Introduction
Lung cancer remains a global health crisis, which
ranks as the second most common malignancy worldwide by incidence
and the leading cause of cancer-related mortality (1,2). In
China, lung cancer holds the highest incidence and mortality rates
among all cancer types (1,2). Non-small cell lung cancer (NSCLC),
which accounts for ~85% of lung cancer cases, is characterized by
its insidious onset and lack of early symptoms, which results in
>70% of patients being diagnosed at advanced stages when
surgical resection is no longer feasible (3). For these patients, multimodal
therapies, including chemotherapy, radiotherapy, targeted therapy,
immunotherapy and anti-angiogenic agents form the cornerstone of
treatment (4).
The gemcitabine-cisplatin (GP) regimen is a
first-line chemotherapy option for advanced NSCLC. However, the
long-term application of GP is limited by cumulative toxicity and
variable efficacy, which underscores the need for safer and more
effective therapeutic strategies (4). Tislelizumab, a humanized monoclonal
antibody targeting programmed cell death protein-1 (PD-1), has
emerged as a potential immunotherapeutic agent. PD-1, expressed on
activated T cells, binds to its ligands programmed death ligand
(PD-L)1 and PD-L2, which are often upregulated on tumor cells and
other cells within the tumor microenvironment, such as
antigen-presenting cells including macrophages and dendritic cells
(5). This interaction delivers an
inhibitory signal that dampens T-cell effector functions, such as
cytokine production and cytotoxicity, which allows cancer cells to
evade immune destruction (5,6). By
blocking PD-1-mediated immunosuppression, tislelizumab prevents
this inhibitory signaling, thereby unleashing antitumor T-cell
activity and potentiating tumor cell apoptosis. Initially approved
for relapsed Hodgkin's lymphoma, tislelizumab has demonstrated
efficacy in gastrointestinal cancer types, esophageal carcinoma and
NSCLC, which offers potential survival benefits (7). Ongoing research continues to explore
the efficacy of tislelizumab in diverse cancer types and
combination strategies, which aim to further optimize its clinical
application and patient outcomes (8).
Serum biomarkers such as CEA, CA125 and cytokeratin
19 fragment (CYFRA21-1) serve key roles in cancer monitoring
(9). CEA, a glycoprotein associated
with epithelial malignancies, is elevated in 30–60% of NSCLC cases,
and has been associated with tumor burden and progression (10). CA125, traditionally associated with
ovarian cancer, is also aberrantly expressed in NSCLC, which
reflects pleural involvement or metastatic spread (11). CYFRA21-1, a structural component of
epithelial cell cytokeratins, facilitates tumor cell detachment and
metastasis by disrupting intercellular adhesion (12). These biomarkers collectively provide
a non-invasive tool for the assessment of therapeutic response and
prognosis.
Therefore, the primary aim of the present study was
to compare the efficacy, long-term survival and safety of
tislelizumab + GP chemotherapy against GP chemotherapy alone in
patients with advanced NSCLC. As a secondary objective, the present
study sought to address the gap in monitoring tools by analyzing
the predictive value of a combined panel of serum CEA, CA125 and
CYFRA21-1 to evaluate the short-term efficacy of the
tislelizumab-based regimen.
Patients and methods
Study design
The present study protocol was approved by the
Ethics Committee of Fourth Affiliated Hospital of Harbin Medical
University (approval no. HRB-2020-23; Harbin, China) and was
performed in accordance with The Declaration of Helsinki. Written
informed consent was obtained from all study subjects before
enrollment. Sample size was estimated based on an anticipated 20%
improvement in objective response rate (ORR) with the addition of
tislelizumab (from 25 to 45%), which required ≤42 patients per
group to achieve 80% power at a two-sided α level of 0.05. To
account for potential dropouts, 45 patients per group were enrolled
in the present study.
A total of 90 patients diagnosed with advanced NSCLC
between January 2021 and December 2024 were enrolled in the present
study. Participants were randomly assigned in a 1:1 ratio to either
the study group (n=45) or the control group (n=45) using a
computer-generated randomization sequence. Allocation concealment
was ensured by the Investigational Drug Service pharmacy of the
Fourth Affiliated Hospital of Harbin Medical University, which
dispensed the study drugs according to the computer-generated
randomization sequence. Inclusion criteria required patients to
meet the diagnostic standards for NSCLC outlined in the Chinese
Clinical Guidelines for Radiotherapy of Non-Small Cell Lung Cancer
(2020 Edition) (13), confirmed
through lung biopsy and imaging studies. Eligible patients had TNM
stage (14) IIIa-IIIc disease
without surgical indications, an Eastern Cooperative Oncology Group
performance status (15) of 0 or 1
and were candidates for chemotherapy, and exhibited no
contraindications to tislelizumab, gemcitabine or cisplatin.
Additional criteria included an expected survival period of >3
months and provision of written informed consent. Exclusion
criteria encompassed severe infections, concurrent primary
malignancies, notable organ dysfunction (for example, heart, liver
or kidney failure), cognitive impairments affecting treatment
compliance, prior chemotherapy exposure, treatment interruptions
during the present study, loss to follow-up, hematopoietic or
systemic dysfunction, and pregnancy or lactation.
Interventions
The control group received the GP chemotherapy
regimen. Gemcitabine hydrochloride (0.2 g/vial; Harbin Yulian
Pharmaceutical Co., Ltd.) was administered intravenously at 1.2
g/m2 diluted in 100 ml 0.9% sodium chloride on days 1
and 8 of a 21-day cycle. Cisplatin (10 ml/10 mg; Guangdong Lingnan
Pharmaceutical Co., Ltd.) was administered intravenously at 75
mg/m2 diluted in 500 ml 0.9% sodium chloride on day 1 of
each cycle. Treatment continued for 4–6 cycles (3–4.5 months, with
biomarker assessment after 3 months as planned).
