Introduction
Lung cancer is the most common malignant tumor
worldwide (1). In 2022, there were
2.48 million new cases of lung cancer worldwide (accounting for
12.4% of all new cancer cases) and 1.81 million deaths (accounting
for 18.7% of all cancer deaths in 2022), both ranking first among
all malignant tumors (2). Lung
cancer is classified into small cell and non-small cell lung cancer
(NSCLC) on the basis of pathology, and NSCLC accounts for 80–85% of
all lung cancer cases (3). The
primary histological subtypes of NSCLC include adenocarcinoma,
squamous cell carcinoma, adenosquamous carcinoma, large cell
carcinoma and not otherwise specified NSCLC (4). Patients with early-stage lung cancer
usually have no symptoms. Most confirmed cases cannot be resected
or have metastasized and the 5-year survival period of these
patients is short (5).
Platinum-based drug therapy is currently the main
treatment option for the first-line treatment of NSCLC. Treatment
with monotherapy drugs may lead to drug resistance (6). At present, the first-line treatment
for NSCLC is immunotherapy combined with chemotherapy for tumors
with PD-L1 expression <50%. For tumors with PD-L1 ≥50%,
immunotherapy is administered. Studies (7–9) have
shown that after first-line immunotherapy, patients with advanced
NSCLC and high PD-L1 expression (PD-L1 ≥50%) treated with
platinum-based drugs as second-line therapy experience improved
overall survival (OS), progression-free survival (PFS) and
objective response Rate (ORR). To the best of our knowledge,
however, the effect of chemotherapy and immunotherapy in these
patients has not been analyzed. Therefore, the present study aimed
to analyze the use of chemotherapy combined with immunotherapy in
patients with advanced NSCLC harboring tumors with high, medium and
low PD-L1 expression (10).
A number of clinical studies have confirmed that
third-generation chemotherapy drugs paclitaxel, pemetrexed,
gemcitabine and vincristine combined with platinum are effective
treatment regimens for NSCLC (11,12).
The paclitaxel + carboplatin regimen is one of the most widely used
regimens in clinical practice (13).
The immune system is the primary defense mechanism
in humans against pathogens and abnormal cells, and T cells serve a
key role in immune surveillance and attacking tumor cells (14). However, tumor cells often evade the
surveillance of the immune system through various mechanisms (such
as defects in antigen presentation function.), and thus
proliferate. Immune checkpoints are key regulatory factors for
maintaining immune tolerance and regulating immune responses. Tumor
cells use these checkpoints to inhibit the activity of T cells,
thereby evading immune attack. Immune checkpoint inhibitors (ICIs)
restore the immune surveillance function and enhance recognition
and attack of tumor cells, thereby improving the prognosis of
patients (15). ICIs relieve the
immunosuppression of T cells and restore their anti-tumor ability
by blocking these inhibitory signals. The programmed death receptor
1 (PD-1)/PD-L1 pathway is an important mechanism for tumor cells to
escape immunity (16). PD-1 is one
of the key targets of ICIs (17).
To explore whether the addition of
immunosuppressants in platinum-based regimens has a more obvious
effect compared with immunosuppressants alone, the present study
aimed to analyze studies that examined paclitaxel + platinum-based
treatment regimens with and without immunosuppressants for patients
with NSCLC in terms of patient survival, asymptomatic survival and
adverse reactions.
Materials and methods
Literature retrieval
Randomized controlled trials (RCTs) on the clinical
efficacy of paclitaxel + carboplatin with and without
immunosuppressants in patients with NSCLC were searched in PubMed
(pubmed.ncbi.nlm.nih.gov/), The Cochrane
Library (cochranelibrary.com/), Embase
(embase.com), China National Knowledge Infrastructure
(cnki.net/), Wanfang Data (wanfangdata.com.cn/), VIP (cqvip.com/)
and China Biology Medicine (sinomed.ac.cn) from the establishment
of the databases to May 2025. The search method used subject words
+ free words, including Chinese key words ‘immunosuppressant,
paclitaxel, carboplatin, non-small cell lung cancer, lung’ and the
English search keywords ‘paclitaxel, carboplatin, non-small cell
lung cancer, atezolizumab, pembrolizumab’. Literature retrieval was
conducted independently by two people.
A total of two researchers screened the selected
literature by reading the full text and then cross-checked the
results. Inclusion criteria were as follows: i) Published RCTs; ii)
patients with NSCLC confirmed by pathology or cytology; iii)
pathological stage III to V; and iv) treatment measure for the
experimental group was paclitaxel + platinum + anti-PD-1/L1 and the
treatment for the control group was paclitaxel + platinum. For
publications containing overlapping data, the most comprehensive
report was included. Exclusion criteria were as follows: i)
County-level research centers; ii) animal experiments; iii) studies
with missing or incomplete data; and iv) ongoing clinical trials.
