Introduction
Bladder cancer is the seventh most commonly
diagnosed cancer in men worldwide and the tenth most common in both
sexes, with ~550,000 new cases each year (1–4). The
most common location of cancer in the urinary tract is the bladder.
Histologically, urothelial carcinoma is the most common type of
bladder cancer. According to the European Association of Urology,
urothelial carcinoma accounts for >90% of all bladder cancer
cases (1). Bladder cancer is a
heterogeneous disease and represents a spectrum of lesions with
varying degrees of malignancy. Urothelial bladder cancer is more
common in developed countries. Additionally, mortality rates are
the highest in certain parts of Europe and North Africa, and lowest
in Asia, Central America and Central Africa (1–3).
Urothelial bladder cancer is known to be linked to several major
risk factors, such as cigarette use, infections with the parasite
Schistosoma haematobium and occupational contact with
chemicals such as aromatic amines and polycyclic aromatic
hydrocarbons (1–3). After radical treatment of bladder
cancer, metastases are diagnosed in up to 50% of patients and are
typically found within the first 2–3 years after surgery. Local and
distant recurrences have a poor prognosis (1,3–7).
Penile metastasis is extremely rare and is typically considered a
late sign of disease dissemination (3–7). The
primary source of penile metastasis is typically the genitourinary
tract, and less commonly the gastrointestinal tract (4–7).
Case report
A 65-year-old man visited the oncology clinic of the
Maria Skłodowska-Curie National Research Institute of Oncology in
Warsaw (Poland) in January 2024 due to periodic bleeding from a
suspicious lesion on the foreskin and glans penis near the external
urethral orifice. The lesion was noticed and observed by the
patient for ~6 months. The patient was chronically treated for type
2 diabetes, osteoarthritis, CNS vascular dementia and irritable
bowel syndrome. The patient was taking two permanent medications:
Betaserc (24 mg once a day_ for the treatment of vertigo and
Metformax (500 mg twice a day) to manage diabetes mellitus.
Historically, the patient had undergone an appendectomy. As for the
family history, it was found that the patient's sister had died of
colon cancer.
The patient had presented to a urology clinic in
Prof. Witold Orłowski Clinical Hospital (Warsaw, Poland) due to
hematuria 15 years earlier. According to the medical history
provided by the patient, from 2019 the patient underwent multiple
transurethral resection of bladder tumor (TURBT) procedures due to
recurrent urothelial non-muscle-invasive bladder cancer. Due to the
patient receiving treatment at another hospital, it is not possible
to determine the exact number of TURBT procedures performed or the
location of the resected lesions during the procedures. Due to
recurrent pTa low-grade bladder cancer, the patient was offered and
subsequently qualified for Bacillus Calmette-Guérin
live-attenuated bacteria intravesical vaccine (so called BCG
therapy), which ultimately failed and resulted in recurrence. After
another TURBT, the histopathological examination revealed pTa
high-grade (HG) bladder cancer, leading to the discontinuation of
infusions. Meanwhile, in 2019 and at the same urology clinic, with
the use of a transrectal biopsy, the patient was diagnosed with
Gleason 6 (3+3) cT1c prostate cancer, according to the Union for
International Cancer Control, Tumor, Node, Metastasis
classification (8). For this
reason, the patient underwent active surveillance. Due to the lack
of response to BCG therapy, the concurrent diagnosis of prostate
cancer and a marked functional impairment of the bladder manifested
by increased lower urinary tract symptoms that originated from poor
tolerance to BCG therapy, the patient qualified for
cystoprostatectomy.