The study group received tislelizumab combined with
the same GP regimen. Tislelizumab (10 ml/100 mg; Boehringer
Ingelheim Biopharmaceuticals) was administered at 200 mg per dose,
diluted in 100 ml 0.9% sodium chloride, via intravenous infusion
every 3 weeks. The treatment duration and chemotherapy protocol
matched those of the control group.
Outcome measures
Short-term efficacy was evaluated according to the
Response Evaluation Criteria in Solid Tumors (version 1.1)
(16), referenced alongside the
Chinese Expert Consensus on PD-L1 Immunohistochemical Testing in
NSCLC (17). Complete response (CR)
was defined as the disappearance of all target lesions maintained
for ≤4 weeks, while partial response (PR) referred to a ≥30%
reduction in the sum of diameters of target lesions. Stable disease
(SD) indicated neither sufficient shrinkage to qualify for PR nor
sufficient increase to qualify for PD, and progressive disease (PD)
was characterized by ≥20% increase in the sum of diameters of
target lesions or the appearance of novel metastases. ORR (%) and
disease control rate [DCR (%)] were calculated as (CR + PR)/total
cases ×100 and (CR + PR + SD)/total cases ×100, respectively.
Long-term efficacy outcomes included
progression-free survival (PFS), defined as the time from
randomization to disease progression or death from any cause, and
overall survival (OS), defined as the time from randomization to
death from any cause. Patients were followed up every 3 months. The
data cut-off date for the present analysis was May 31, 2025.
Serum levels of CEA, CA125 and CYFRA21-1 were
measured pre-treatment and 3 months post-treatment. Fasting venous
blood (4 ml) was centrifuged at 1,500 × g for 10 min at room
temperature and serum was analyzed via electrochemiluminescence
using the Cobas e601 immunoassay analyzer and corresponding kits
(Elecsys CEA Immunoassay, cat. no. YDLC-15737; Elecsys CA125 II
Immunoassay, cat. no. YDLC-15799; Elecsys CYFRA 21-1 Immunoassay,
cat. no. YDLC-9257; all from Shanghai Yuduo Biotechnology Co.,
Ltd.). Each biomarker was evaluated in triplicate, with means
recorded. Positive thresholds were defined as >5 ng/ml for CEA,
>35 U/ml for CA125 and >3.3 ng/ml for CYFRA21-1.
Adverse events (AEs) were classified using the
Common Terminology Criteria for Adverse Events by the National
Cancer Institute (version 5.0) (18). Receiver operating characteristic
(ROC) curve analysis evaluated the predictive value of biomarkers
for short-term therapeutic efficacy.
Statistical analysis
SPSS (version 26.0; IBM Corp.) was employed for data
analysis. Normally distributed continuous variables were expressed
as the mean ± SD and compared using unpaired Student's t-test,
while non-normally distributed variables were reported as the
median (interquartile range) and analyzed using Mann-Whitney U
test. Categorical data were described as frequencies (%) and
assessed via χ2 or Fisher's exact tests as appropriate.
PFS and OS were estimated using the Kaplan-Meier method and
differences between groups were assessed using the log-rank test.
Hazard ratios (HRs) and their 95% CIs were calculated using Cox
proportional hazards models. ROC curves determined sensitivity,
specificity and area under the curve (AUC) for biomarkers. The
DeLong test was used to assess the statistical significance of the
differences between the AUC of the combined biomarker panel and
those of the individual markers. P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline characteristics of the two
groups
No statistically significant differences were
observed between the study and control groups in terms of age, sex,
smoking history, alcohol consumption, clinical stage or
histological subtypes (P>0.05), as detailed in Table I. The mean age was 53.23±8.03 years
in the control group vs. 53.09±8.76 years in the study group
(t=−0.079; P=0.937). Male/female ratios were comparable (31/14 vs.
33/12; χ2=0.216; P=0.642), as were smoking (12 vs. 14)
and alcohol consumption (9 vs. 13) rates. Both groups exhibited
similar distributions of advanced-stage disease (Stage III, 17 vs.
16; Stage IV, 28 vs. 29) and histological classifications (squamous
carcinoma, 16 vs. 14; adenocarcinoma, 29 vs. 31). Tumor
localization and laterality (left lung, 19 vs. 17; right lung, 24
vs. 27; bilateral, 2 vs. 1) also demonstrated no significant
differences (P>0.05 for all comparisons).
 | Table I.Comparison of general patient
characteristics between the two groups. |
Table I.
Comparison of general patient
characteristics between the two groups.
| Characteristic | Control group
(n=45) | Study group
(n=45) |
t/χ2 | P-value |
|---|
| Age, years | 53.23±8.03 | 53.09±8.76 | −0.079 | 0.937 |
| Male/female sex,
n | 31/14 | 33/12 | 0.216 | 0.642 |
| Smoking, n | 12 | 14 | 0.216 | 0.642 |
| Alcohol
consumption, n | 9 | 13 | 0.963 | 0.326 |
| Clinical stage (I),
n |
|
| 0.048 | 0.827 |
| Stage
III | 17 | 16 |
|
|
| Stage
IV | 28 | 29 |
|
|
| Pathological type,
n |
|
| 0.200 | 0.655 |
|
Squamous carcinoma | 16 | 14 |
|
|
|
Adenocarcinoma | 29 | 31 |
|
|
| Primary site,
n |
|
| 0.563 | 0.905 |
| Upper
lobe | 5 | 6 |
|
|
| Middle
lobe | 24 | 26 |
|
|
| Lower
lobe | 7 | 5 |
|
|
|
Overlap | 9 | 8 |
|
|
| Tumor location,
n |
|
| - | 0.745a |
| Left
lung | 19 | 17 |
|
|
| Right
lung | 24 | 27 |
|
|
|
Bilateral | 2 | 1 |
|
|
Short-term efficacy outcomes
The study group demonstrated numerically higher ORR
(33.33 vs. 22.22%) and DCR (77.78 vs. 60.00%) compared with the
control group; however, these differences did not reach statistical
significance (P=0.239 and P=0.069, respectively) (Table II). In the study group, 5 patients
achieved CR and 10 demonstrated PR, whereas the control group had 3
CR and 7 PR cases. SD was observed in 20 patients in the study
group vs. 17 in the control group, and PD occurred in 10 patients
in the study group vs. 19 in the control group, which favored the
tislelizumab combination regimen.
 | Table II.Comparison of short-term treatment
efficacy between the two groups. |
Table II.
