|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
3
|
Gridelli C, Maione P, Rossi A, Ferrara ML,
Castaldo V, Palazzolo G and Mazzeo N: Treatment of advanced
non-small-cell lung cancer in the elderly. Lung Cancer. 66:282–286.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen P, Liu Y, Wen Y and Zhou C:
Non-small-cell lung cancerin China. Cancer Commun (Lond).
42:937–970. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saad HM, Tourky GF, Al-Kuraishy HM,
Al-Gareeb AI, Khattab AM, Elmasry SA, Alsayegh AA, Hakami ZH,
Alsulimani A, Sabatier JM, et al: The potential role of MUC16
(CA125) biomarker in lung cancer: A magic biomarker but with
adversity. Diagnostics (Basel). 12:29852022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Five-year outcomes with pembrolizumab versus chemotherapy
for metastatic non-small-cell lung cancer with PD-L1 tumor
proportion score ≥ 50. J Clin Oncol. 39:2339–2349. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hellmann MD, Paz-Ares L, Bernabe Caro R,
Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A,
Lupinacci L, de la Mora Jimenez E, et al: Nivolumab plus ipilimumab
in advanced non-small-cell lung cancer. N Engl J Med.
381:2020–2031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olivares-Hernández A, Posado-Domínguez L,
Redondo-González JC, Corvo-Félix L, Bellido Hernández L,
Fonseca-Sánchez E and Del Barco-Morillo E: Response to
platinum-based therapies in second-line after immunotherapy in
advanced or metastatic non-small-cell lung cancer PD-L1 ≥50. Transl
Lung Cancer Res. 13:2649–2659. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garassino MC, Gadgeel S, Speranza G, Felip
E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled
N, et al: Pembrolizumab plus pemetrexed and platinum in nonsquamous
non-small-cell lung cancer: 5-Year outcomes from the phase 3
KEYNOTE-189 study. J Clin Oncol. 41:1992–1998. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Non-oncogene-addicted metastatic non-small-cell lung cancer:
ESMO clinical practice guideline for diagnosis, treatment and
follow-up. Ann Oncol. 34:358–376. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gadgeel S, Rodríguez-Abreu D, Speranza G,
Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell
SF, et al: Updated analysis from KEYNOTE-189: Pembrolizumab or
placebo plus pemetrexed and platinum for previously untreated
metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol.
38:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma A, Jasrotia S and Kumar A: Effects
of chemotherapy on the immune system: Implications for cancer
treatment and patient outcomes. Naunyn Schmiedebergs Arch
Pharmacol. 397:2551–2566. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiaoxi Q, Jiajin W and Hong W:
Immune-related adverse events induced by ICIs in advanced NSCLC: A
meta-analysis and systematic review. Zhongguo Fei Ai Za Zhi.
23:772–791. 2020.(In Chinese). PubMed/NCBI
|
|
16
|
Huang MY, Jiang XM, Wang BL, Sun Y and Lu
JJ: Combination therapy with ANTI-PD-1/PD-L1 blockade in non-small
cell lung cancer: Strategies and mechanisms. Pharmacol Ther.
219:1076942021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Onoi K, Chihara Y, Uchino J, Shimamoto T,
Morimoto Y, Iwasaku M, Kaneko Y, Yamada T and Takayama K: Immune
checkpoint inhibitors for lung cancer treatment: A review. J Clin
Med. 9:13622020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou
J, Lu Y, Shi Y, Wang Z, Jiang L, et al: Anlotinib as a third-line
therapy in patients with refractory advanced non-small-cell lung
cancer: A multicentre, randomised phase II trial (ALTER0302). Br J
Cancer. 118:654–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
Randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang S, Liang H, Liu Z, Zhao S, Liu J,
Xie Z, Wang W, Zhang Y, Han B, He J and Liang W: The impact of
anlotinib on brain metastases of non-small cell lung cancer: Post
hoc analysis of a phase III Randomized control trial (ALTER0303).