Outcome indicators included OS and PFS. The safety indicator was
the incidence of side effects. PFS is defined as from the start of
treatment to the onset of disease progression or death), and OS was
defined as the time from the initiation of chemotherapy to the
patient death. Certain studies contained three groups as follows:
PD-1/PD-L1 + NAB-paclitaxel (Group ICICa) and carboplatin (Group
ICICb); Or paclitaxel plus carboplatin (Group Che). We conducted a
secondary group comparison of the research, namely:PD-1/L1 combined
with paclitaxel and carboplatin (Group ICICa) vs. paclitaxel and
carboplatin (Group Che).PD-1/L1 combined with NAB-paclitaxel and
carboplatin (Group ICICb) VS paclitaxel and carboplatin (group
Che).
A total of two researchers independently screened
the literature and extracted the data, including: i) First author
and country; ii) patient and tumor characteristics, including age,
pathological classification, tumor stage and treatment methods;
iii) outcome/safety indicators; and iv) evaluation indicators of
risk of bias.
Risk of bias assessment
RevMan 5.4 (Revman International Inc.) was used for
risk of bias assessment. The assessment included the following: i)
Random allocation method; ii) allocation sequence concealment; iii)
blinding method; iv) integrity of outcome data; v) selective
outcome reporting; and vi) other sources of bias.
Statistical analysis
Data were analyzed using RevMan5.4 software.
Heterogeneity was evaluated by the Q test, with P<0.1 or
I2≥50% indicating heterogeneity, and the random-effects
model was used; when heterogeneity was absent, the fixed-effect
model was used (18–20). OS, PFS and adverse reactions were
expressed using hazard ratio (HR) or the relative odds ratio (OR).
HR and OR >1 indicated that the effect size of the experimental
group was larger than that of the control group. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Literature screening
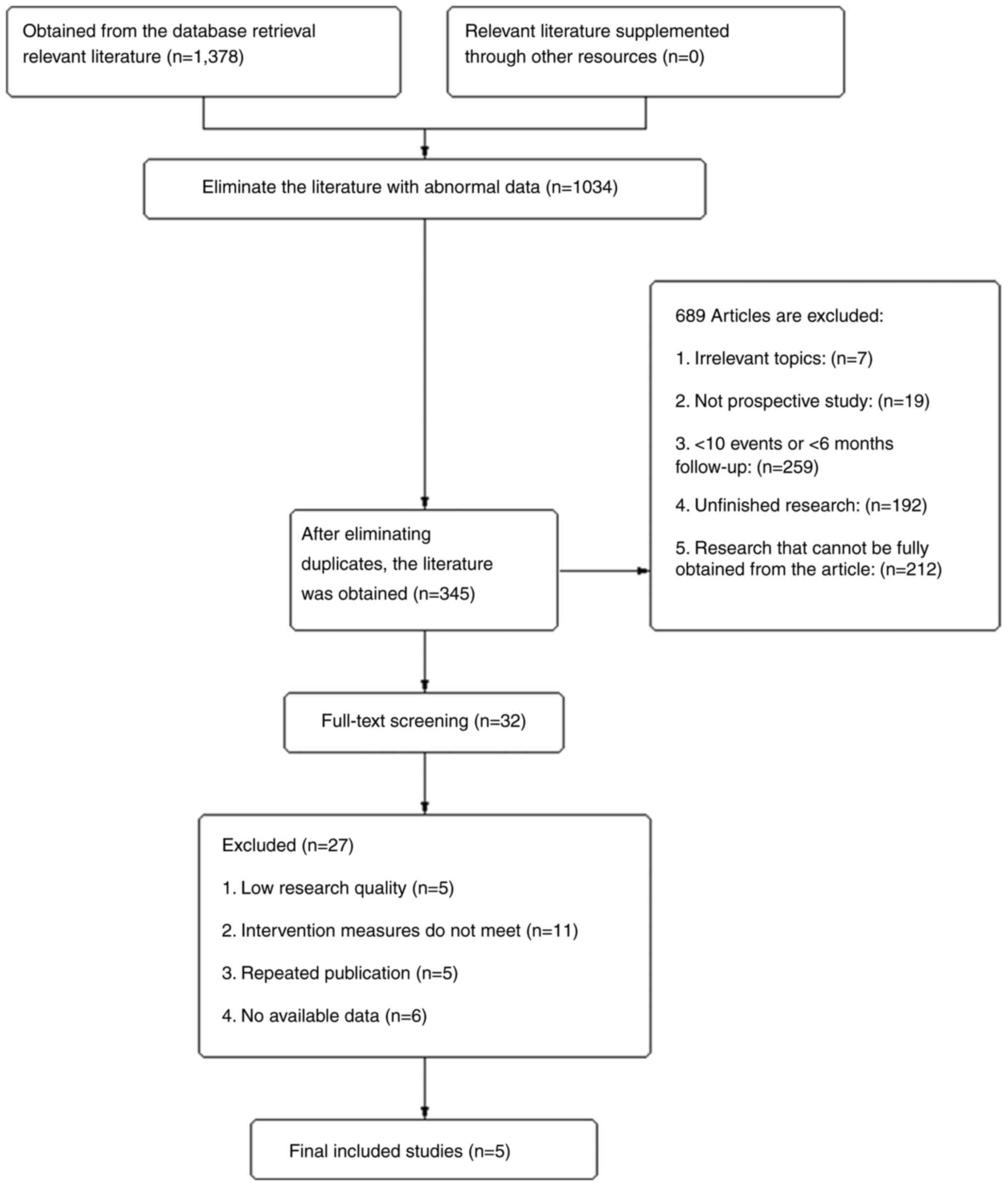
A total of 1,378 studies were obtained by searching
the databases (Fig. 1). Duplicate
studies were removed and irrelevant literature was screened and
removed after reading the titles and abstracts. After reading the
full text, five clinical studies were included, all of which were
RCTs (21–25). Of 3,683 included patients, there
were 2,069 patients in the test (T) group and 1,614 patients in the
control (C) group (Table I). There
were 1,447 (69.93%) male patients in the T group and 1,141 (70.69%)
in the C group. The mean age range of patients in the T group was
60.0–69.5 years and that in the C group was 62–69 years. A total of
1,593 patients (76.99%) in the T group and 1,302 (81.78%) in the C
group had a smoking history. There were 718 patients with an ECOG
score of 0 in the T group (34%).
