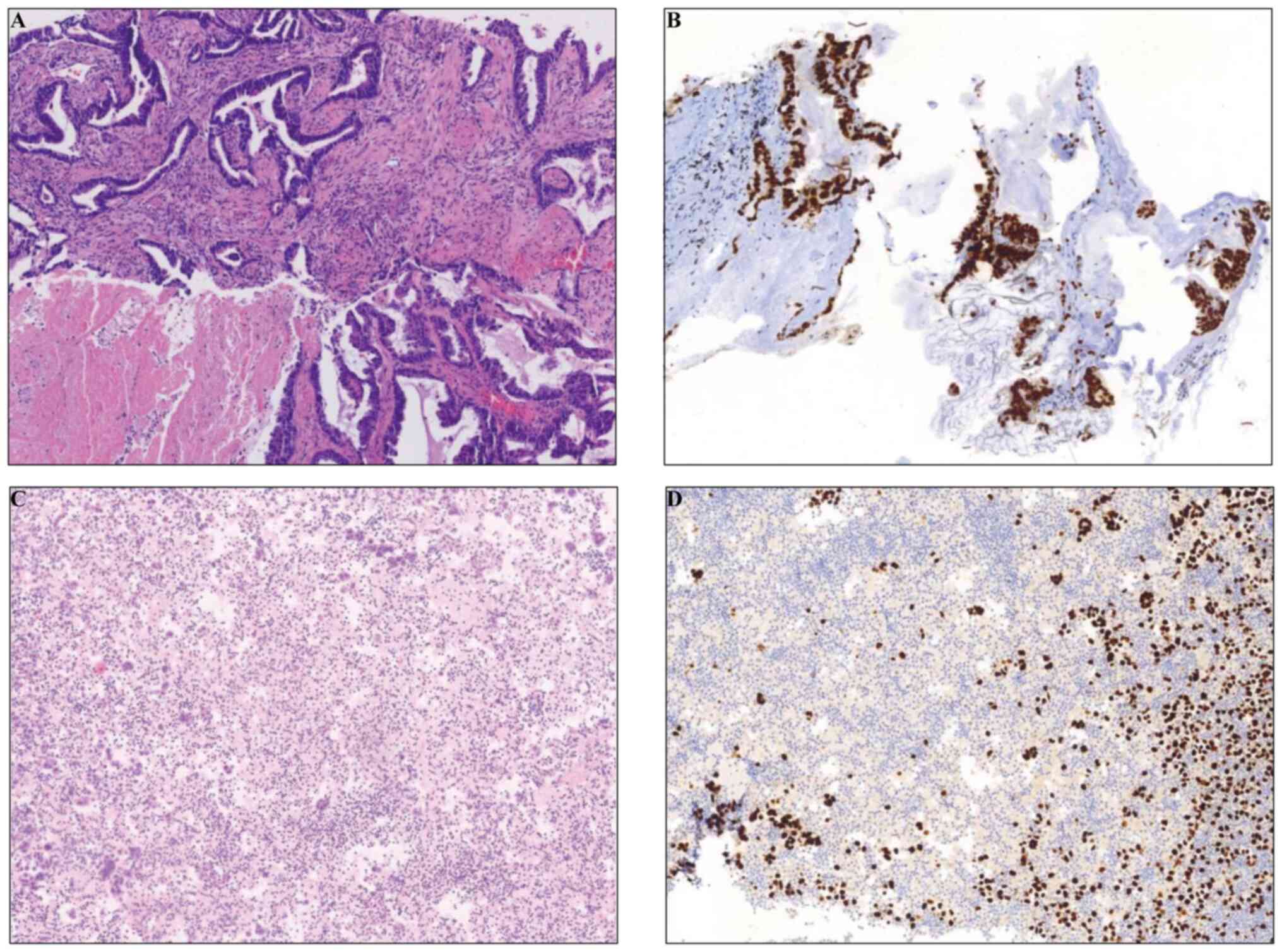
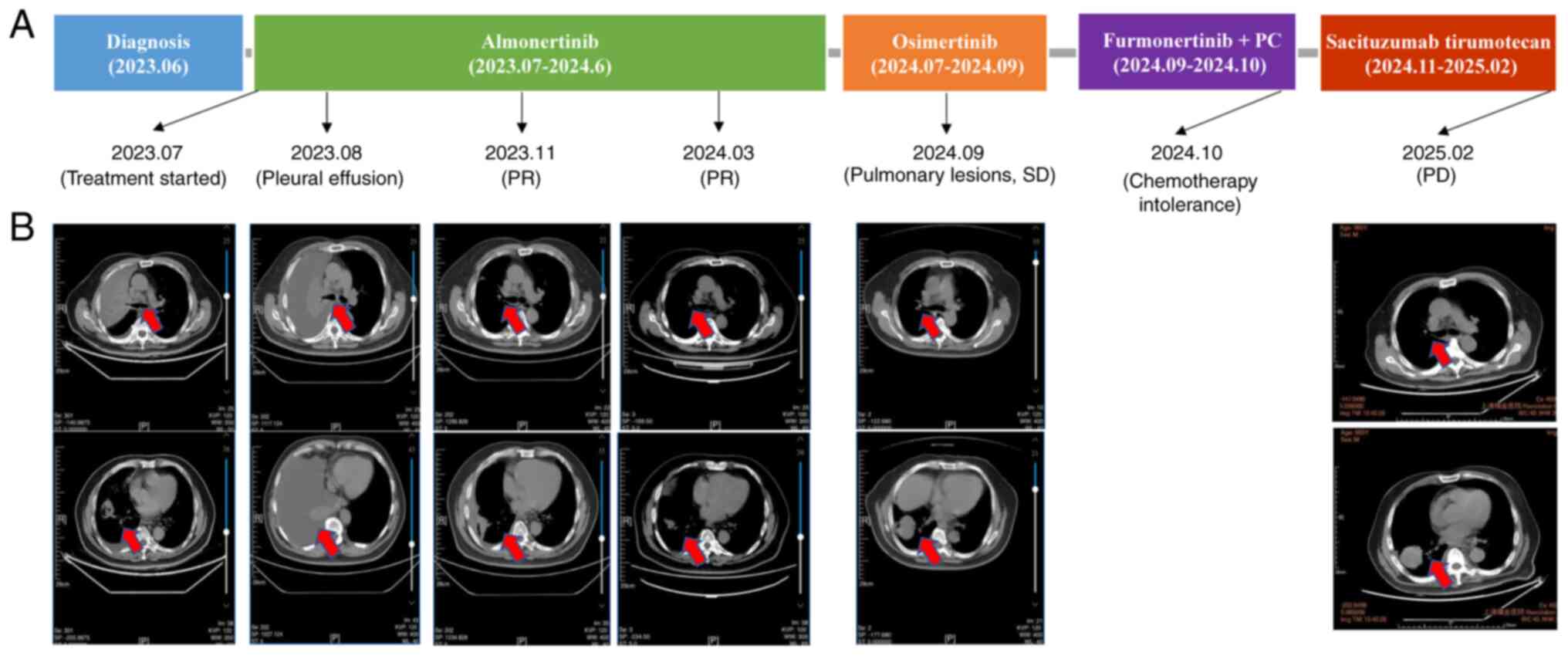
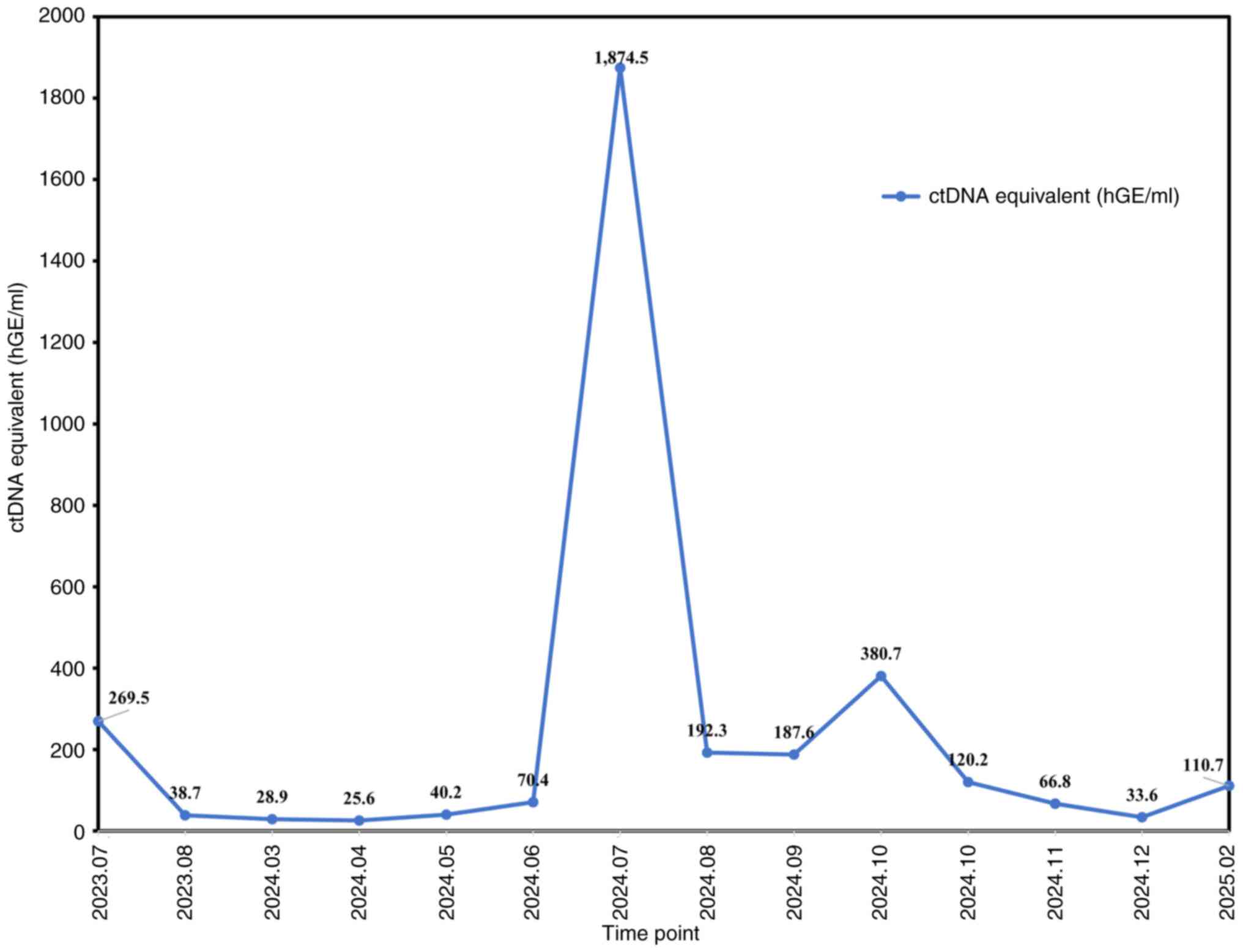
|
1
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukuda A and Okuma Y: From rarity to
reality: Osimertinib's promising horizon in treating uncommon EGFR
mutations in Non-Small cell lung cancer. Clin Cancer Res.
30:3128–3136. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okuma Y, Kubota K, Shimokawa M, Hashimoto
K, Kawashima Y, Sakamoto T, Wakui H, Murakami S, Okishio K,
Hayashihara K, et al: First-line osimertinib for previously
untreated patients with NSCLC and uncommon EGFR mutations: The
UNICORN phase 2 nonrandomized clinical trial. JAMA Oncol. 10:43–51.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JC, Sequist LV, Geater SL, Tsai CM,
Mok TS, Schuler M, Yamamoto N, Yu CJ, Ou SH, Zhou C, et al:
Clinical activity of afatinib in patients with advanced
non-small-cell lung cancer harbouring uncommon EGFR mutations: A
combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung
6. Lancet Oncol. 16:830–838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Miao J, Wang Y, Xing W, Xu X and
Wu R: Comparison of the efficacy, safety of first-line treatments
for of advanced EGFR mutation-positive non-small-cell lung cancer
in Asian populations: A systematic review and network
meta-analysis. Front Pharmacol. 14:12123132023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hofman P, Calabrese F, Kern I, Adam J,
Alarcão A, Alborelli I, Anton NT, Arndt A, Avdalyan A, Barberis M,
et al: Real-world EGFR testing practices for non-small-cell lung
cancer by thoracic pathology laboratories across Europe. ESMO Open.
