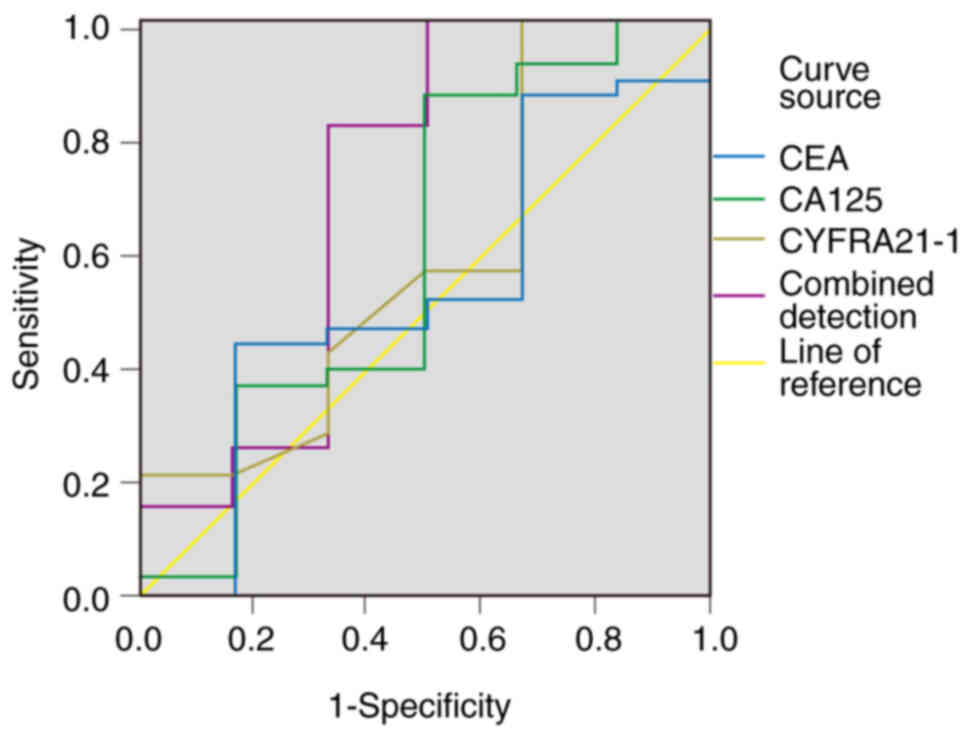
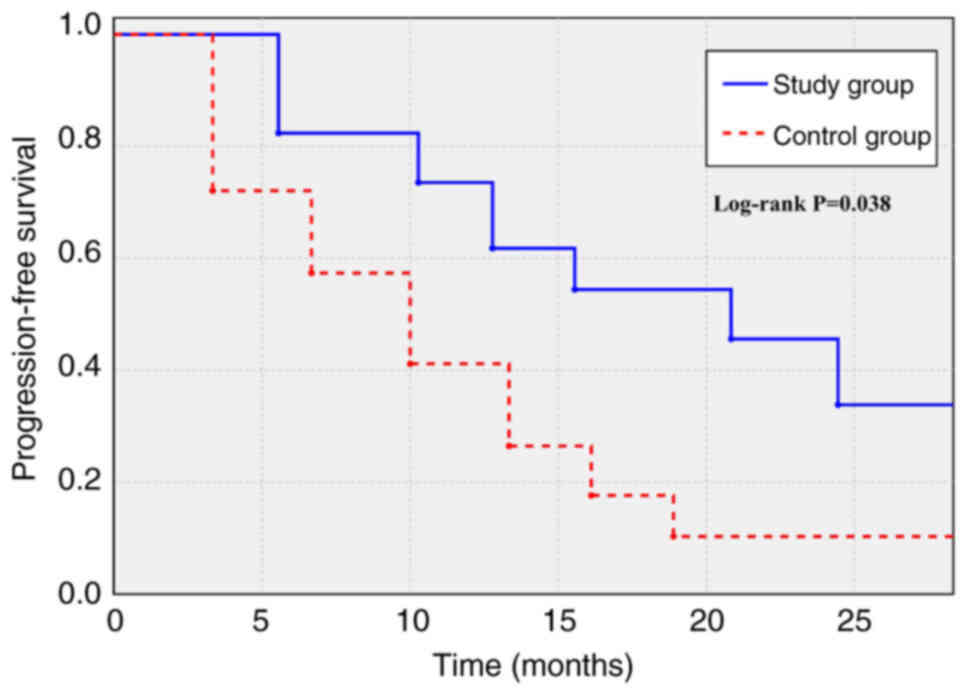
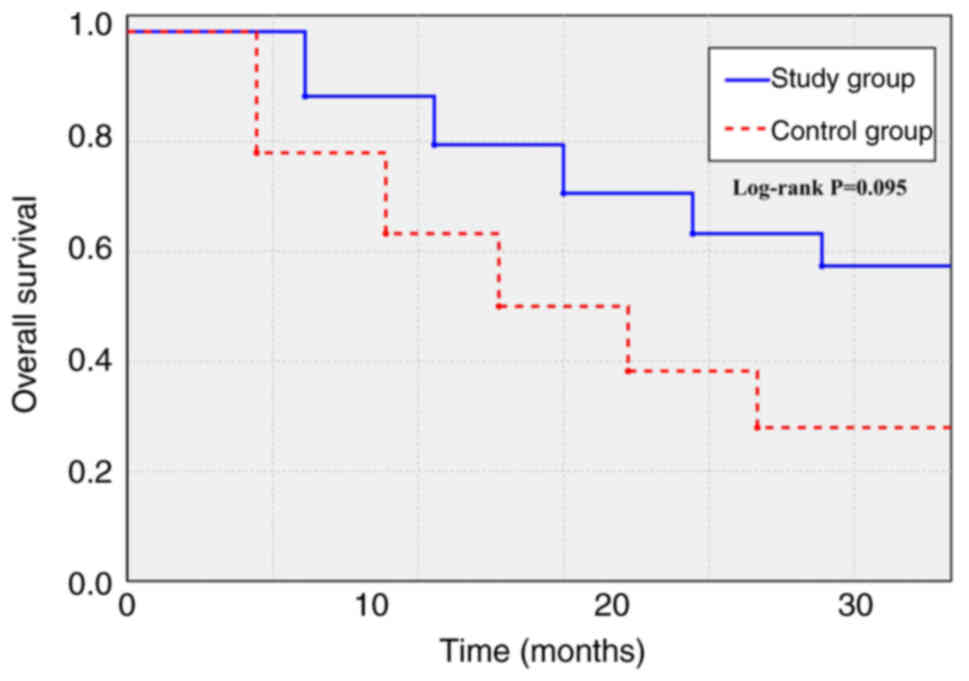
|
1
|
Riely GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non-small cell lung cancer, version 4.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:249–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang
T, Li W and Xia Y: Management of locally advanced non-small cell
lung cancer: State of the art and future directions. Cancer Commun
(Lond). 44:23–46. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guan H, He Y, Wei Z, Wang J, He L, Mu X
and Peng X: Assessment of induction chemotherapy regimen TPF vs GP
followed by concurrent chemoradiotherapy in locally advanced
nasopharyngeal carcinoma: A retrospective cohort study of 160
patients. Clin Otolaryngol. 45:274–279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dermani FK, Samadi P, Rahmani G, Kohlan AK
and Najafi R: PD-1/PD-L1 immune checkpoint: Potential target for
cancer therapy. J Cell Physiol. 234:1313–1325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang C, McKay RM, Lee SY, Romo CG,
Blakeley JO, Haniffa M, Serra E, Steensma MR, Largaespada D and Le
LQ: Cutaneous neurofibroma heterogeneity: Factors that influence
tumor burden in neurofibromatosis type 1. J Invest Dermatol.