 | Table I.Randomized control trials included in
meta-analysis. |
Table I.
Randomized control trials included in
meta-analysis.
|
|
| Number of cases | Median age, years
(range) | Male/female (%) | ECOG | History of smoking
(%) |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | Country | T | C | T | C | T | C | T (%) | C (%) | T | C | (Refs.) |
|---|
| Wang et al,
2024 | China (ICICa-Che-C
Group) | 120.0 | 121.0 | 60.0 (41.0–74.0) | 62.0 (34.0–74.0) | 107.0 (89.2) | 111.0 (91.7) | 31.0 (25.8) | 32.0 (26.4) | 96.0 (80.0) | 98.0 (81.0) | (21) |
|
| China (ICICb-Che
Group) | 119.0 | 121.0 | 63.0 (38.0–74.0) | 62.0 (34.0–74.0) | 112.0 (94.1) | 111.0 (91.7) | 22.0 (18.5) | 32.0 (26.4) | 107.0 (89.9) | 98 (81.0) |
|
| Jotte et al,
2020 | USA (ICICa-Che
Group) | 338.0 | 340.0 | 66.0
(43.0–85.0) | 65.0
(38.0–86.0) | 278.0 (82.2) | 277.0 (81.5) | 109.0 (32.2) | 110.0 (32.4) | 308.0 (91.1) | 316.0 (92.9) | (22) |
|
| USA (ICICb-Che
Group) | 343.0 | 340.0 | 65.0
(23.0–83.0) | 65.0
(38.0–86.0) | 280.0 (81.6) | 277.0 (81.5) | 115.0 (33.5) | 110.0 (32.4) | 311.0 (90.7) | 316.0 (92.9) |
|
| Sugawara et
al, 2023 | Japan | 22.0 | 28.0 | 69.5
(45.0–87.0) | 69.0
(43.0–82.0) | 19.0 (86) | 24.0 (86) | 10.0 (45) | 13.0 (46.0) | 21.0 (95.0) | 26.0 (93.0) | (23) |
| Ren et al,
2022 | China | 193.0 | 196.0 | 64.0
(34.0–74.0) | 62.0
(34.0–74.0) | 179.0 (93.0) | 180.0 (92.0) | 38.0 (20.0) | 43.0 (22.0) | 171.0 (89.0) | 173.0 (88.0) | (24) |
| West et al,
2019 | USA (ICICa-Che
Group) | 483.0 | 240.0 | 64.0
(18.0–86.0) | 65.0
(38.0–85.0) | 206.0 (43.0) | 138.0 (58.0) | 204.0 (42.0) | 93.0 (39.0) | 160.0 (33.0) | 73.0 (30.0) | (25) |
|
| USA (ICICb-Che
Group) | 451.0 | 228.0 | 64.0
(18.0–86.0) | 65.0
(38.0–85.0) | 266.0 (59.0) | 134.0 (59.0) | 189.0 (42.0) | 91.0 (40.0) | 419.0 (87.0) | 220.0 (92.0) |
|
Basic characteristics of the
patients
Tumor tissue in the T group showed high-medium PD-L1
expression in 464 cases (22.43%), low PD-L1 expression in 685 cases
(33.11%), and negative or unknown PD-L1 expression in 920 cases
(44.47%; Table II). In the C
group, there were 370 cases (22.992%) with high-medium PD-L1
expression, 556 (34.45%) with low PD-L1 expression and 688 cases
(42.63%) with negative or unknown PD-L1 expression. In the T group,
there were 132 cases (6.38%) of stage IIIb (According to the INM
classification method for malignant tumors) (26) and 1,937 cases (93.62%) of stage IV
cancer, while in the C group, there were 143 cases (8.86%) of stage
IIIb and 1,471 cases (91.14%) of stage IV cancer.
 | Table II.Expression of PD1/L1 and tumor
staging. |
Table II.