8:1016282023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borgeaud M, Parikh K, Banna GL, Kim F,
Olivier T, Le X and Addeo A: Unveiling the landscape of uncommon
EGFR mutations in NSCLC-A systematic review. J Thorac Oncol.
19:973–983. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu Y, Yao F, Wang J, Huang Z, Hu T, Zhu C
and Lu S: An in-depth exploration of four heterogeneity
structure-based EGFR mutation subgroups in Chinese non-small cell
lung cancer. BMC Pulm Med. 25:3162025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao W, Song A, Xu Y, Wu Q, Liu C, Yin JC,
Ou Q, Wu X, Shao Y and Zhao X: Rare mutation-dominant compound
EGFR-positive NSCLC is associated with enriched kinase
domain-resided variants of uncertain significance and poor clinical
outcomes. BMC Med. 21:732023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu S, Dong X, Jian H, Chen J, Chen G, Sun
Y, Ji Y, Wang Z, Shi J, Lu J, et al: AENEAS: A randomized phase III
trial of aumolertinib versus gefitinib as first-line therapy for
locally advanced or metastatic non-small-cell lung cancer with EGFR
Exon 19 deletion or L858R mutations. J Clin Oncol. 40:3162–3171.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang W, Cheng Y, Chen Z, Wang W, Li Y, Yin
Y, Li X, Xu X, Yu G, Mi Y, et al: Abstract CT247: Updated efficacy
and safety of anti-TROP2 ADC SKB264 (MK-2870) for previously
treated advanced NSCLC in Phase 2 study. Cancer Res. 84:CT2472024.
View Article : Google Scholar
|
|
12
|
Riely GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non-small cell lung cancer, version 4.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:249–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Qiu T and Ren S: Updates to the
2024 CSCO advanced non-small cell lung cancer guidelines. Cancer
Biol Med. 22:77–82. 2025.PubMed/NCBI
|
|
14
|
Brice K, Arline C, Raez LE, Dumais K and
Block M: Molecular profiling using Next-generation sequencing of
sufficient endobronchial ultrasound-guided transbronchial needle
aspiration and liquid biopsy samples in patients with advanced lung
cancer. J Clin Transl Pathol. 4:110–114. 2024.
|
|
15
|
Zhang YC, Zhou Q and Wu YL: The emerging
roles of NGS-based liquid biopsy in non-small cell lung cancer. J
Hematol Oncol. 10:1672017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moding EJ, Nabet BY, Alizadeh AA and Diehn
M: Detecting liquid remnants of solid tumors: Circulating tumor DNA
minimal residual disease. Cancer Discov. 11:2968–2986. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chaudhuri AA, Chabon JJ, Lovejoy AF,
Newman AM, Stehr H, Azad TD, Khodadoust MS, Esfahani MS, Liu CL,
Zhou L, et al: Early detection of molecular residual disease in
localized lung cancer by circulating tumor DNA profiling. Cancer
Discov. 7:1394–1403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng ML, Lau CJ, Milan MSD, Supplee JG,
Riess JW, Bradbury PA, Jänne PA, Oxnard GR and Paweletz CP: Plasma
ctDNA response is an early marker of treatment effect in advanced
NSCLC. JCO Precis Oncol. 5:PO.20.00419, 2021.
|
|
20
|
Leite da Silva LF, Saldanha EF, de Menezes
JSA, Halamy Pereira L, de Bragança Dos Santos JAR, Buonopane IR, de
Souza EM, de Menezes CUG and Lopes G: Plasma ctDNA kinetics as a
predictor of systemic therapy response for advanced non-small cell
lung cancer: A systematic review and meta-analysis. Oncologist.
30:oyae3442025. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi K, Wang G, Pei J, Zhang J, Wang J,
Ouyang L, Wang Y and Li W: Emerging strategies to overcome
resistance to third-generation EGFR inhibitors. J Hematol Oncol.
15:942022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He J, Huang Z, Han L, Gong Y and Xie C:
Mechanisms and management of 3rd-generation EGFR-TKI resistance in
advanced non-small cell lung cancer (review). Int J Oncol.
