al: Atezolizumab, vemurafenib, and cobimetinib as
first-line treatment for unresectable advanced BRAFV600
mutation-positive melanoma (IMspire150): Primary analysis of the
randomised, double-blind, placebo-controlled, phase 3 trial.
Lancet. 395:1835–1844. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Atkins MB, Lee SJ, Chmielowski B, Tarhini
AA, Cohen GI, Truong TG, Moon HH, Davar D, O'Rourke M, Stephenson
JJ, et al: Combination dabrafenib and trametinib versus combination
nivolumab and ipilimumab for patients with advanced BRAF-mutant
melanoma: The DREAMseq trial-ECOG-ACRIN EA6134. J Clin Oncol.
41:186–197. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ziogas DC, Martinos A, Petsiou DP,
Anastasopoulou A and Gogas H: Beyond immunotherapy: Seizing the
momentum of oncolytic viruses in the ideal platform of skin
cancers. Cancers (Basel). 14:28732022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Puzanov I, Milhem MM, Minor D, Hamid O, Li
A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, et al:
Talimogene laherparepvec in combination with ipilimumab in
previously untreated, unresectable stage IIIB-IV melanoma. J Clin
Oncol. 34:2619–2626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gorry C, McCullagh L, O'Donnell H, Barrett
S, Schmitz S, Barry M, Curtin K, Beausang E, Barry R and Coyne I:
Neoadjuvant treatment for stage III and IV cutaneous melanoma.
Cochrane Database Syst Rev. 1:CD0129742023.PubMed/NCBI
|
|
63
|
Patel SP, Othus M, Chen Y, Wright GP Jr,
Yost KJ, Hyngstrom JR, Hu-Lieskovan S, Lao CD, Fecher LA, Truong
TG, et al: Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in
advanced melanoma. N Engl J Med. 388:813–823. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Amaria RN, Prieto PA, Tetzlaff MT, Reuben
A, Andrews MC, Ross MI, Glitza IC, Cormier J, Hwu WJ, Tawbi HA, et
al: Neoadjuvant plus adjuvant dabrafenib and trametinib versus
standard of care in patients with high-risk, surgically resectable
melanoma: A single-centre, open-label, randomised, phase 2 trial.
Lancet Oncol. 19:181–193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Amaria RN, Menzies AM, Burton EM, Scolyer
RA, Tetzlaff MT, Antdbacka R, Ariyan C, Bassett R, Carter B, Daud
A, et al: Neoadjuvant systemic therapy in melanoma: Recommendations
of the international neoadjuvant melanoma consortium. Lancet Oncol.
20:e378–e389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Swetter SM, Johnson D, Albertini MR,
Barker CA, Bateni S, Baumgartner J, Bhatia S, Bichakjian C, Boland
G, Chandra S, et al: NCCN guidelines® insights:
Melanoma: Cutaneous, version 2.2024. J Natl Compr Canc Netw.
22:290–298. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Spagnolo F, Ghiorzo P, Orgiano L,
Pastorino L, Picasso V, Tornari E, Ottaviano V and Queirolo P:
BRAF-mutant melanoma: Treatment approaches, resistance mechanisms,
and diagnostic strategies. OncoTargets Ther. 8:157–168. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Haugh AM and Johnson DB: Management of
V600E and V600K BRAF-mutant melanoma. Curr Treat Options Oncol.
20:812019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Long GV, Trefzer U, Davies MA, Kefford RF,
Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Algazi
A, et al: Dabrafenib in patients with Val600Glu or Val600Lys
BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A
multicentre, open-label, phase 2 trial. Lancet Oncol. 13:1087–1095.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dummer R, Goldinger SM, Turtschi CP,
Eggmann NB, Michielin O, Mitchell L, Veronese L, Hilfiker PR,
Felderer L and Rinderknecht JD: Vemurafenib in patients with
BRAF(V600) mutation-positive melanoma with symptomatic brain
metastases: Final results of an open-label pilot study. Eur J
Cancer. 50:611–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Davies MA, Saiag P, Robert C, Grob JJ,
Flaherty KT, Arance A, Chiarion-Sileni V, Thomas L, Lesimple T,
Mortier L, et al: Dabrafenib plus trametinib in patients with
BRAFV600-mutant melanoma brain metastases (COMBI-MB): A
multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol.
18:863–873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Margolin K, Ernstoff MS, Hamid O, Lawrence
D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF,
et al: Ipilimumab in patients with melanoma and brain metastases:
An open-label, phase 2 trial. Lancet Oncol. 13:459–465. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jensen IS, Zacherle E, Blanchette CM,
Zhang J and Yin W: Evaluating cost benefits of combination
therapies for advanced melanoma. Drugs Context. 5:2122972016.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Paraiso KHT, Xiang Y, Rebecca VW, Abel EV,
Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR, et
al: PTEN loss confers BRAF inhibitor resistance to melanoma cells
through the suppression of BIM expression. Cancer Res.
71:2750–2760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xing F, Persaud Y, Pratilas CA, Taylor BS,
Janakiraman M, She QB, Gallardo H, Liu C, Merghoub T, Hefter B, et
al: Concurrent loss of the PTEN and RB1 tumor suppressors
attenuates RAF dependence in melanomas harboring (V600E)BRAF.
Oncogene. 31:446–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Whittaker SR, Theurillat JP, Van Allen E,
Wagle N, Hsiao J, Cowley GS, Schadendorf D, Root DE and Garraway
LA: A genome-scale RNA interference screen implicates NF1 loss in
resistance to RAF inhibition. Cancer Discov. 3:350–362. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nissan MH, Pratilas CA, Jones AM, Ramirez
R, Won H, Liu C, Tiwari S, Kong L, Hanrahan AJ, Yao Z, et al: Loss
of NF1 in cutaneous melanoma is associated with RAS activation and
MEK dependence. Cancer Res. 74:2340–2350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pratilas CA, Taylor BS, Ye Q, Viale A,
Sander C, Solit DB and Rosen N: (V600E)BRAF is associated with
disabled feedback inhibition of RAF-MEK signaling and elevated
transcriptional output of the pathway. Proc Natl Acad Sci USA.
106:4519–4524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shi H, Moriceau G, Kong X, Koya RC,
Nazarian R, Pupo GM, Bacchiocchi A, Dahlman KB, Chmielowski B,
Sosman JA, et al: Preexisting MEK1 exon 3 mutations in V600E/KBRAF
melanomas do not confer resistance to BRAF inhibitors. Cancer
Discov. 2:414–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Poulikakos PI, Persaud Y, Janakiraman M,
Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, et al:
RAF inhibitor resistance is mediated by dimerization of aberrantly
spliced BRAF(V600E). Nature. 480:387–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shi H, Moriceau G, Kong X, Lee MK, Lee H,
Koya RC, Ng C, Chodon T, Scolyer RA, Dahlman KB, et al: Melanoma
whole-exome sequencing identifies (V600E)B-RAF
amplification-mediated acquired B-RAF inhibitor resistance. Nat
Commun. 3:7242012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Augustin RC and Luke JJ: Rapidly evolving
pre- and post-surgical systemic treatment of melanoma. Am J Clin
Dermatol. 25:421–434. 2024. View Article : Google Scholar : PubMed/NCBI
|