Expression of PD1/L1 and tumor
staging.
|
|
| T PD-1/L1
expression (%) | C PD-1/L1
expression (%) | Stage IIIb (%) | Stage IV (%) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | Country | High-medium | Low |
Negative/unknown | High-medium | Low |
Negative/unknown | T | C | T | C | (Refs.) |
|---|
| Wang et al,
2024 | China (ICICa-Che
Group) | 42.0 (35.0) | 42.0 (35.0) | 47.0 (39.2) | 41.0 (33.9) | 31.0 (25.6) | 49.0 (37.2) | 3.08.0 (31.7) | 44.0 (36.4) | 82.0 (68.3) | 77.0 (63.6) | (21) |
|
| China (ICICa-Che
Group) | 42.0 (35.3) | 30.0 (25.2) | 46.0 (38.7) | 41.0 (33.9) | 31.0 (25.6) | 49.0 (37.2) | 40.0 (33.6) | 44.0 (36.4) | 79.0 (66.4) | 77.0 (63.6) |
|
| Jotte et al,
2020 | USA (ICICa-Che
Group) | 48.0 (14.2) | 110.0 (35.5) | 170.0 (50.3) | 44.0 (12.9) | 125.0 (36.8) | 171.0 (50.3) | NA | NA | 338.0 (100) | 340.0 (100) | (22) |
|
| USA (ICICb-Che
Group) | 47.0 (13.7) | 136.0 (39.7) | 160.0 (46.6) | 44.0 (12.9) | 125.0 (36.8) | 171.0 (50.3) | NA | NA | 343.0 (100) | 340.0 (100) |
|
| Sugawara et
al, 2023 | Japan | 13.0
(59.0) | 9.0 (41.0) | 2.0 (9.0) | 22
(78.6) | 6.0 (21.0) | 0.00 | NA | NA | 22.0 (100.0) | 28.0 (100.0) | (23) |
| Ren et al,
2022 | China | 95
(68.3) | 91.0 (47.0) | 7 (4) | 93
(66.0) | 97.0 (49.0) | 6.0 (3.0) | 54.0 (28.0) | 55.0 (28.0) | 139.0 (72.0) | 141.0 (72.0) | (24) |
| West et al,
2019 | USA (ICICa-Che
Group) | 91.0 (19.0) | 139.0 (29.0) | 253.0 (52.0) | 43.0 (18.0) | 68.0 (28.0) | 129.0 (54.0) | NA | NA | 483.0 (100.0) | 240.0 (100.0) | (25) |
|
| USA (ICICb-Che
Group) | 88.0 (20.0) | 128.0 (28.0) | 235.0 (52.0) | 42.0 (18.0) | 65.0 (29.0) | 121.0 (53.0) | NA | NA | 451.0 (100.0) | 228.0 (100.0) |
|
Bias risk assessment
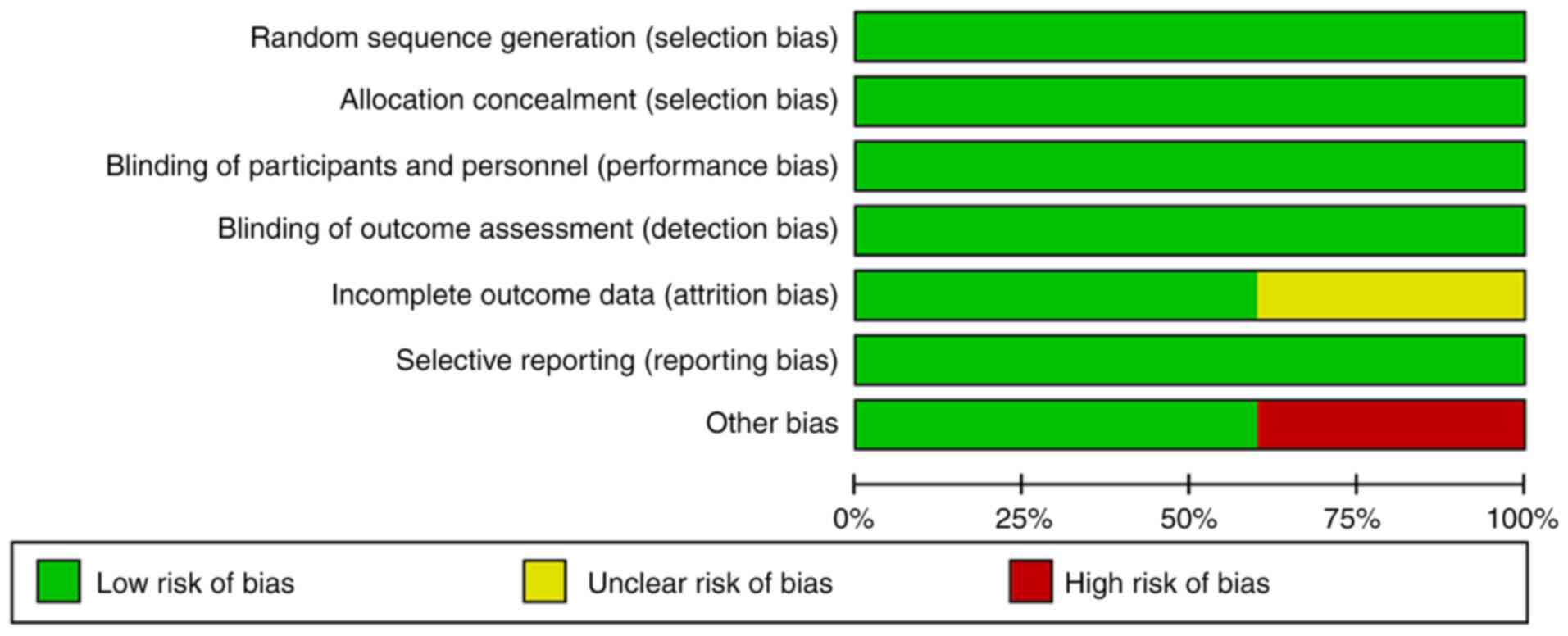
Publication bias assessment was not performed as
<10 studies were included (27).