Comparison of short-term treatment
efficacy between the two groups.
| Outcome
measure | Control group
(n=45) | Study group
(n=45) |
t/χ2 | P-value |
|---|
| CR, n | 3 | 5 |
|
|
| PR, n | 7 | 10 |
|
|
| SD, n | 17 | 20 |
|
|
| PD, n | 19 | 10 |
|
|
| ORR, n (%) | 10 (22.22) | 15 (33.33) | 1.385 | 0.239 |
| DCR, n (%) | 27 (60.00) | 35 (77.78) | 3.318 | 0.069 |
Predictive value of serum biomarkers
for short-term therapeutic efficacy
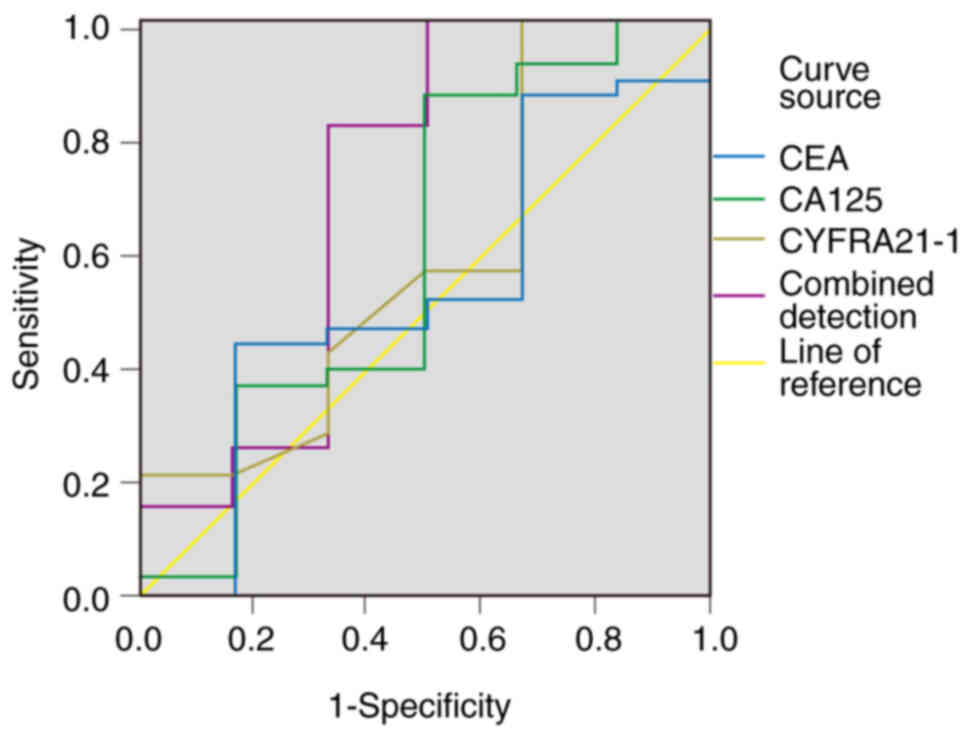
ROC curve analysis demonstrated that the combination
of CEA, CA125 and CYFRA21-1 levels provided notable predictive
performance for evaluating the short-term efficacy of tislelizumab
+ GP chemotherapy in patients with advanced NSCLC, compared with
individual biomarkers (Table III;
Fig. 1). The combined model
achieved the highest AUC (0.705; 95% CI, 0.411–0.999), sensitivity
(87.16%) and specificity (88.35%), which significantly outperformed
individual markers (χ2=9.021; P<0.05). By contrast,
no significant differences were observed among the individual
biomarkers: i) CEA demonstrated an AUC of 0.530 (95% CI,
0.269–0.791), sensitivity of 73.23%, specificity of 68.79% at a
cut-off of 89.15 pg/ml; ii) CA125 demonstrated an AUC of 0.594 (95%
CI, 0.297–0.891), sensitivity of 76.56%, specificity of 70.67% at
31.4 U/ml; iii) CYFRA21-1 demonstrated an AUC of 0.583 (95% CI,
0.312–0.855), sensitivity of 74.64%, specificity of 69.67% at 19.05
ng/ml.
 | Table III.Evaluation of the efficacy of CEA,
CA125, CYFRA21-1 levels and combined detection for the short-term
treatment of non-small cell lung cancer with tislelizumab combined
with gemcitabine-cisplatin chemotherapy. |
Table III.