In October 2020, a radical cystoprostatectomy with
urinary diversion using Bricker's method was performed at the
urology center in Prof. Witold Orłowski Clinical Hospital. The
histopathology result following this procedure revealed pTa HG N0
R0 urothelial carcinoma of the bladder and Gleason 7 (3+4) pT2 N0
R0 prostate cancer. The patient did not undergo urethrectomy during
cystoprostatectomy due to the lack of previously diagnosed tumor
in situ. Additionally, the documentation provided by the
patient did not describe the location of the lesions in the bladder
neck and prostatic urethra, which would be an indication for
ureterectomy. During the follow-up after bladder and prostate
removal surgery, no recurrence was found via imaging studies up to
36 months post-surgery. During a visit to an oncologist at the
Maria Skłodowska-Curie National Research Institute of Oncology
(Warsaw, Poland) in October 2023, a computed tomography (CT) of the
chest, abdomen and pelvis was performed, which indicated no signs
of local recurrence or metastatic changes in the abdominal cavity
and pelvis. Hyperdense fatty tissue in the penile region was
observed and it was noted that further diagnosis by magnetic
resonance imaging should be considered. No lymphadenopathy was
found. The patient only provided a description of the examination
and therefore images are not shown here. At the urology visit
scheduled by the oncologist in October 2023 at the Maria
Skłodowska-Curie National Research Institute of Oncology, the
patient was in good general condition, with good circulatory and
respiratory function and no enlarged peripheral lymph nodes. The
urostomy was draining normal urine. Red exophytic lesions suspected
of malignancy were found on the foreskin and anterior surface of
the penile glans. The patient was not diagnosed with phimosis.
There were no enlarged palpable lymph nodes in the inguinal area on
either side.
After a urological consultation at Maria
Skłodowska-Curie National Research Institute of Oncology in
February 2024, due to the macroscopic appearance of the lesions on
the glans penis, the patient qualified for and underwent a partial
penectomy. The surgical specimen was fixed in 10% neutrally
buffered formalin at room temperature (20–30°C) for 24 h. Then, the
representative samples were paraffin-embedded, sliced into 4 µm
thick sections, stained with hematoxylin and eosin (Mar-Four) at
room temperature for 6–10 min and examined using conventional light
microscopy. Immunohistochemistry was performed on formalin-fixed,
paraffin-embedded tissue sections (3 µm). Slides were
deparaffinized in xylene and rehydrated through graded ethanol to
water. Heat-induced epitope retrieval was carried out using
EnVision™ FLEX Target Retrieval Solution on a Dako Omnis or
Autostainer Link 48 platform with PT Link (preheat/cool 65°C,
incubation 20 min at 97°C; Dako; Agilent Technologies, Inc.) or
Cell Conditioning 1 buffer for 64 min at 95°C on a BenchMark ULTRA
automated stainer (Roche Tissue Diagnostics), according to the
manufacturers' protocols. Endogenous peroxidase activity was
blocked with the system-specific peroxidase-blocking reagent, then
ready-to-use or diluted primary antibodies were applied and
incubated at room temperature (list of markers; clones, dilutions
and suppliers are summarized in Table
I). Antibody binding was visualized using a polymer-based
HRP/DAB detection system (EnVision™ FLEX, Dako, Agilent
Technologies, Inc. or UltraView DAB IHC Detection Kit, Roche Tissue
Diagnostics). Slides were counterstained with hematoxylin (~5 min),
dehydrated, cleared and mounted in a permanent, non-aqueous medium.