As all the studies used centralized randomization allocation
schemes for RCTs and blinding of both researchers and participants,
selection bias and implementation bias were judged as low-risk
(Fig. 2). All studies reported the
methods of randomization. The outcome data studies were complete,
and it was not clear whether there were other sources of bias. One
cohort study (24) did not report
the number of occurrences of specific side effects in the follow-up
population, indicating a certain degree of bias. One study
(23) had a small number of
participants, poor representativeness and selection bias.
Meta-analysis results
Median OS and PFS
There was statistical heterogeneity between these
studies. (P=0.07, I2=49; Fig. 3). A random-effects model was used
for meta-analysis; OS of the T group was significantly higher than
that of the C group [OR=20.43, 95% confidence interval (CI) (18.81,
22.19), P<0.001]. There was no statistical heterogeneity among
studies on PFS. (P=0.14, I2=38; Fig. 4). A random-effects model was used
for meta-analysis; PFS of the T group was significantly higher than
that of the C group [HR=7.24, 95% CI (6.93, 7.78), P<0.001].
![Forest plot of the median overall
survival rate of patients with non-small cell lung cancer. There
was no statistical heterogeneity among the studies (P=0.07,
I2=49). A random-effects model was used for
meta-analysis; overall survival of the T group was significantly
higher than that of the C group [OR=20.43, 95%CI (18.81, 22.19),
P<0.001]. C, control; T, test;-HR, Hazard Ratio; df, degrees of
freedom; IV, Information Value; CI, confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g02.jpg) | Figure 3.Forest plot of the median overall
survival rate of patients with non-small cell lung cancer. There
was no statistical heterogeneity among the studies (P=0.07,
I2=49). A random-effects model was used for
meta-analysis; overall survival of the T group was significantly
higher than that of the C group [OR=20.43, 95%CI (18.81, 22.19),
P<0.001]. C, control; T, test;-HR, Hazard Ratio; df, degrees of
freedom; IV, Information Value; CI, confidence interval. |
![Forest plots of the median
asymptomatic survival of patients with non-small cell lung cancer.
There was no statistical heterogeneity among the studies (P=0.14,
I2=38). A random-effects model was used for
meta-analysis; asymptomatic survival of the T group was
significantly higher than that of the C group [OR=7.24, 95%CI
(6.93, 7.78), P<0.001]. C, control group; T, the test group; HR,
Hazard Ratio; df, degrees of freedom; IV, Information Value; CI,
confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g03.jpg) | Figure 4.Forest plots of the median
asymptomatic survival of patients with non-small cell lung cancer.
There was no statistical heterogeneity among the studies (P=0.14,
I2=38). A random-effects model was used for
meta-analysis; asymptomatic survival of the T group was
significantly higher than that of the C group [OR=7.24, 95%CI
(6.93, 7.78), P<0.001]. C, control group; T, the test group; HR,
Hazard Ratio; df, degrees of freedom; IV, Information Value; CI,
confidence interval. |
Safety indicators
The safety assessment of patient medication mainly
included the evaluation of the incidence of adverse events. There
was no statistical heterogeneity among the studies (P<0.00001,
I2=84; Fig. 5). A
random-effects model was used for meta-analysis; incidence of
adverse events in the T group was significantly lower than that in
the C group [OR=2.47, 95% CI (1.16, 5.25), P=0.02].
![Forest plots of the occurrence of
adverse reactions in patients with non-small cell lung cancer.
There was no statistical heterogeneity among the studies
(P<0.00001, I2=84). A random-effects model was used
for meta-analysis; incidence of adverse events in the T group was
significantly lower than that in the C group [OR=2.47, 95%CI (1.16,
5.25), P=0.02]. C, control; T, test; OR, odds ratio; df, degrees of
freedom; IV, Information Value; CI, confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g04.jpg) | Figure 5.Forest plots of the occurrence of
adverse reactions in patients with non-small cell lung cancer.