Evaluation of the efficacy of CEA,
CA125, CYFRA21-1 levels and combined detection for the short-term
treatment of non-small cell lung cancer with tislelizumab combined
with gemcitabine-cisplatin chemotherapy.
| Factor | AUC (95% CI) | Key value | Sensitivity, % | Specificity, % |
|---|
| CEA, pg/ml | 0.530
(0.269–0.791) | 89.15 | 73.23 | 68.79 |
| CA125, U/ml | 0.594
(0.297–0.891) | 31.4 | 76.56 | 70.67 |
| CYFRA21-1,
ng/ml | 0.583
(0.312–0.855) | 19.05 | 74.64 | 69.67 |
| Combined
detection | 0.705
(0.411–0.999) | - | 87.16 | 88.35 |
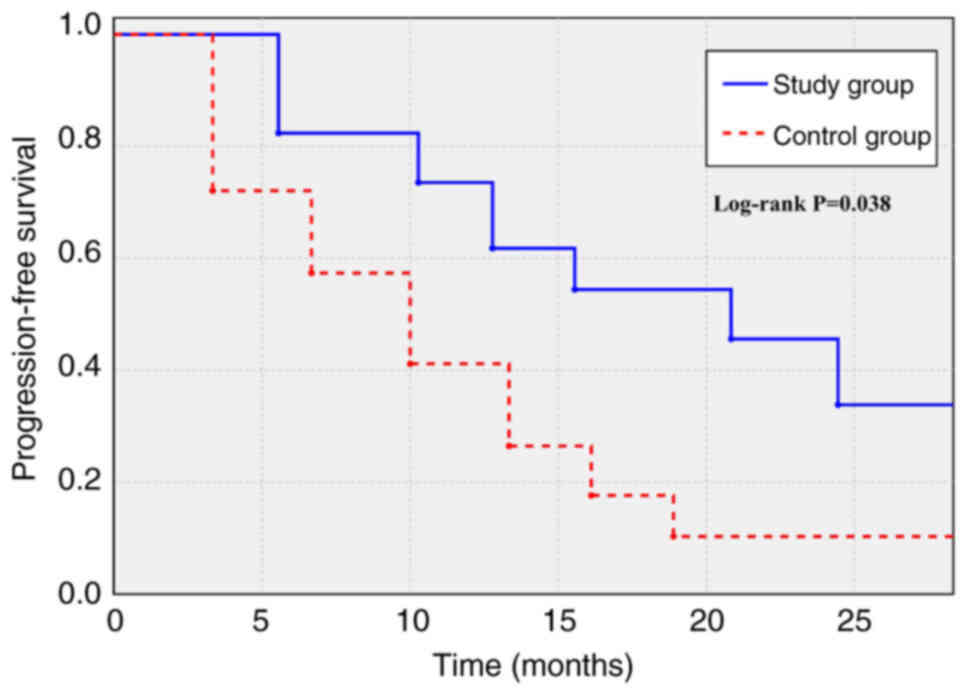
Long-term efficacy outcomes
The median follow-up duration for the entire cohort
was 23.5 months (range, 5.0–52.0 months). Long-term follow-up data
revealed notable survival outcomes in the study group compared with
those in the control group. As shown in the Kaplan-Meier analysis
for PFS (Fig. 2), the median PFS
(the time at which 50% of patients were progression-free) was
significantly longer in the study group (20.8 months; 95% CI,
13.6–25.4) compared with in the control group (10.0 months; 95% CI,
7.1–12.9) (HR, 0.66; 95% CI, 0.43–0.98; log-rank P=0.038).
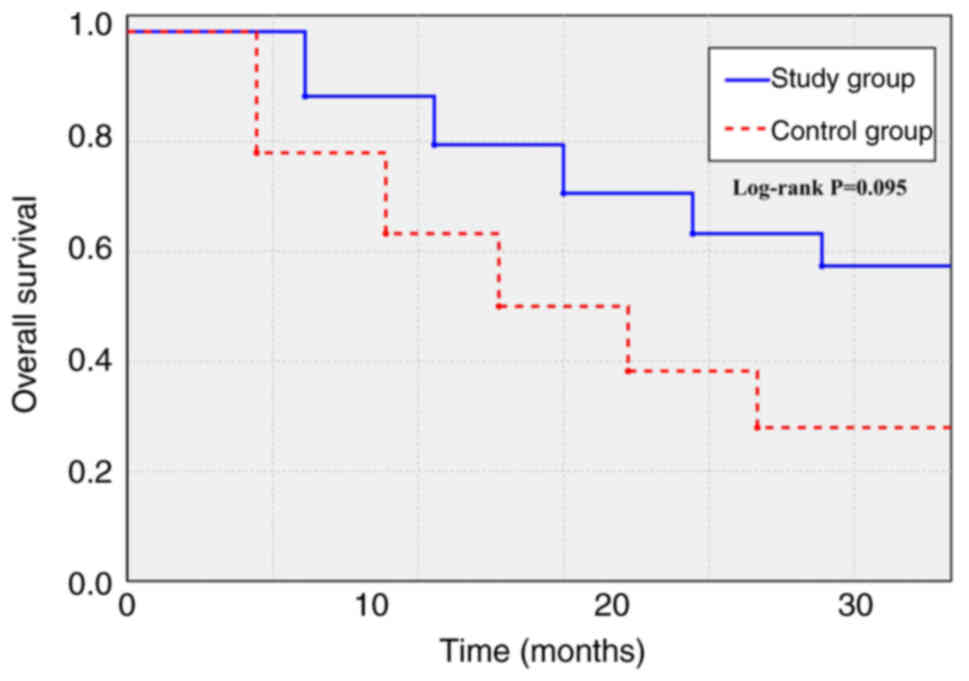
Regarding OS (Fig. 3), the study
group demonstrated a trend towards improvement, with a median OS
(the time at which 50% of patients remained alive) that was not
reached (95% CI, 19.8-not reached) compared with 16.0 months (95%
CI, 13.5–18.5) in the control group (HR, 0.71; 95% CI, 0.47–1.07;
log-rank P=0.095).