59:902021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sichuan Kelun-Biotech Biopharmaceutical
Co.Ltd, . Kelun-Biotech's TROP2 ADC Sacituzumab Tirumotecan
(sac-TMT) Approved for Marketing in Second Indication by NMPA for
EGFRm NSCLC. Mar 10–2025.
|
|
25
|
Le Rhun E, Guckenberger M, Smits M, Dummer
R, Bachelot T, Sahm F, Galldiks N, de Azambuja E, Berghoff AS,
Metellus P, et al: EANO-ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up of patients with brain
metastasis from solid tumours. Ann Oncol. 32:1332–1347. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choudhury NJ, Marra A, Sui JSY, Flynn J,
Yang SR, Falcon CJ, Selenica P, Schoenfeld AJ, Rekhtman N, Gomez D,
et al: Molecular biomarkers of disease outcomes and mechanisms of
acquired resistance to First-Line osimertinib in advanced
EGFR-mutant lung cancers. J Thorac Oncol. 18:463–475. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Offin M, Chan JM, Tenet M, Rizvi HA, Shen
R, Riely GJ, Rekhtman N, Daneshbod Y, Quintanal-Villalonga A,
Penson A, et al: Concurrent RB1 and TP53 alterations define a
subset of EGFR-mutant lung cancers at risk for histologic
transformation and inferior clinical outcomes. J Thorac Oncol.
14:1784–1793. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang T, Wan B, Zhao Y, Li C, Liu H, Lv T,
Zhan P and Song Y: Treatment of uncommon EGFR mutations in
non-small cell lung cancer: New evidence and treatment. Transl Lung
Cancer Res. 8:302–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duffy MJ: Circulating tumor DNA (ctDNA) as
a biomarker for lung cancer: Early detection, monitoring and
therapy prediction. Tumour Biol. 46 (Suppl 1):S283–S295. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mok T, Janne PA, Nishio M, Novello S, Reck
M, Steuer C, Wu YL, Fougeray R, Fan PD, Meng J, et al:
HERTHENA-Lung02: Phase III study of patritumab deruxtecan in
advanced EGFR-mutated NSCLC after a third-generation EGFR TKI.
Future Oncol. 20:969–980. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oxnard GR, Thress KS, Alden RS, Lawrance
R, Paweletz CP, Cantarini M, Yang JC, Barrett JC and Jänne PA:
Association between plasma genotyping and outcomes of treatment
with osimertinib (AZD9291) in advanced Non-small-cell lung cancer.
J Clin Oncol. 34:3375–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mack PC, Miao J, Redman MW, Moon J,
Goldberg SB, Herbst RS, Melnick MA, Walther Z, Hirsch FR, Politi K,
et al: Circulating tumor DNA kinetics predict Progression-free and
overall survival in EGFR TKI-Treated patients with EGFR-Mutant
NSCLC (SWOG S1403). Clin Cancer Res. 28:3752–3760. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andrews HS, Zariffa N, Nishimura KK, Choi
SH, Deng S, Eisele M, Espenschied CR, Goren EM, Guha M, Hong S, et
al: ctDNA clearance as an early indicator of improved clinical
outcomes in advanced NSCLC treated with TKI: Findings from an
aggregate analysis of eight clinical trials. Clin Cancer Res.
31:2162–2172. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abbosh C, Birkbak NJ, Wilson GA,
Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA,
Veeriah S, Rosenthal R, et al: Phylogenetic ctDNA analysis depicts
early-stage lung cancer evolution. Nature. 545:446–451. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong TH, Hwang S, Dasgupta A, Abbosh C,
Hung T, Bredno J, Walker J, Shi X, Milenkova T, Horn L, et al:
Clinical utility of Tumor-naive presurgical circulating tumor DNA
detection in Early-stage NSCLC. J Thorac Oncol. 19:1512–1524. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhong R, Gao R, Fu W, Li C, Huo Z, Gao Y,
Lu Y, Li F, Ge F, Tu H, et al: Accuracy of minimal residual disease
detection by circulating tumor DNA profiling in lung cancer: A
meta-analysis. BMC Med. 21:1802023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JH, Long GV, Menzies AM, Lo S,
Guminski A, Whitbourne K, Peranec M, Scolyer R, Kefford RF, Rizos H
and Carlino MS: Association between circulating tumor DNA and
pseudoprogression in patients with metastatic melanoma treated with
Anti-programmed cell death 1 antibodies. JAMA Oncol. 4:717–721.
2018. View Article : Google Scholar : PubMed/NCBI
|