There was no statistical heterogeneity among the studies
(P<0.00001, I2=84). A random-effects model was used
for meta-analysis; incidence of adverse events in the T group was
significantly lower than that in the C group [OR=2.47, 95%CI (1.16,
5.25), P=0.02]. C, control; T, test; OR, odds ratio; df, degrees of
freedom; IV, Information Value; CI, confidence interval. |
Discussion
The meta-analysis showed that in terms of
controlling the progression of NSCLC, the anti-PD-1/L1 +
paclitaxel/albumin-paclitaxel + carboplatin treatment regimen was
significantly superior to the paclitaxel/albumin-paclitaxel +
carboplatin treatment regimen. Several reasons may explain the
improved efficacy of anti-PD-1/L1 + paclitaxel/albumin-paclitaxel +
carboplatin treatment. The regime enhances the anti-tumor immune
response: Chemotherapy induces immunogenic cell death, releasing
tumor antigens, while immunotherapy eliminates the inhibition of T
cells, enabling the immune system to recognize and attack tumor
cells, forming a cycle of antigen release-immune activation
(28). The regime overcomes
chemotherapy resistance: Certain tumor cells may develop resistance
through mutations or other mechanisms during chemotherapy. However,
immunotherapy does not rely on chemotherapy drugs that directly
kill tumor cells, but activates immune pathways, such as T cells,
to attack drug-resistant cells and overcome chemotherapy resistance
(29). The regime reduces the risk
of recurrence and metastasis: Chemotherapy combined with
immunotherapy not only enhances the efficacy of the initial
treatment but also decreases the risk of tumor recurrence and
metastasis by establishing a lasting immune memory. Especially in
cases where minimal residual lesions are difficult to eliminate
through chemotherapy, immunotherapy provides long-term protection
(30).
The present study analyzed OS, PFS and the incidence
of adverse events, and verified the hypothesis that the combination
of chemotherapy and immunotherapy alters the antigen release-immune
activation cycle. Chemotherapy creates the prerequisite conditions
for immunotherapy to take effect, and immunotherapy converts the
short-term killing effect of chemotherapy into long-term, and
potentially curative immune memory. Chemoimmunotherapy improved the
initial treatment effect and decreased the risk of tumor recurrence
and metastasis (22). Since both
the OS and PFS data are medians with significant differences, and
there are certain errors in the meta-analysis of survival data
using the original data rows obtained from the literature, a random
mode was adopted for comparative analysis. The median PFS in terms
of Teff high was 6.9 months in the T group and 5.6 months in the C
group [HR 0.61, 95% CI (0.46–0.81)]. The median PFS in Teff low was
6.0 months in the T and 5.7 months in the C group [HR 0.84, 95% CI
(0.67–1.05)]. The median OS in Teff high was 17 months in the T
group and 15.9 months in the C group [HR 0.88, 95% CI (0.66–0.9)].
The median PFS in terms of Teff low was 13.2 months in the T group
and 12.6 months in the C group [HR 0.86, 95% CI (0.69–1.13)].
The meta-analysis showed that patients in the T
group had a longer survival time than those in the C group,
reflected in the median OS and PFS. Although anti-PD-1/L1 combined
with paclitaxel/albumin and platinum-based drugs better controls
the condition and prolongs the survival time of patients, it is
essential to recheck indicators such as blood cells, coagulation
function, electrolytes, liver and kidney function and blood
pressure during medication. For patients with severe adverse
reactions, daily monitoring is suitable. Patients experiencing
particularly severe adverse reactions need to decrease the dosage,
stop the medication or switch regimens to avoid worsening condition
and death. For those with serious related diseases before
chemotherapy, medication should be used with caution or avoided
altogether.
The majority of patients with NSCLC are elderly, and
their physical functions, organ condition and psychological states
are different from those of younger patients. They have a poorer
tolerance to toxic drugs (31) and
a lower acceptance of large fluctuations in condition, making them
more likely to be negative or resistant to medication. Therefore,
clinicians need to be cautious when administering drugs to this
patient population.
The present study has several limitations. Only five
articles were included, resulting in a small overall sample size
that lacked representativeness. As the included studies had already
excluded patients with contraindications during the trial design
stage, such as those with a bleeding tendency, the results cannot
be applied to all patients with NSCLC. The overall disease control
and survival rates of patients with NSCLC in the clinic are lower
than the research data (32,33).
For patients with a tendency to bleed, the use of
immunosuppressants should be applied with caution and a switch to
other targeted drugs should be considered. Differences in date,
region and ethnicity between the included studies introduce
bias.
Compared with the paclitaxel/albumin-paclitaxel +
carboplatin regimen, the anti-PD-1/L1 +
paclitaxel/albumin-paclitaxel + platinum regimen prolonged the
survival time of patients with NSCLC. Although patients treated
with anti-PD-1/L1 + paclitaxel/albumin-paclitaxel + platinum were
more prone to drug toxicity and side effects such as leukopenia,
bleeding, proteinuria, and hypertension, in patients with no
serious toxic side effects, anti-PD-1/L1+
paclitaxel/albumin-paclitaxel + platinum is a more effective
choice. The cost of immunosuppressants remains high, and a large
number of patients do not adhere to regular and adequate use. The
identification of alternative drugs or strategies using
combinations of immune drugs to decrease the dose of a single
immune drug may improve the treatment efficacy, shortening the
treatment cycle and lowering the cost. This treatment plan can be
promoted and used in patients with advanced NSCLC. PFS may also be
improved and the side effects of this treatment plan may be
decreased. More research into the side effects and methods to
alleviate the toxic and side effects of this treatment plan is
required. Overall, the present study provides support for the use
of chemoimmunotherapy in patients with advanced NSCLC and low
expression of PD-L1.