Serum biomarker levels
Pretreatment levels of CEA, CA125 and CYFRA21-1 were
comparable between groups (P>0.05) (Table IV). At 3 months post-treatment, the
study group exhibited significantly lower biomarker levels compared
with the control group for CEA [4.02±0.82 ng/ml vs. 4.61±0.89 ng/ml
(t=−3.270; P=0.002)], CA125 [42.18±5.82 U/ml vs. 49.76±5.66 U/ml
(t=−6.263; P<0.001)] and CYFRA21-1 [3.42±0.43 ng/ml vs.
4.39±0.56 ng/ml (t=9.216; P<0.001)].
 | Table IV.Comparison of CEA, CA125 and
CYFRA21-1 levels between the two groups. |
Table IV.
Comparison of CEA, CA125 and
CYFRA21-1 levels between the two groups.
| A, CEA, ng/ml |
|---|
|
|---|
| Time | Control group
(n=45) | Study group
(n=45) | t | P-value |
|---|
| Before treatment (1
day) | 6.23±0.94 | 6.19±0.97 | −0.199 | 0.843 |
| 3 months after
treatment | 4.61±0.89 | 4.02±0.82 | −3.270 | 0.002 |
|
| B, CA125,
U/ml |
|
| Time | Control group
(n=45) | Study group
(n=45) | t | P-value |
|
| Before treatment (1
day) | 63.98±5.32 | 64.23±5.86 | 0.212 | 0.833 |
| 3 months after
treatment | 49.76±5.66 | 42.18±5.82 | −6.263 | <0.001 |
|
| C, CYFRA21-1,
ng/ml |
|
| Time | Control group
(n=45) | Study group
(n=45) | t | P-value |
|
| Before treatment (1
day) | 6.34±0.76 | 6.29±0.81 | −0.302 | 0.763 |
| 3 months after
treatment | 4.39±0.56 | 3.42±0.43 | −9.216 | <0.001 |
Comparison of AEs
The incidence of AEs in the study group was
numerically lower compared with that in the control group, although
no statistically significant differences were observed (P>0.05
for all comparisons) (Table V).
Grade I anemia occurred in 4 patients in the control group vs. 2
patients in the study group (χ2=0.714; P=0.398), whereas
Grade II anemia was reported in 2 vs. 1 cases (χ2=0.322;
P=0.570). Neutropenia rates were similarly comparable: Grade I (3
vs. 2; χ2=0.212; P=0.645) and Grade II (2 vs. 1;
χ2=0.345; P=0.557). Elevated alkaline phosphatase levels
also indicated no significant intergroup differences, with similar
numbers of Grade I (3 vs. 2) and Grade II (2 vs. 1) events.
Cutaneous reactions such as Grade I maculopapular rash (1 vs. 0;
χ2=1.011; P=0.315) and gastrointestinal toxicities,
including Grade I nausea (12 vs. 8; χ2=1.029; P=0.310),
Grade II nausea (8 vs. 11; χ2=0.600; P=0.439) and Grade
I vomiting (9 vs. 5; χ2=1.353; P=0.245), were marginally
reduced in the study group but did not reach statistical
significance.
 | Table V.Comparison of adverse reactions
between the control (n=45) and study (n=45) groups. |
Table V.
Comparison of adverse reactions
between the control (n=45) and study (n=45) groups.
| Adverse event | Control group
(n=45) | Study group
(n=45) | P-value |
|---|
| Anemia |
|
|
|
| Grade
I | 4 | 2 | 0.438ª |
| Grade
II | 2 | 1 | 0.616ª |
| Neutropenia |
|
|
|
| Grade
I | 3 | 2 | 0.695ª |
| Grade
II | 2 | 1 | 0.616ª |
| Alkaline
phosphatase elevation |
|
|
|
| Grade
I | 3 | 2 | 0.695ª |
| Grade
II | 2 | 1 | 0.616ª |
| Grade I rash | 1 | 0 | 0.494ª |
| Nausea |
|
|
|
| Grade
I | 12 | 8 | 0.294b |
| Grade
II | 8 | 11 | 0.439b |
| Grade I
vomiting | 9 | 5 | 0.245b |
Discussion
NSCLC is a highly aggressive malignancy
characterized by insidious onset, rapid progression and a lack of
effective early diagnostic methods. Most patients are diagnosed at
advanced stages (III or IV) with local or distant metastases,
resulting in a poor prognosis, with the 5-year survival rate for
distant-stage disease being ~7% (19). Currently, the GP chemotherapy
regimen remains a common first-line treatment for advanced NSCLC.
While it improves local control rates, survival rates and reduces
distant metastases to some extent, its efficacy is limited by
factors such as tumor sensitivity, drug resistance, dose-limiting
toxicity and compromised patient performance status (20). Furthermore, chemotherapy-induced
toxicities, such as damage to germ, gastrointestinal epithelial and
immune cells (for example, lymphocytes and hematopoietic cells)
exacerbate immune dysfunction, impair antitumor responses and
accelerate disease progression (21).
Recent advances in immunotherapy, particularly
immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1, have
revolutionized NSCLC management (22–24).
Tumor cells evade immune surveillance by expressing PD-L1, which
binds to PD-1 on T cells, suppressing their activity. ICIs block
this interaction, which restores T-cell-mediated antitumor immunity
and inhibits cancer progression (25). Tislelizumab, a novel PD-1 monoclonal
antibody developed in China, exhibits enhanced antitumor activity
compared with traditional PD-1 inhibitors, such as pembrolizumab
and nivolumab, due to structural modifications in its
crystallizable fragment (Fc) region, which minimizes binding to Fcγ
receptors on macrophages, thereby reducing antibody-dependent
phagocytosis of T cells (26). It
demonstrates lower IC50 values, higher binding affinity
and improved safety profiles, particularly reduced incidences of
capillary hyperplasia, compared with other agents such as
camrelizumab. Additionally, the cost-effectiveness of tislelizumab
compared with imported PD-1 inhibitors (for example, pembrolizumab
and atezolizumab) enhances patient accessibility (25,27).