Oncologist. 25:e870–e874. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Lu S, Yu X, Hu Y, Zhao J, Sun M,
Yu Y, Hu C, Yang K, Song Y, et al: Tislelizumab plus chemotherapy
versus chemotherapy alone as first-line treatment for advanced
squamous non-small-cell lung cancer: final analysis of the
randomized, phase III RATIONALE-307 trial. ESMO Open. 9:1037272024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jotte R, Cappuzzo F, Vynnychenko I,
Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, Soo R, Conter HJ,
Kozuki T, Huang KC, et al: Atezolizumab in combination with
carboplatin and nab-paclitaxel in advanced squamous NSCLC
(IMpower131): Results from a randomized phase III trial. J Thorac
Oncol. 15:1351–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugawara S, Tanaka K, Imamura F, Yamamoto
N, Nishio M, Okishio K, Hirashima T, Tanaka H, Fukuhara T, Nakahara
Y, et al: Pembrolizumab plus chemotherapy in Japanese patients with
metastatic squamous non-small-cell lung cancer in KEYNOTE-407.
Cancer Sci. 114:3330–3341. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren S, Chen J, Xu X, Jiang T, Cheng Y,
Chen G, Pan Y, Fang Y, Wang Q, Huang Y, et al: Camrelizumab plus
carboplatin and paclitaxel as first-line treatment for advanced
squamous NSCLC (CameL-Sq): A phase 3 trial. J Thorac Oncol.
17:544–557. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
West H, McCleod M, Hussein M, Morabito A,
Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et
al: Atezolizumab in combination with carboplatin plus
nab-paclitaxel chemotherapy compared with chemotherapy alone as
first-line treatment for metastatic non-squamous non-small-cell
lung cancer (IMpower130): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:924–937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan JJ, Mai HQ, Ng WT, Hu CS, Li JG, Chen
XZ, Chow JCH, Wong E, Lee V, Ma LY, et al: Ninth version of the
AJCC and UICC nasopharyngeal cancer TNM staging classification.
JAMA Oncol. 10:1627–1635. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salas-Benito D, Pérez-Gracia JL,
Ponz-Sarvisé M, Rodriguez-Ruiz ME, Martínez-Forero I, Castañón E,
López-Picazo JM, Sanmamed MF and Melero I: Paradigmson immune
therapy combinations with chemotherap. Cancer Discov. 11:1353–1367.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Larroquette M, Domblides C, Lefort F,
Lasserre M, Quivy A, Sionneau B, Bertolaso P, Gross-Goupil M,
Ravaud A and Daste A: Combining immune check point inhibitors with
chemotherapy in advanced solid tumours: A review. Eur J Cancer.
158:47–62. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Butterfield LH and Najjar YG:
Immunotherapy combination approaches:Mechanisms, biomarkers and
clinical observations. Nat Rev Immunol. 24:399–416. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adus MR, Natividad J, Mai A, Ouyang Y,
Lambrecht N, Szabo S, Ge L, Hoa N and Dacosta-Iyer MG: Lung cancer:
A classic example of tumor escape and progression while providing
opportunities for immunological intervention. Clin Dev Immunol.
2012.1607242012.PubMed/NCBI
|
|
32
|
Lu S, Zhang W, Wu L, Wang W, Zhang P;
Neotorch Investigators, ; Fang W, Xing W, Chen Q, Yang L, et al:
Perioperative toripalimab plus chemotherapy for patients with
resectable non-small cell lung cancer: The neotorch randomized
clinical trial. JAMA. 331:201–211. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Z, Hackshaw A, Feng Q, Fu X, Zhang Y,
Mao C and Tang J: Comparison of gefitinib, erlotinib and afatinib
in non-small cell lung cancer: A meta-analysis. Int J Cancer.