Acknowledgements
The authors would like to thank Mrs Gabrielle White
Wolf for editing the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in this study may be requested
from the corresponding author.
Authors' contributions
FL wrote and revised the manuscript, performed the
literature review and constructed figures. LL and FL performed the
literature review, designed the study and revised the manuscript.
SY and LL analyzed data. All authors have read and approved the
final manuscript. FL, LL and SY confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
3
|
Gridelli C, Maione P, Rossi A, Ferrara ML,
Castaldo V, Palazzolo G and Mazzeo N: Treatment of advanced
non-small-cell lung cancer in the elderly. Lung Cancer. 66:282–286.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen P, Liu Y, Wen Y and Zhou C:
Non-small-cell lung cancerin China. Cancer Commun (Lond).
42:937–970. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saad HM, Tourky GF, Al-Kuraishy HM,
Al-Gareeb AI, Khattab AM, Elmasry SA, Alsayegh AA, Hakami ZH,
Alsulimani A, Sabatier JM, et al: The potential role of MUC16
(CA125) biomarker in lung cancer: A magic biomarker but with
adversity. Diagnostics (Basel). 12:29852022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Five-year outcomes with pembrolizumab versus chemotherapy
for metastatic non-small-cell lung cancer with PD-L1 tumor
proportion score ≥ 50. J Clin Oncol. 39:2339–2349. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hellmann MD, Paz-Ares L, Bernabe Caro R,
Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A,
Lupinacci L, de la Mora Jimenez E, et al: Nivolumab plus ipilimumab
in advanced non-small-cell lung cancer. N Engl J Med.
381:2020–2031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olivares-Hernández A, Posado-Domínguez L,
Redondo-González JC, Corvo-Félix L, Bellido Hernández L,
Fonseca-Sánchez E and Del Barco-Morillo E: Response to
platinum-based therapies in second-line after immunotherapy in
advanced or metastatic non-small-cell lung cancer PD-L1 ≥50. Transl
Lung Cancer Res. 13:2649–2659. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garassino MC, Gadgeel S, Speranza G, Felip
E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled
N, et al: Pembrolizumab plus pemetrexed and platinum in nonsquamous
non-small-cell lung cancer: 5-Year outcomes from the phase 3
KEYNOTE-189 study. J Clin Oncol. 41:1992–1998. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Non-oncogene-addicted metastatic non-small-cell lung cancer:
ESMO clinical practice guideline for diagnosis, treatment and
follow-up. Ann Oncol. 34:358–376. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gadgeel S, Rodríguez-Abreu D, Speranza G,
Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell
SF, et al: Updated analysis from KEYNOTE-189: Pembrolizumab or
placebo plus pemetrexed and platinum for previously untreated
metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol.
38:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma A, Jasrotia S and Kumar A: Effects
of chemotherapy on the immune system: Implications for cancer
treatment and patient outcomes. Naunyn Schmiedebergs Arch
Pharmacol. 397:2551–2566. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiaoxi Q, Jiajin W and Hong W:
Immune-related adverse events induced by ICIs in advanced NSCLC: A
meta-analysis and systematic review. Zhongguo Fei Ai Za Zhi.
23:772–791. 2020.(In Chinese). PubMed/NCBI
|
|
16
|
Huang MY, Jiang XM, Wang BL, Sun Y and Lu
JJ: Combination therapy with ANTI-PD-1/PD-L1 blockade in non-small
cell lung cancer: Strategies and mechanisms. Pharmacol Ther.
219:1076942021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Onoi K, Chihara Y, Uchino J, Shimamoto T,
Morimoto Y, Iwasaku M, Kaneko Y, Yamada T and Takayama K: Immune
checkpoint inhibitors for lung cancer treatment: A review. J Clin
Med. 9:13622020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou
J, Lu Y, Shi Y, Wang Z, Jiang L, et al: Anlotinib as a third-line
therapy in patients with refractory advanced non-small-cell lung
cancer: A multicentre, randomised phase II trial (ALTER0302). Br J
Cancer. 118:654–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
Randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang S, Liang H, Liu Z, Zhao S, Liu J,
Xie Z, Wang W, Zhang Y, Han B, He J and Liang W: The impact of
anlotinib on brain metastases of non-small cell lung cancer: Post
hoc analysis of a phase III Randomized control trial (ALTER0303).