143:1369–1377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abushanab AK, Mustafa MT, Mousa MT,
Qawaqzeh RA, Alqudah GN and Albanawi RF: Efficacy and safety of
tislelizumab for malignant solid tumor: A systematic review and
meta-analysis of phase III randomized trials. Expert Rev Clin
Pharmacol. 16:1153–1161. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu S: Value of Combined Detection of
CYFRA21-1, CA125, CEA, NSE and SCCA Serum in the Diagnosis of Lung
Cancer. Guide China Med. 22:47–50. 2024.(In Chinese).
|
|
10
|
Kobayashi K, Ono Y, Kitano Y, Oba A, Sato
T, Ito H, Mise Y, Shinozaki E, Inoue Y, Yamaguchi K, et al:
Prognostic impact of tumor markers (CEA and CA19-9) on patients
with resectable colorectal liver metastases stratified by tumor
number and size: Potentially valuable biologic markers for
preoperative treatment. Ann Surg Oncol. 30:7338–7347. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang ZH, Han YW, Liang H and Wang LM:
Prognostic value of serum CYFRA21-1 and CEA for non-small-cell lung
cancer. Cancer Med. 4:1633–1638. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen F and Zhang X: Predictive value of
serum SCCA and CYFRA21-1 levels on radiotherapy efficacy and
prognosis in patients with non-small cell lung cancer. Biotechnol
Genet Eng Rev. 40:4205–4214. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radiation Oncology Branch of Chinese
Medical Association, Radiation Oncologist Branch of Chinese Medical
Doctor Association Professional Committee on Radiation Oncology,
China Anti-Cancer Association Experts Committee on Radiation
Oncology and Radiation Oncologist Branch of Chinese Society of
Clinical Oncology, . Clinical practice guideline for radiation
therapy in small cell lung cancer (2020 version). Chin J Radiat
Oncol. 29:608–614. 2020.(In Chinese).
|
|
14
|
Fleming ID: AJCC/TNM cancer staging,
present and future. J Surg Oncol. 77:233–236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chinese Anti-Cancer Association, Lung
Cancer Study Group of Committee of Oncopathology, Chinese Society
of Lung Cancer and Expert Group on PD-L1 Testing Consensus, .
Chinese Expert Consensus on Standards of PD-L1 Immunohistochemistry
Testing for Non-small Cell Lung Cancer. Chin J Lung Cancer.
23:733–740. 2020.(In Chinese).
|
|
18
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE)
version 5.0, 2017. https://dctd.cancer.gov/research/ctep-trials/for-sites/adverse-events/ctcae-v5-5×7.pdfJune
15–2025
|
|
19
|
Spitaleri G, Trillo Aliaga P, Attili I,
Del Signore E, Corvaja C, Corti C, Crimini E, Passaro A and de
Marinis F: Sustained improvement in the management of patients with
non-small-cell lung cancer (NSCLC) harboring ALK translocation:
where are we running? Curr Oncol. 30:5072–5092. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv P and Liu M: Meta-analysis of the
clinical effect of Kanglaite injection-assisted gemcitabine plus
cisplatin regimen on non-small cell lung cancer. Am J Transl Res.
15:2999–3012. 2023.PubMed/NCBI
|
|
21
|
Wakelee H: Chemotherapy and immunotherapy
in early-stage NSCLC: Neoadjuvant vs adjuvant therapy. Clin Adv
Hematol Oncol. 21:648–651. 2023.PubMed/NCBI
|
|
22
|
Olivares-Hernández A, González Del
Portillo E, Tamayo-Velasco Á, Figuero-Pérez L, Zhilina-Zhilina S,
Fonseca-Sánchez E and Miramontes-González JP: Immune checkpoint
inhibitors in non-small cell lung cancer: From current perspectives
to future treatments-a systematic review. Ann Transl Med.
11:3542023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizuno T, Katsuya Y, Sato J, Koyama T,
Shimizu T and Yamamoto N: Emerging PD-1/PD-L1 targeting
immunotherapy in non-small cell lung cancer: Current status and
future perspective in Japan, US, EU, and China. Front Oncol.
12:9259382022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim SM, Hong MH and Kim HR: Immunotherapy
for non-small cell lung cancer: Current landscape and future
perspectives. Immune Netw. 20:e102020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jackson M, Ahmari N, Wu J, Rizvi TA,
Fugate E, Kim MO, Dombi E, Arnhof H, Boehmelt G, Düchs MJ, et al:
Combining SOS1 and MEK inhibitors in a murine model of plexiform
neurofibroma results in tumor shrinkage. J Pharmacol Exp Ther.