All immunostained slides were evaluated using conventional light
microscopy. The pathology report demonstrated high-grade urothelial
carcinoma (papillary configuration and architectural disorder and
enlarged pleomorphic oval shaped hyperchromatic nuclei, with
prominent nucleoli and frequent mitotic figures) of the urethra
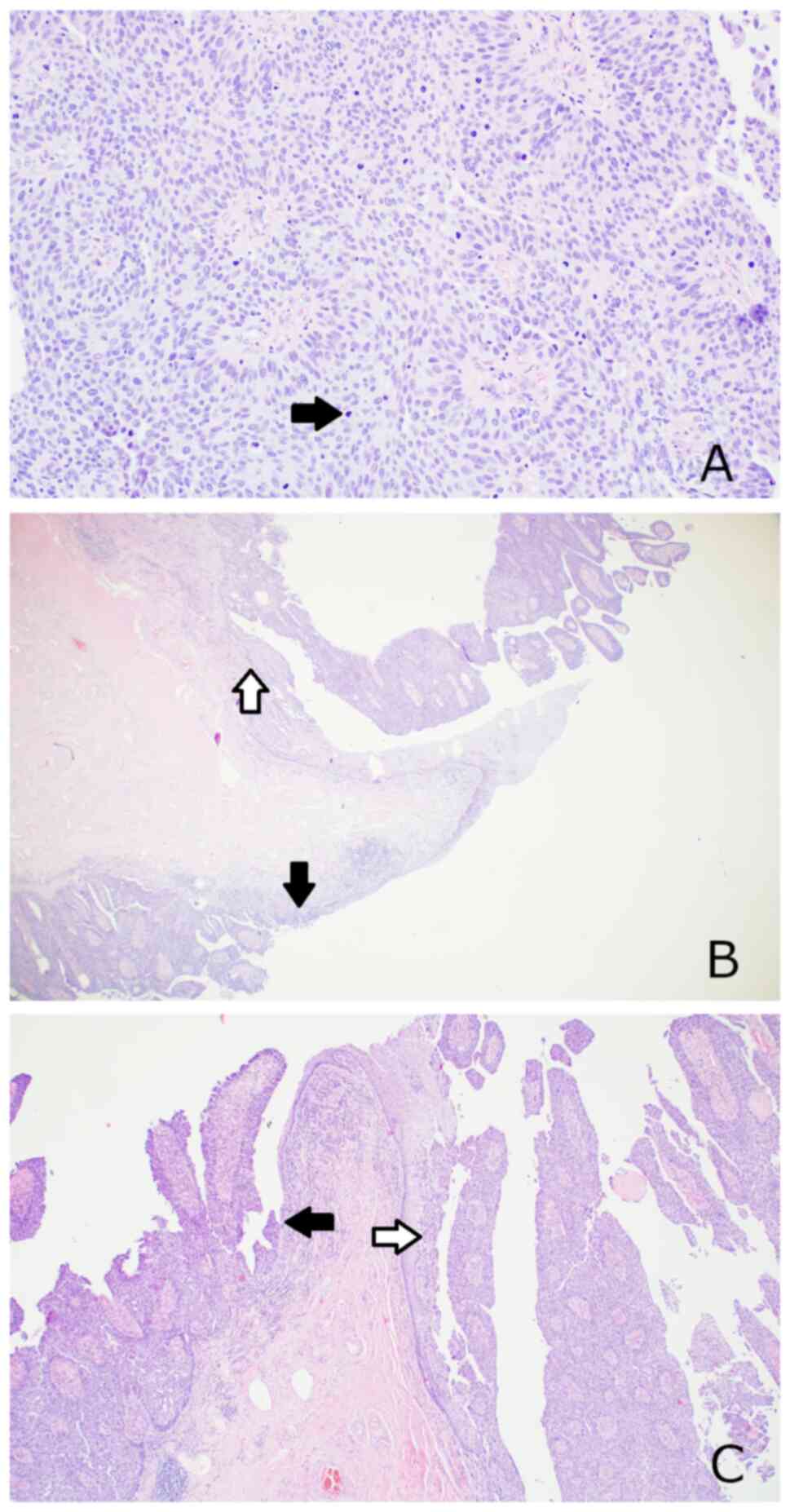
with focal underlying connective tissue invasion (Fig. 1A and B). There was no direct
connection between the squamous epithelium lesion and the urethral
tumor (Fig. 1C). No angioinvasion
or perineural invasion was identified. Immunohistochemically, tumor
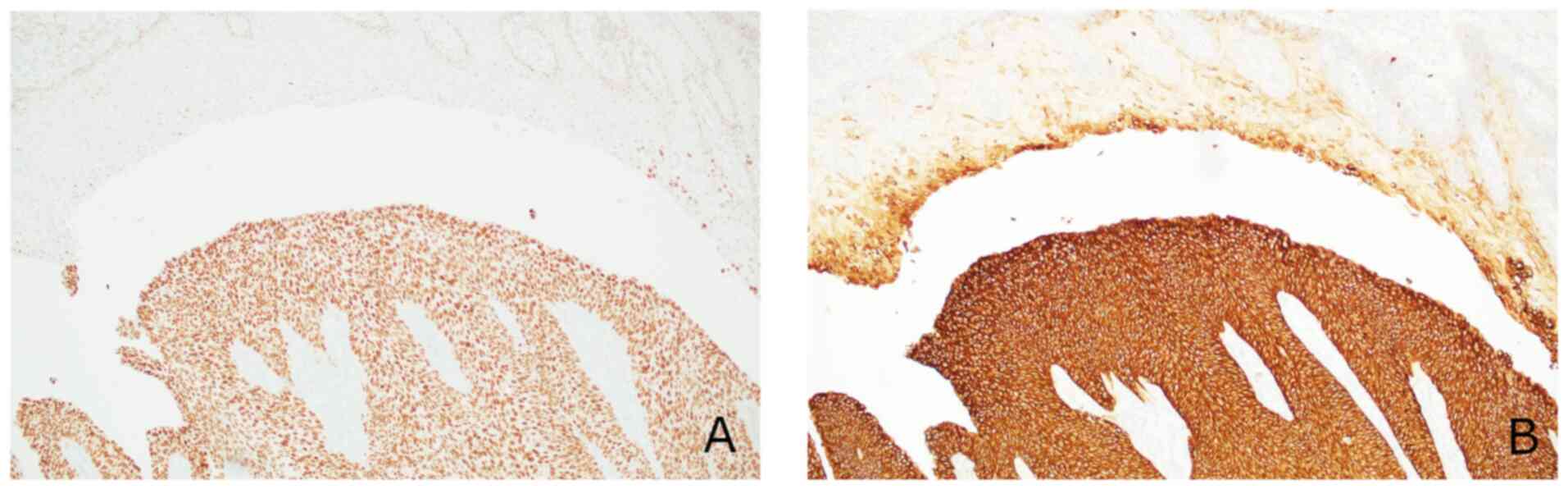
cells showed strong diffuse expression of GATA binding protein 3
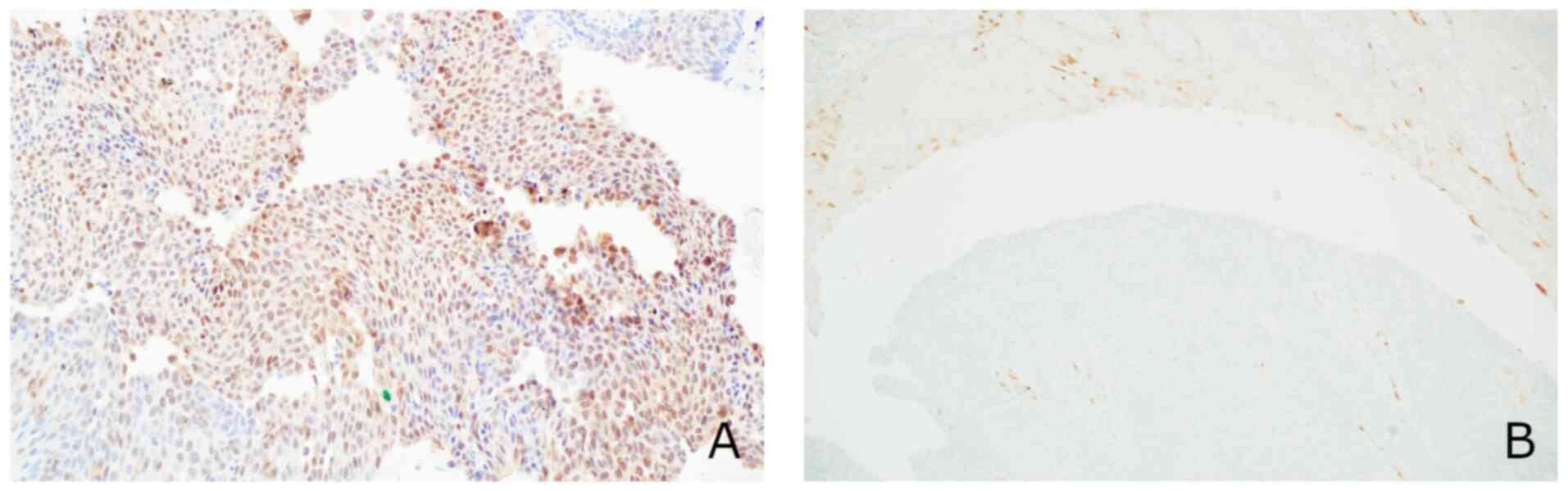
and cytokeratin 7 (Fig. 2), focal
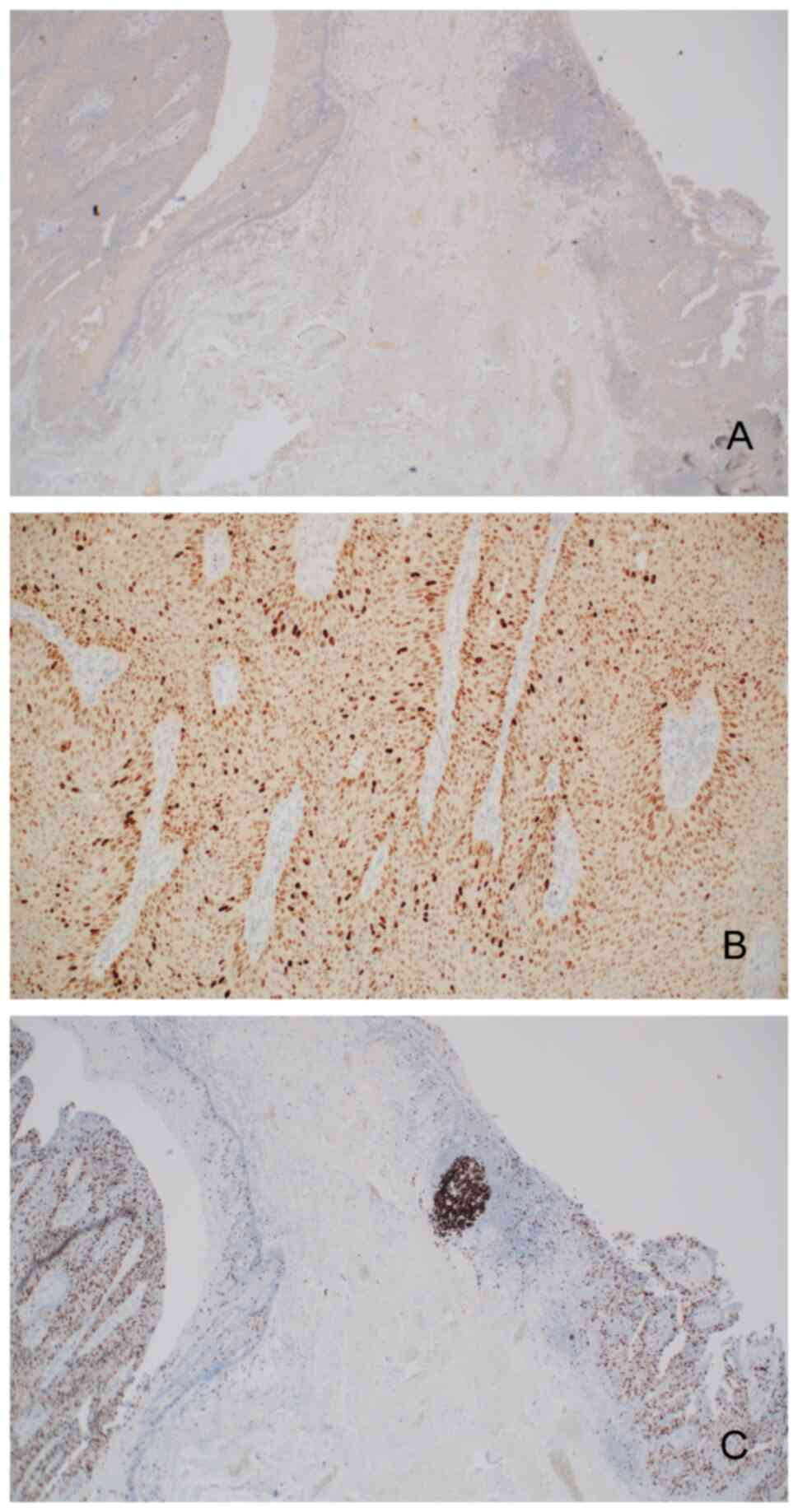