The combination of immunotherapy with chemotherapy
is founded on several potential synergistic mechanisms.
Chemotherapy can induce immunogenic cell death, which releases
tumor antigens (such as CALR and HMGB1) and damage-associated
molecular patterns (including ATP and Hsp70/90) that can prime and
enhance antitumor T-cell responses (28). Furthermore, chemotherapy may
modulate the tumor microenvironment by reducing the numbers of
immunosuppressive cells such as regulatory T cells and
myeloid-derived suppressor cells and by upregulating PD-L1
expression on tumor cells, potentially sensitizing them to PD-1
blockade (29). Tislelizumab, by
invigorating the T-cell response, can then more effectively target
and eliminate tumor cells rendered vulnerable by chemotherapy.
Although chemotherapy alone can induce some level of immunogenic
cell death, its clinical success is limited because this effect is
often transient and insufficient to overcome the highly
immunosuppressive tumor microenvironment. Tumors employ multiple
escape mechanisms, of which the chief mechanism is the upregulation
of checkpoint proteins such as PD-L1, which actively inhibit T-cell
function and lead to T-cell exhaustion. Therefore, even if
chemotherapy enhances antigen presentation, the responding T cells
remain suppressed. This limitation highlights the rationale for
combination therapy: Chemotherapy acts to increase tumor
immunogenicity, while tislelizumab simultaneously blocks the
PD-1/PD-L1 inhibitory axis, thereby unleashing a potent and durable
antitumor T-cell response that neither agent could achieve alone
(30).
The present study findings regarding short-term
efficacy (ORR and DCR) exhibited numerically higher rates in the
tislelizumab-GP group; however, these differences were not
statistically significant. This lack of statistical significance
might be attributed to the relatively small sample size. However,
the observed trend, coupled with the significant improvement in
median PFS (11.8 vs. 8.7 months; P=0.038) and the positive trend in
OS (median 23.5 vs. 18.2 months; P=0.095) at a median follow-up of
23.5 months, suggested a clinically meaningful benefit from the
addition of tislelizumab to GP chemotherapy. This aligns with
findings from several large-scale trials but also highlights the
ongoing debate, as outcomes can vary based on study design, patient
populations and treatment regimens. For example, Santoro et
al (31) evaluated
spartalizumab and various chemotherapy backbones in a
PD-L1-unselected metastatic NSCLC population, and provided a
relevant comparison. Their gemcitabine/cisplatin group (Group A)
reported a higher ORR (57.6%) compared with the present study
(33.33%); however, the median PFS (7.5 months) was shorter compared
with 11.8 months from the present study results, which suggests
potential differences in drug-specific activity or patient
characteristics. By contrast, a meta-analysis by Zhou et al
(32) offers a different
perspective, which focused on the neoadjuvant setting for
resectable NSCLC. It concluded that the addition of adjuvant
immunotherapy to a neoadjuvant chemoimmunotherapy regimen did not
improve PFS or OS; however, this previous study had a different
clinical question and setting compared with the focus of the
present study on first-line treatment for advanced, unresectable
disease. Furthermore, Mathew et al (33) outlined the preclinical rationale for
these combinations in a review article, but did not provide
comparative clinical trial data. A notable comparison is the
EMPOWER-Lung 3 trial by Makharadze et al (34), which evaluated cemiplimab +
chemotherapy in first-line, advanced NSCLC irrespective of PD-L1
status. This previous study reported an ORR of 43.6% and a median
PFS of 8.2 months (HR, 0.55), with a median OS of 21.1 months.
Although the ORR in this previous study was higher, the present
study exhibited a more favorable median PFS (11.8 months), a
difference that might be attributed to the specific GP regimen,
regional population differences or other unmeasured patient
factors. Finally, Zhu et al (35) also evaluated a distinct setting:
Neoadjuvant toripalimab + chemotherapy in resectable stage II–III
NSCLC. Its primary endpoints were the major pathological response
rate (55.6%) and R0 resection rate (100%), which are not applicable
to the palliative-intent treatment of the advanced-stage cohort in
the present study. This highlights the key importance of treatment
setting when comparing efficacy outcomes.
Serum biomarkers such as CEA, CA125 and CYFRA21-1
are key for monitoring therapeutic efficacy. The present study
corroborated findings from Strum et al (36) and Clevers et al (37), which reported markedly reduced
post-treatment levels of all three biomarkers in the
tislelizumab-GP group (P<0.05). These reductions are considered
clinically relevant as they may reflect a decrease in tumor burden.
The ROC analysis in the present study further revealed that the
multi-biomarker panel outperformed individual markers in the
prediction of short-term therapeutic outcomes, which underscores
their clinical utility for early response assessment.
Safety is a key consideration. In the present study
cohort, AEs were manageable and comparable between groups, which
supports the tolerability of the combination regimen. This
practical implication, that the addition of tislelizumab does not
markedly exacerbate toxicity, makes it a viable option for patients
who can tolerate standard chemotherapy. Despite these potential
synergies, the widespread adoption of tislelizumab and other ICIs
faces challenges. A primary concern is the occurrence of
immune-related AEs (irAEs), which can affect any organ system and
include pneumonitis, colitis, hepatitis and endocrinopathies.
Although often manageable, these irAEs can be severe or even
life-threatening, which requires vigilant monitoring and
specialized management, with fatal outcomes reported particularly
for pneumonitis, hepatitis, colitis and myocarditis (38). Furthermore, a notable portion of
patients exhibit primary or acquired resistance to ICI therapy,
which limits its long-term benefit, with resistance rates varying
markedly between monotherapies (for example, ~30% for PD-1
inhibitors) and combination therapies (for example, 60–70% for
CTLA-4 inhibitors or combinations) (39). The modest predictive power of
biomarkers such as PD-L1 expression indicates that patient
selection remains suboptimal due to the lack of reliable biomarkers
(39,40). These factors, combined with the high
cost of immunotherapy, contribute to the complexities of its
integration into routine clinical practice (39).