385:106–116. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong
W, Zhang Y, Zhou X, Wang Z, Wang Y, et al: The binding of an
anti-PD-1 antibody to FcγRI has a profound impact on its biological
functions. Cancer Immunol Immunother. 67:1079–1090. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kresbach C, Dottermusch M, Eckhardt A,
Ristow I, Paplomatas P, Altendorf L, Wefers AK, Bockmayr M,
Belakhoua S, Tran I, et al: Atypical neurofibromas reveal distinct
epigenetic features with proximity to benign peripheral nerve
sheath tumor entities. Neuro Oncol. 25:1644–1655. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galluzzi L, Buqué A, Kepp O, Zitvogel L
and Kroemer G: Immunogenic cell death in cancer and infectious
disease. Nat Rev Immunol. 17:97–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poon E, Mullins S, Watkins A, Williams GS,
Koopmann JO, Di Genova G, Cumberbatch M, Veldman-Jones M,
Grosskurth SE, Sah V, et al: The MEK inhibitor selumetinib
complements CTLA-4 blockade by reprogramming the tumor immune
microenvironment. J Immunother Cancer. 5:632017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang X, Chen X, Li H and Li Y:
Tislelizumab plus chemotherapy is more cost-effective than
chemotherapy alone as first-line therapy for advanced non-squamous
non-small cell lung cancer. Front Public Health. 11:10099202023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santoro A, Pilar G, Tan DSW, Zugazagoitia
J, Shepherd FA, Bearz A, Barlesi F, Kim TM, Overbeck TR, Felip E,
et al: Spartalizumab in combination with platinum-doublet
chemotherapy with or without canakinumab in patients with
PD-L1-unselected, metastatic NSCLC. BMC Cancer. 24:13072024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Li A, Yu H, Wang Y, Zhang X, Qiu
H, Du W, Luo L, Fu S, Zhang L and Hong S: Neoadjuvant-adjuvant vs
neoadjuvant-only PD-1 and PD-L1 inhibitors for patients with
resectable NSCLC: An indirect meta-analysis. JAMA Netw Open.
7:e2412852024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mathew M, Enzler T, Shu CA and Rizvi NA:
Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther.
186:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Makharadze T, Gogishvili M, Melkadze T,
Baramidze A, Giorgadze D, Penkov K, Laktionov K, Nemsadze G,
Nechaeva M, Rozhkova I, et al: Cemiplimab plus chemotherapy versus
chemotherapy alone in advanced NSCLC: 2-year follow-up from the
phase 3 EMPOWER-lung 3 part 2 trial. J Thorac Oncol. 18:755–768.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu X, Sun L, Song N, He W, Xie B, Hu J,
Zhang J, Yang J, Dai J, Bian D, et al: Safety and effectiveness of
neoadjuvant PD-1 inhibitor (toripalimab) plus chemotherapy in stage
II–III NSCLC (LungMate 002): An open-label, single-arm, phase 2
trial. BMC Med. 20:4932022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Strum S, Vincent M, Gipson M, McArthur E
and Breadner D: Assessment of serum tumor markers CEA, CA-125, and
CA19-9 as adjuncts in non-small cell lung cancer management.
Oncotarget. 15:381–388. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clevers MR, Kastelijn EA, Peters BJM,
Kelder H and Schramel FMNH: Evaluation of serum biomarker CEA and
Ca-125 as immunotherapy response predictors in metastatic non-small
cell lung cancer. Anticancer Res. 41:869–876. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
Toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naing A, Hajjar J, Gulley JL, Atkins MB,
Ciliberto G, Meric-Bernstam F and Hwu P: Strategies for improving
the management of immune-related adverse events. J Immunother
Cancer. 8:e0017542020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Imai H, Kaira K and Kagamu H: Advanced
research on immune checkpoint inhibitor therapy. J Clin Med.
11:53922022. View Article : Google Scholar : PubMed/NCBI
|