expression of S100 calcium binding protein P (Fig. 3A), no expression of p16 (Fig. 3B) or cytokeratin 20 (Fig. 4A) and upregulation of p53 (Fig. 4B). The proliferation index defined
by the expression of Ki-67 (MIB-1) was high (in ~70% of tumor
cells) (Fig. 4C). Furthermore, a
field of non-invasive carcinoma was found within the glans and
foreskin epithelium, presenting the same morphology and
immunohistochemical profile as the urethral tumor. We consider that
the aforementioned microscopic description should be interpreted as
urothelial carcinoma implantation into the glans and foreskin
epithelium, most likely due to the migration of neoplastic cells
originating from the bladder or urethra tumor via urine.
 | Table I.Details of immunohistochemical
staining. |
Table I.
Details of immunohistochemical
staining.
| Protein | Cat. no. | Clone | Dilution | Supplier | Platform |
|---|
| CK7 | IR619 | OV-TL 12/30 | Ready to use | Dako (Agilent
Technologies, Inc.) | Autostainer |
| GATA3 | 7107749001 | L50-823 | Ready to use | Roche
Diagnostics | Ventana
BenchMark |
| S100P | 06523935001 | 16/f5 | 1:500 | Roche
Diagnostics | Manual |
| Ki-67 | GA626 | MIB-1 | Ready to use | Dako (Agilent
Technologies, Inc.) | Omnis |
| p53 | M7001 | D-07 | Ready to use | Dako (Agilent
Technologies, Inc.) | Omnis |
| p16 | 06695248001 | E6H4 | Ready to use | Roche
Diagnostics | Ventana
BenchMark |
| CK20 | IR777 | Ks20.8 | Ready to use | Dako (Agilent
Technologies, Inc.) | Autostainer |
After receiving the histopathology results, the
patient qualified for urethroscopy and diagnostic dissemination. On
April 10, 2024, the patient underwent a follow-up urethroscopy,
during which the urethra was found to be normal. Therefore, no
specimens were collected for histopathology examination.
Macroscopically, the physical examination also showed no suspicious
changes in the penile stump. CT scans of the chest, abdomen and
pelvis revealed no lesions of recurrent disease or distant
metastases. The patient had three follow-up visits by August 2025
during which no recurrence of the disease was found.
Discussion
Urothelial carcinoma is a major histological subtype
that accounts for nearly 90% of the diagnosed cases of bladder
cancer (1–3). The incidence and prevalence of
urothelial carcinoma increases with age, peaking in the eighth
decade of life (1). Risk factors
for developing urothelial carcinoma include age, sex (men are 3–4
times more likely than women to develop this type of cancer),
smoking (which accounts for at least half of all cases), exposure
to environmental toxins, inflammation and infection of the bladder,
genetic predisposition and exposure to radiation and chemotherapy
(1–4). The main symptom of urothelial
carcinoma is gross hematuria (especially with clots) or hematuria.