The present study had several limitations. Firstly,
the sample size was relatively small, which may have limited the
statistical power to detect significant differences in some
secondary outcomes, such as ORR, DCR and 2-year survival rates, and
may have affected the generalizability of the findings. Secondly,
it was a single-center study, which potentially introduced
selection bias. Thirdly, while the present study incorporated
long-term follow-up for PFS and OS, further follow-up would have
been beneficial to ascertain the durability of the observed
survival benefits. Finally, PD-L1 expression status was not
uniformly assessed or associated with outcomes. Future multicenter
studies with larger sample sizes and comprehensive biomarker
analyses are warranted.
In conclusion, the addition of tislelizumab to GP
chemotherapy markedly improved PFS and exhibited a favorable trend
towards improved OS in advanced NSCLC, with a manageable safety
profile. The combined assessment of serum CEA, CA125 and CYFRA21-1
provides robust predictive value for short-term therapeutic
monitoring. These findings advocate for the consideration of
tislelizumab-based combinations in NSCLC, which offers a more
effective therapeutic option, while the biomarker panel can
potentially aid in early clinical decision-making in the
future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LW conceptualized the study, designed the
methodology and prepared the original draft of the manuscript. TG
performed data acquisition and analysis, and contributed to the
preparation of the original draft. YC performed data analysis and
data visualization. ML provided supervision and performed the
statistical analyses using the appropriate software. YW contributed
to the development of the methodology, and also reviewed and edited
the manuscript. LW and TG confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
relevant Ethics Committee of Fourth Affiliated Hospital of Harbin
Medical University (approval no. HRB-2020-23; Harbin, China). The
study was performed in accordance with The Declaration of Helsinki
and written informed consent was obtained from all of the present
study subjects before enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Riely GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non-small cell lung cancer, version 4.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:249–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang
T, Li W and Xia Y: Management of locally advanced non-small cell
lung cancer: State of the art and future directions. Cancer Commun
(Lond). 44:23–46. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guan H, He Y, Wei Z, Wang J, He L, Mu X
and Peng X: Assessment of induction chemotherapy regimen TPF vs GP
followed by concurrent chemoradiotherapy in locally advanced
nasopharyngeal carcinoma: A retrospective cohort study of 160
patients. Clin Otolaryngol. 45:274–279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dermani FK, Samadi P, Rahmani G, Kohlan AK
and Najafi R: PD-1/PD-L1 immune checkpoint: Potential target for
cancer therapy. J Cell Physiol. 234:1313–1325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang C, McKay RM, Lee SY, Romo CG,
Blakeley JO, Haniffa M, Serra E, Steensma MR, Largaespada D and Le
LQ: Cutaneous neurofibroma heterogeneity: Factors that influence
tumor burden in neurofibromatosis type 1. J Invest Dermatol.
143:1369–1377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abushanab AK, Mustafa MT, Mousa MT,
Qawaqzeh RA, Alqudah GN and Albanawi RF: Efficacy and safety of
tislelizumab for malignant solid tumor: A systematic review and
meta-analysis of phase III randomized trials. Expert Rev Clin
Pharmacol. 16:1153–1161. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu S: Value of Combined Detection of
CYFRA21-1, CA125, CEA, NSE and SCCA Serum in the Diagnosis of Lung
Cancer. Guide China Med. 22:47–50. 2024.(In Chinese).
|
|
10
|
Kobayashi K, Ono Y, Kitano Y, Oba A, Sato
T, Ito H, Mise Y, Shinozaki E, Inoue Y, Yamaguchi K, et al:
Prognostic impact of tumor markers (CEA and CA19-9) on patients
with resectable colorectal liver metastases stratified by tumor
number and size: Potentially valuable biologic markers for
preoperative treatment. Ann Surg Oncol. 30:7338–7347. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang ZH, Han YW, Liang H and Wang LM:
Prognostic value of serum CYFRA21-1 and CEA for non-small-cell lung
cancer. Cancer Med. 4:1633–1638. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen F and Zhang X: Predictive value of
serum SCCA and CYFRA21-1 levels on radiotherapy efficacy and
prognosis in patients with non-small cell lung cancer. Biotechnol
Genet Eng Rev. 40:4205–4214. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radiation Oncology Branch of Chinese
Medical Association, Radiation Oncologist Branch of Chinese Medical
Doctor Association Professional Committee on Radiation Oncology,
China Anti-Cancer Association Experts Committee on Radiation
Oncology and Radiation Oncologist Branch of Chinese Society of
Clinical Oncology, . Clinical practice guideline for radiation
therapy in small cell lung cancer (2020 version). Chin J Radiat
Oncol. 29:608–614. 2020.(In Chinese).
|
|
14
|
Fleming ID: AJCC/TNM cancer staging,
present and future. J Surg Oncol. 77:233–236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chinese Anti-Cancer Association, Lung
Cancer Study Group of Committee of Oncopathology, Chinese Society
of Lung Cancer and Expert Group on PD-L1 Testing Consensus, .
Chinese Expert Consensus on Standards of PD-L1 Immunohistochemistry
Testing for Non-small Cell Lung Cancer. Chin J Lung Cancer.