140:2805–2819. 2017. View Article : Google Scholar : PubMed/NCBI
|















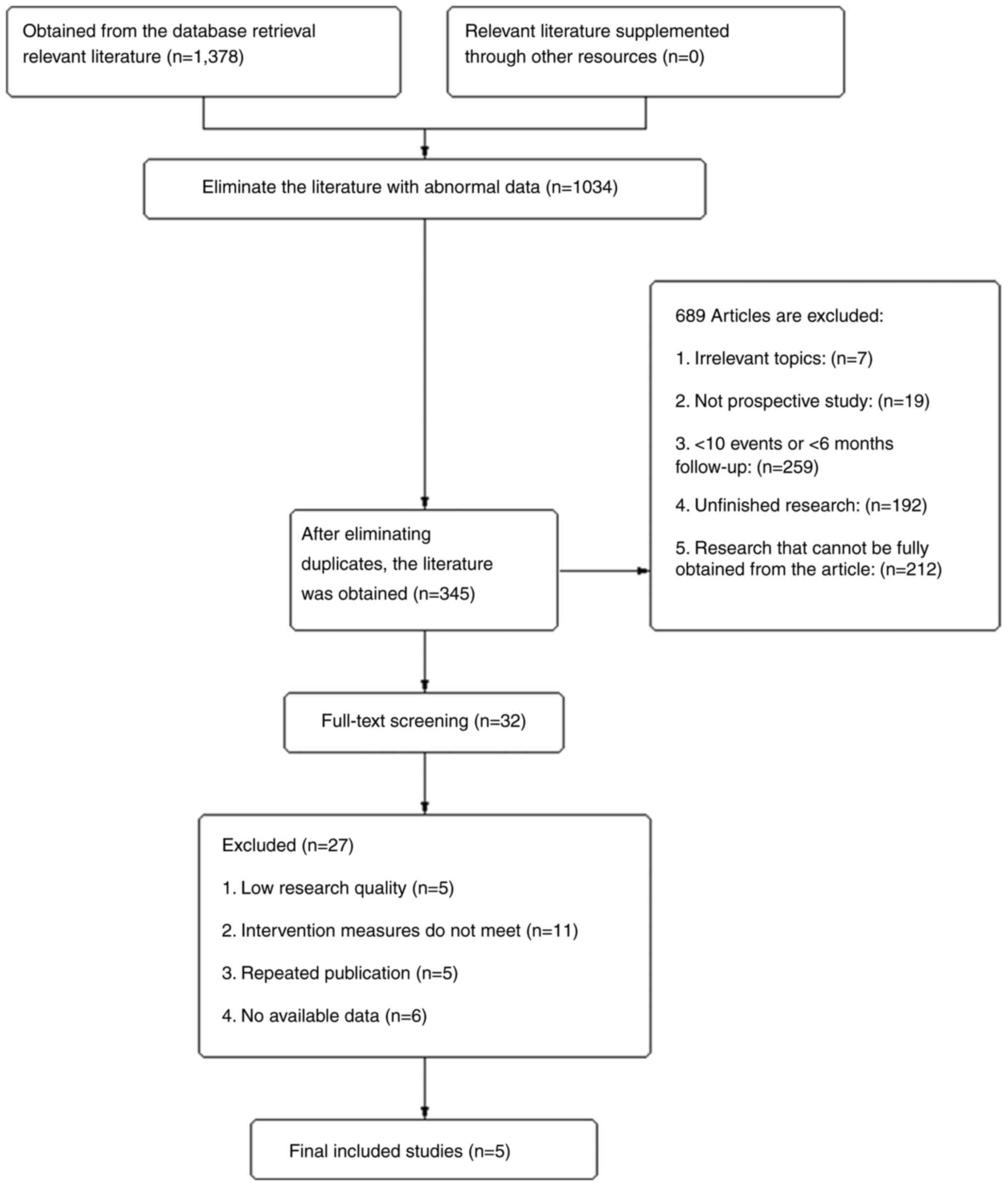
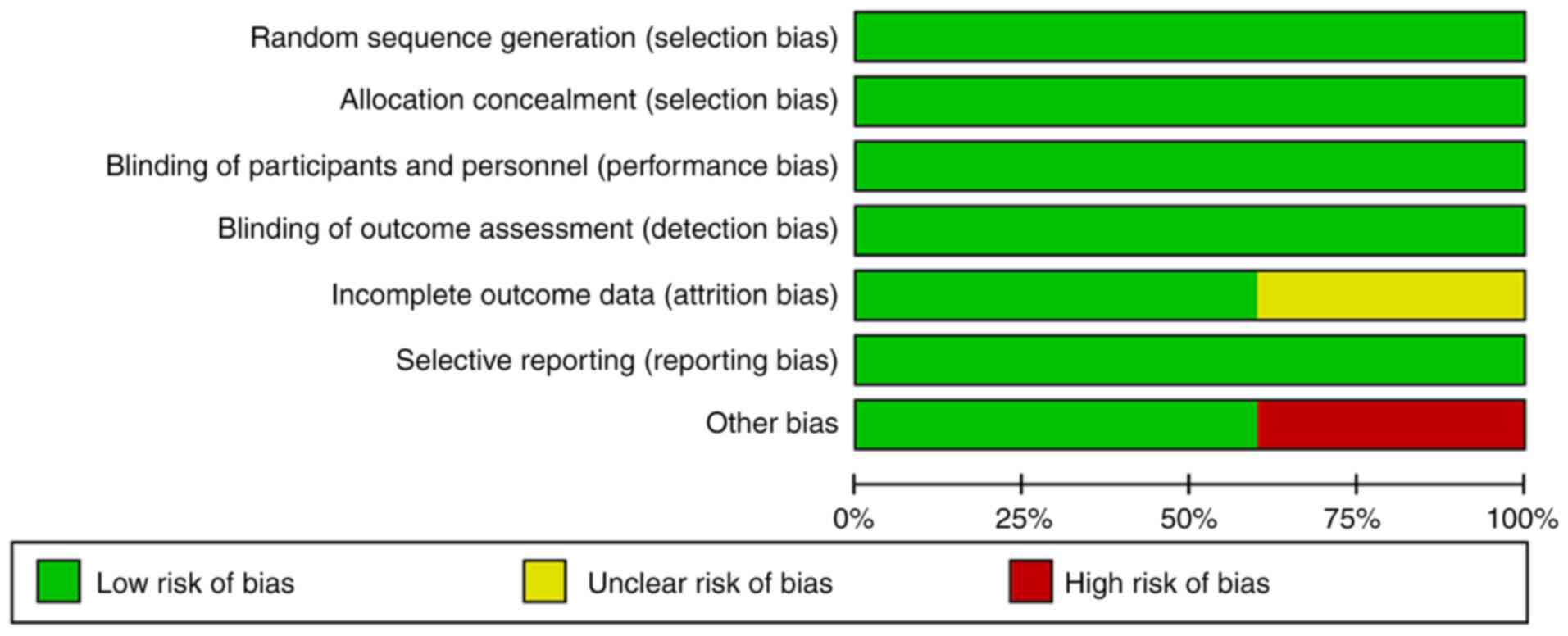
![Forest plot of the median overall
survival rate of patients with non-small cell lung cancer. There
was no statistical heterogeneity among the studies (P=0.07,
I2=49). A random-effects model was used for
meta-analysis; overall survival of the T group was significantly
higher than that of the C group [OR=20.43, 95%CI (18.81, 22.19),
P<0.001]. C, control; T, test;-HR, Hazard Ratio; df, degrees of
freedom; IV, Information Value; CI, confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g02.jpg)
![Forest plots of the median
asymptomatic survival of patients with non-small cell lung cancer.
There was no statistical heterogeneity among the studies (P=0.14,
I2=38). A random-effects model was used for
meta-analysis; asymptomatic survival of the T group was
significantly higher than that of the C group [OR=7.24, 95%CI
(6.93, 7.78), P<0.001]. C, control group; T, the test group; HR,
Hazard Ratio; df, degrees of freedom; IV, Information Value; CI,
confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g03.jpg)
![Forest plots of the occurrence of
adverse reactions in patients with non-small cell lung cancer.
There was no statistical heterogeneity among the studies
(P<0.00001, I2=84). A random-effects model was used
for meta-analysis; incidence of adverse events in the T group was
significantly lower than that in the C group [OR=2.47, 95%CI (1.16,
5.25), P=0.02]. C, control; T, test; OR, odds ratio; df, degrees of
freedom; IV, Information Value; CI, confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g04.jpg)