Less common symptoms include bladder inflammation, difficulty
urinating or frequent urination. Some urothelial carcinomas are
detected incidentally on imaging studies, such as ultrasound or CT,
performed for unrelated reasons. The prognosis of urothelial
carcinoma varies and depends on a number of factors, such as tumor
size, number of lesions found by cystoscopy, T category in the TNM
staging, tumor grade and coexisting carcinoma in situ
(1). Patients undergoing cystectomy
require long-term monitoring for recurrence. Distant spread of
bladder cancer typically occurs through lymphatic pathways. The
finding of metastasis in the lymph nodes serves as a key element in
establishing the survival prognosis of patients and dictates the
requirement for treatment (1,4). The
basic mechanisms by which metastases can spread to the skin include
direct invasion from an underlying tumor, implantation from a
surgical scar and spreading via the lymphatic system or blood
vessels (1,5–7,9–13).
Distant relapse affects ~50% of patients and is generally detected
within 24 months following operation. The most frequent sites for
these recurrences are the lymph nodes located above the aortic
split, the pulmonary tissue, the hepatic system and the bones
(1,5,9).
The occurrence of metastatic lesions in the penile
region is a very rare phenomenon and is typically associated with
the spread of cancer via the blood vessels. Typically, the first
symptom of penile metastatic lesions is a palpable penile lump or
mass (51%), priapism (27%), lower urinary tract symptoms (27%),
penile pain (17%), urinary retention (13%) and skin changes such as
redness or ulceration on the penis (11%) (5–7,9,14).
Although penile metastasis can be a late sign of cancer progression
and spread, systemic treatment (chemotherapy and immunotherapy) can
improve the prognosis of patients (6,8,9,14).
Other therapeutic options for lesions located on the penis include
conservative treatment, partial or total penectomy, radiation
therapy or systemic treatment. In most cases, due to local
advancement and/or evidence of spread, chemotherapy or palliative
care is the only treatment option for these patients (5,7,9,14).
In cases of uncontrolled local symptoms, such as untreatable pain
or bleeding, palliative surgery (partial or total penectomy) may be
advised (14).
It is possible for urothelial carcinoma cells to
implant along the urinary tract during micturition (10,12);
however, implantation of the tumor into the foreskin and the
epithelium of the glans is extremely rare (5). The uniqueness of the present case lies
not only in this rare localization, but also in the fact that the
patient had undergone cystoprostatectomy and therefore no longer
had a bladder. In such circumstances, any implantation with urine
should be located in the Bricker loop or urostomy. An alternative
hypothesis for the neoplastic changes that occurred in the
patient's urethra and foreskin may be the iatrogenic transfer of
cancer cells to the glans penis via surgical instruments or gloves.
However, we consider this unlikely in the present case due to the
high standards of sterility maintained during the procedures and
the 3-year period from cystoprostatectomy until the tumor
recurrence was confirmed. The lack of CT images and pathology from
the diagnosis and treatment of the patient prior to the occurrence
of metastases to the glans penis may be considered a limitation of
the present case report. However, recurrence of the disease
associated with the transfer of tumor cells within urine therefore
confirms the high malignancy of urothelial carcinoma and the need
for close observation of patients after radical treatment to detect
disease recurrence or dissemination. In terms of the upper urinary
tract, there is a hypothesis that cancer cells located in the upper
sections, such as the renal pelvis or ureter, may nest in the
bladder while not being present in the lower parts of the ureter
(1,4). To the best of our knowledge, there is
no data to suggest that the presence of a tumor in the distal part
of the urethra guarantees the presence of a tumor in the proximal
part of the urethra; however, in each case, the entire urethra
should be examined. In patients who have undergone
cystoprostatectomy or cystectomy with Bricker urinary diversion for
oncological reasons, a physical examination of the penis should not
be overlooked. In cases where the tumor location in the preceding
TURBT was in the bladder neck or near the proximal part of the
urethra, it would be reasonable to consider performing follow-up
urethroscopy for more accurate monitoring of the oncological
disease. This would improve the detection of potential urothelial
cancer recurrences and the patient's quality of life. At present,
increasingly more treatments are available for the disease in the
generalized stage, prolonging the lives of patients and enabling
them to achieve long-term remissions. Although the location of
metastatic lesions in the penis is encountered occasionally, any
occurrence of urethral bleeding should be diagnosed and the penis
should be evaluated to exclude this location of metastatic lesions.