23:733–740. 2020.(In Chinese).
|
|
18
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE)
version 5.0, 2017. https://dctd.cancer.gov/research/ctep-trials/for-sites/adverse-events/ctcae-v5-5×7.pdfJune
15–2025
|
|
19
|
Spitaleri G, Trillo Aliaga P, Attili I,
Del Signore E, Corvaja C, Corti C, Crimini E, Passaro A and de
Marinis F: Sustained improvement in the management of patients with
non-small-cell lung cancer (NSCLC) harboring ALK translocation:
where are we running? Curr Oncol. 30:5072–5092. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv P and Liu M: Meta-analysis of the
clinical effect of Kanglaite injection-assisted gemcitabine plus
cisplatin regimen on non-small cell lung cancer. Am J Transl Res.
15:2999–3012. 2023.PubMed/NCBI
|
|
21
|
Wakelee H: Chemotherapy and immunotherapy
in early-stage NSCLC: Neoadjuvant vs adjuvant therapy. Clin Adv
Hematol Oncol. 21:648–651. 2023.PubMed/NCBI
|
|
22
|
Olivares-Hernández A, González Del
Portillo E, Tamayo-Velasco Á, Figuero-Pérez L, Zhilina-Zhilina S,
Fonseca-Sánchez E and Miramontes-González JP: Immune checkpoint
inhibitors in non-small cell lung cancer: From current perspectives
to future treatments-a systematic review. Ann Transl Med.
11:3542023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizuno T, Katsuya Y, Sato J, Koyama T,
Shimizu T and Yamamoto N: Emerging PD-1/PD-L1 targeting
immunotherapy in non-small cell lung cancer: Current status and
future perspective in Japan, US, EU, and China. Front Oncol.
12:9259382022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim SM, Hong MH and Kim HR: Immunotherapy
for non-small cell lung cancer: Current landscape and future
perspectives. Immune Netw. 20:e102020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jackson M, Ahmari N, Wu J, Rizvi TA,
Fugate E, Kim MO, Dombi E, Arnhof H, Boehmelt G, Düchs MJ, et al:
Combining SOS1 and MEK inhibitors in a murine model of plexiform
neurofibroma results in tumor shrinkage. J Pharmacol Exp Ther.
385:106–116. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong
W, Zhang Y, Zhou X, Wang Z, Wang Y, et al: The binding of an
anti-PD-1 antibody to FcγRI has a profound impact on its biological
functions. Cancer Immunol Immunother. 67:1079–1090. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kresbach C, Dottermusch M, Eckhardt A,
Ristow I, Paplomatas P, Altendorf L, Wefers AK, Bockmayr M,
Belakhoua S, Tran I, et al: Atypical neurofibromas reveal distinct
epigenetic features with proximity to benign peripheral nerve
sheath tumor entities. Neuro Oncol. 25:1644–1655. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galluzzi L, Buqué A, Kepp O, Zitvogel L
and Kroemer G: Immunogenic cell death in cancer and infectious
disease. Nat Rev Immunol. 17:97–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poon E, Mullins S, Watkins A, Williams GS,
Koopmann JO, Di Genova G, Cumberbatch M, Veldman-Jones M,
Grosskurth SE, Sah V, et al: The MEK inhibitor selumetinib
complements CTLA-4 blockade by reprogramming the tumor immune
microenvironment. J Immunother Cancer. 5:632017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang X, Chen X, Li H and Li Y:
Tislelizumab plus chemotherapy is more cost-effective than
chemotherapy alone as first-line therapy for advanced non-squamous
non-small cell lung cancer. Front Public Health. 11:10099202023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santoro A, Pilar G, Tan DSW, Zugazagoitia
J, Shepherd FA, Bearz A, Barlesi F, Kim TM, Overbeck TR, Felip E,
et al: Spartalizumab in combination with platinum-doublet
chemotherapy with or without canakinumab in patients with
PD-L1-unselected, metastatic NSCLC. BMC Cancer. 24:13072024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Li A, Yu H, Wang Y, Zhang X, Qiu
H, Du W, Luo L, Fu S, Zhang L and Hong S: Neoadjuvant-adjuvant vs
neoadjuvant-only PD-1 and PD-L1 inhibitors for patients with
resectable NSCLC: An indirect meta-analysis. JAMA Netw Open.
7:e2412852024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mathew M, Enzler T, Shu CA and Rizvi NA:
Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther.
186:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Makharadze T, Gogishvili M, Melkadze T,
Baramidze A, Giorgadze D, Penkov K, Laktionov K, Nemsadze G,
Nechaeva M, Rozhkova I, et al: Cemiplimab plus chemotherapy versus
chemotherapy alone in advanced NSCLC: 2-year follow-up from the
phase 3 EMPOWER-lung 3 part 2 trial. J Thorac Oncol. 18:755–768.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu X, Sun L, Song N, He W, Xie B, Hu J,
Zhang J, Yang J, Dai J, Bian D, et al: Safety and effectiveness of
neoadjuvant PD-1 inhibitor (toripalimab) plus chemotherapy in stage
II–III NSCLC (LungMate 002): An open-label, single-arm, phase 2
trial. BMC Med. 20:4932022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Strum S, Vincent M, Gipson M, McArthur E
and Breadner D: Assessment of serum tumor markers CEA, CA-125, and
CA19-9 as adjuncts in non-small cell lung cancer management.
Oncotarget. 15:381–388. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clevers MR, Kastelijn EA, Peters BJM,
Kelder H and Schramel FMNH: Evaluation of serum biomarker CEA and
Ca-125 as immunotherapy response predictors in metastatic non-small
cell lung cancer. Anticancer Res. 41:869–876. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
Toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naing A, Hajjar J, Gulley JL, Atkins MB,
Ciliberto G, Meric-Bernstam F and Hwu P: Strategies for improving
the management of immune-related adverse events. J Immunother
Cancer. 8:e0017542020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Imai H, Kaira K and Kagamu H: Advanced
research on immune checkpoint inhibitor therapy. J Clin Med.
11:53922022. View Article : Google Scholar : PubMed/NCBI
|