Oncologist. 25:e870–e874. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Lu S, Yu X, Hu Y, Zhao J, Sun M,
Yu Y, Hu C, Yang K, Song Y, et al: Tislelizumab plus chemotherapy
versus chemotherapy alone as first-line treatment for advanced
squamous non-small-cell lung cancer: final analysis of the
randomized, phase III RATIONALE-307 trial. ESMO Open. 9:1037272024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jotte R, Cappuzzo F, Vynnychenko I,
Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, Soo R, Conter HJ,
Kozuki T, Huang KC, et al: Atezolizumab in combination with
carboplatin and nab-paclitaxel in advanced squamous NSCLC
(IMpower131): Results from a randomized phase III trial. J Thorac
Oncol. 15:1351–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugawara S, Tanaka K, Imamura F, Yamamoto
N, Nishio M, Okishio K, Hirashima T, Tanaka H, Fukuhara T, Nakahara
Y, et al: Pembrolizumab plus chemotherapy in Japanese patients with
metastatic squamous non-small-cell lung cancer in KEYNOTE-407.
Cancer Sci. 114:3330–3341. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren S, Chen J, Xu X, Jiang T, Cheng Y,
Chen G, Pan Y, Fang Y, Wang Q, Huang Y, et al: Camrelizumab plus
carboplatin and paclitaxel as first-line treatment for advanced
squamous NSCLC (CameL-Sq): A phase 3 trial. J Thorac Oncol.
17:544–557. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
West H, McCleod M, Hussein M, Morabito A,
Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et
al: Atezolizumab in combination with carboplatin plus
nab-paclitaxel chemotherapy compared with chemotherapy alone as
first-line treatment for metastatic non-squamous non-small-cell
lung cancer (IMpower130): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:924–937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan JJ, Mai HQ, Ng WT, Hu CS, Li JG, Chen
XZ, Chow JCH, Wong E, Lee V, Ma LY, et al: Ninth version of the
AJCC and UICC nasopharyngeal cancer TNM staging classification.
JAMA Oncol. 10:1627–1635. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salas-Benito D, Pérez-Gracia JL,
Ponz-Sarvisé M, Rodriguez-Ruiz ME, Martínez-Forero I, Castañón E,
López-Picazo JM, Sanmamed MF and Melero I: Paradigmson immune
therapy combinations with chemotherap. Cancer Discov. 11:1353–1367.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Larroquette M, Domblides C, Lefort F,
Lasserre M, Quivy A, Sionneau B, Bertolaso P, Gross-Goupil M,
Ravaud A and Daste A: Combining immune check point inhibitors with
chemotherapy in advanced solid tumours: A review. Eur J Cancer.
158:47–62. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Butterfield LH and Najjar YG:
Immunotherapy combination approaches:Mechanisms, biomarkers and
clinical observations. Nat Rev Immunol. 24:399–416. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adus MR, Natividad J, Mai A, Ouyang Y,
Lambrecht N, Szabo S, Ge L, Hoa N and Dacosta-Iyer MG: Lung cancer:
A classic example of tumor escape and progression while providing
opportunities for immunological intervention. Clin Dev Immunol.
2012.1607242012.PubMed/NCBI
|
|
32
|
Lu S, Zhang W, Wu L, Wang W, Zhang P;
Neotorch Investigators, ; Fang W, Xing W, Chen Q, Yang L, et al:
Perioperative toripalimab plus chemotherapy for patients with
resectable non-small cell lung cancer: The neotorch randomized
clinical trial. JAMA. 331:201–211. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Z, Hackshaw A, Feng Q, Fu X, Zhang Y,
Mao C and Tang J: Comparison of gefitinib, erlotinib and afatinib
in non-small cell lung cancer: A meta-analysis. Int J Cancer.
140:2805–2819. 2017. View Article : Google Scholar : PubMed/NCBI
|















![Forest plot of the median overall
survival rate of patients with non-small cell lung cancer. There
was no statistical heterogeneity among the studies (P=0.07,
I2=49). A random-effects model was used for
meta-analysis; overall survival of the T group was significantly
higher than that of the C group [OR=20.43, 95%CI (18.81, 22.19),
P<0.001]. C, control; T, test;-HR, Hazard Ratio; df, degrees of
freedom; IV, Information Value; CI, confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g02.jpg)
![Forest plots of the median
asymptomatic survival of patients with non-small cell lung cancer.
There was no statistical heterogeneity among the studies (P=0.14,
I2=38). A random-effects model was used for
meta-analysis; asymptomatic survival of the T group was
significantly higher than that of the C group [OR=7.24, 95%CI
(6.93, 7.78), P<0.001]. C, control group; T, the test group; HR,
Hazard Ratio; df, degrees of freedom; IV, Information Value; CI,
confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g03.jpg)
![Forest plots of the occurrence of
adverse reactions in patients with non-small cell lung cancer.
There was no statistical heterogeneity among the studies
(P<0.00001, I2=84). A random-effects model was used
for meta-analysis; incidence of adverse events in the T group was
significantly lower than that in the C group [OR=2.47, 95%CI (1.16,
5.25), P=0.02]. C, control; T, test; OR, odds ratio; df, degrees of
freedom; IV, Information Value; CI, confidence interval.](/article_images/ol/31/3/ol-31-03-15454-g04.jpg)