Men with a history of bladder cancer should be aware of the
possibility of penile metastasis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PK wrote the manuscript, collected data, and made
substantial contributions to the conception and design of the
study. PK and TK performed the operation, provided tissues that
could be used as material for the study and were involved in the
acquisition of data. OKS and RR carried out the histopathological
and microscopic examinations of the tissue, acquired data and made
substantial contributions to data interpretation. PK and TD made
significant contributions to the conception and content of the
manuscript. PN and BGN made substantial contributions to the
discussion section, and were responsible for the analysis and
interpretation of the data presented in the case report. TK and TD
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent for
participation in the study. The informed consent procedures were in
compliance with The Declaration of Helsinki.
Patient consent for publication
The patient provided written informed consent for
the publication of any data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van der Heijden AG, Bruins HM, Carrion A,
Cathomas R, Compérat E, Dimitropoulos K, Efstathiou JA, Fietkau R,
Kailavasan M, Lorch A, et al: EAU Guidelines on Muscle-invasive and
Metastatic Bladder Cancer. European Association of Urology
Guidelines on Muscle-invasive and Metastatic Bladder Cancer:
Summary of the 2025 Guidelines. Eur Urol. 87:582–600. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI
|
|
3
|
Chavan S, Bray F, Lortet-Tieulent J,
Goodman M and Jemal A: International variations in bladder cancer
incidence and mortality. Eur Urol. 66:59–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mari A, Campi R, Tellini R, Gandaglia G,
Albisinni S, Abufaraj M, Hatzichristodoulou G, Montorsi F, van
Velthoven R, Carini M, et al: Patterns and predictors of recurrence
after open radical cystectomy for bladder cancer: A comprehensive
review of the literature. World J Urol. 36:157–170. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polanco-Pujol L, Caño-Velasco J,
Moralejo-Garate M, Bataller-Monfort V, Subirá-Ríos D and Hernández
FC: Penile metastasis from urothelial carcinoma: Review of
literature. Arch Esp Urol. 74:208–214. 2021.PubMed/NCBI
|
|
6
|
Yu T, Cui X, Liang N, Wu S and Lin C:
Postoperative metachronous metastasis of bladder cancer to penis: A
case report. Arch Esp Urol. 76:622–626. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sönmez NC, Coşkun B, Arisan S, Güney S and
Dalkılıç A: Early penile metastasis from primary bladder cancer as
the first systemic manifestation: A case report. Cases J.
2:72812009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
John Wiley & Sons Ltd.; Oxford: 2017
|
|
9
|
Guvel S, Kilinc F, Torun D, Egilmez T and
Ozkardes H: Malignant priapism secondary to bladder cancer. J
Androl. 24:499–500. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weeratunga GN: Cutaneous metastatic
implantation of ureteric urothelial carcinoma to the glans and
prepuce. Urol Case Rep. 47:1023392023.PubMed/NCBI
|
|
11
|
Ogiso S, Maeno A, Yamashita M, Souma T,
Nakamura K and Okuno H: Micturitional disturbance due to labial
adhesion as a cause of vaginal implantation of bladder urothelial
carcinoma. Int J Urol. 13:1454–1455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Makino T, Kitagawa Y and Namiki M:
Metastatic urothelial carcinoma of the prepuce and glans penis:
Suspected implantation of non-muscle-invasive bladder cancer via
urine. Case Rep Oncol. 7:509–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyamoto T, Ikehara A, Araki M, Akaeda T
and Mihara M: Cutaneous metastatic carcinoma of the penis:
Suspected metastasis implantation from a bladder tumor. J Urol.
163:15192000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
European Association of Urology (EAU), .
ASCO Guidelines on Penile Cancer. EAU Central Office; Arnhem: 2025,
https://uroweb.org/guidelines/penile-cancerAugust
8–2025
|