Introduction
Gastric cancer (GC) ranks fifth worldwide in terms
of cancer incidence and mortality (1). The prevalence and high mortality rate
of GC make it a notable health issue, and its treatment and
management are planned according to the localized or
advanced/metastatic status of the disease. Although surgery is the
primary treatment option for GC, systemic therapies, such as
chemotherapy, targeted therapy and immunotherapy, have increased in
importance. Molecular diagnostic techniques have facilitated
genetic studies of GC and the identification of new potential
molecular targets. Although notable progress has been made in the
treatment of GC, further research and development are needed
(2–4).
In previous years, advantages such as low toxicity
and cost-effectiveness have increased interest in bioactive
compounds as therapeutic agents. Among these, the Nigella
sativa-derived compound thymoquinone (TQ) inhibits cancer cell
proliferation, migration and invasion at different cancer stages
(5). In addition, the anticancer
effects of TQ have been demonstrated in numerous types of cancer,
such as breast, pancreatic and prostate cancer, and it has been
suggested that TQ represents a new pharmacological agent that can
be used in cancer treatment (5–8).
The potent biological activity of TQ may allow
modifications to enhance its activity and applications toward
specific targets when modified with oxime derivatives. Owing to the
favorable properties of oxime bond formation, such as high
efficiency, chemoselectivity, formation in aqueous solvents and
water as the only by-product, oxime chemistry has progressed
rapidly and has been used chiefly for bioconjugation. In addition,
oxime chemistry is a promising field for bioconjugation and
eco-friendly polymer synthesis due to its low environmental impact
(9–11).
Studies have explored the anticancer effects of TQ
and its specific mechanisms on GC. It has been observed that the
antitumor effects of TQ increase when combined with
chemotherapeutics, such as 5-fluorouracil and cisplatin. These
studies have shown that TQ may be a pioneer molecule for treating
GC. However, the number of studies investigating the effect of
TQ-oxime (TQ-ox) on GC is insufficient (12–16).
The present study aimed to investigate the effects
of TQ-ox on the human GC cell line AGS and normal human gastric
epithelial cells (HGEpiCs) cell line. Therefore, the present study
evaluated the cytotoxic, genotoxic and apoptotic effects of TQ-ox,
and its potential to cause DNA damage. Building on our previous
studies (8,11,17)
investigating TQ-ox in other cancer models, the present study
extended that work by examining its cytotoxic, oxidative stress
(OS)-mediated, mitochondrial, apoptotic and genotoxic effects
specifically in AGS cells in comparison with HGEpiCs within a
single integrated experimental framework.
Materials and methods
TQ-ox synthesis
The TQ-ox used in the present study was not newly
synthesized; rather, it was obtained from our previously published
work (8). In the prior study, TQ-ox
was synthesized by nitrosating carvacrol with NaNO2 in
ethanol and hydrochloric acid under an argon atmosphere. The crude
product was purified by sequential washing steps, and its purity
and identity were confirmed through melting-point determination,
thin-layer chromatography analysis, and structural characterization
by 1H-NMR, 13C-NMR and elemental
analysis.
Cell lines and culture conditions
The present study used the AGS human GC cell line
(cat. no. CRL-1739™), which was commercially purchased from the
American Type Culture Collection. AGS cells were cultured in F-12K
medium supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (P/S) (all from Sigma-Aldrich; Merck KGaA).
Primary HGEpiCs were obtained from Innoprot (cat. no. P10778).
These cells were cultured in epithelial cell medium kit (cat. no.
P60106; Innoprot) supplemented with 2% FBS (Sigma-Aldrich; Merck
KGaA), 1% epithelial cell growth supplement (included in the
epithelial cell medium kit) and 1% P/S (Sigma-Aldrich; Merck KGaA),
according to the manufacturer's recommendations.
HGEpiCs were used to determine the effects of the
tested compounds on non-cancerous gastric epithelium and to perform
a comparative toxicity/selectivity assessment with AGS cells.
Unless otherwise specified, both AGS cells and
HGEpiCs were maintained under identical incubation conditions of
37°C in a humidified atmosphere containing 5% CO2 and
95% air. All in vitro experiments described in the present
study including cytotoxicity, intracellular reactive oxygen species
(ROS), GSH, mitochondrial membrane potential (MMP), apoptosis and
comet assays were also performed under these same incubation
conditions. For assessing the effects of TQ-ox on cytotoxicity and
intracellular ROS levels, AGS cells and HGEpiCs (7×103
cells/well) were seeded in 96-well plates; for evaluating the
effect of TQ-ox on apoptosis and DNA damage, 5×104/well
for each AGS cells and HGEpiCs were seeded in 6-well plates and
incubated for 24 h.
Cytotoxic activity
A luminescent ATP test was conducted to determine
the cytotoxic effects of TQ-ox. AGS cells and HGEpiCs were seeded
at 7×103 cells/well in 96-well plates, which was
determined to be the optimal density for these assays. After 24 h
of attachment, the cells were incubated with synthesized TQ-ox at
concentrations ranging from 2.5 to 100 µM for 24 h at 37°C in a
humidified incubator containing 5% CO2 and 95% air. To
detect ATP concentration, a homogeneous method that indicates the
presence of viable cells was performed using a
CellTiterGlo® luminescence cell viability kit (Promega
Corporation), according to the manufacturer's instructions.
Subsequently, measurements were performed after 5 min using a
multiplate reader (Synergy™ HTX Flash Multimode Reader; BioTek;
Agilent Technologies, Inc.). Luminescence emitted in the presence
of ATP was quantified in relative luminescence units. The
half-maximal inhibitory concentration (IC50) values of
TQ-ox were calculated from dose-response curves.
Intracellular ROS detection
The effect of TQ-ox on intracellular ROS was
assessed using the fluorometric method with
2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA;
Sigma-Aldrich; Merck KGaA) fluorescent dye. AGS cells and HGEpiCs
were treated with various concentrations of TQ-ox (2.5–100 µM) for
24 h at 37°C in a humidified incubator containing 5% CO2
and 95% air. After incubation, the medium was aspirated, and the
wells were washed three times with 1X Dulbecco's PBS (dPBS).
Subsequently, 100 µl 10 µM H2DCF-DA prepared in
distilled water was added to each well and the cells were incubated
for 30 min at 37°C. The fluorescence intensity of the resulting DCF
was determined using a fluorescence plate reader (Synergy HTX
Multi-Mode Reader) at an excitation of 488 nm and an emission of
525 nm. The results were analyzed relative to the control group,
which was treated with 0.1% DMSO.
Glutathione (GSH) analysis
The luminometric commercially obtained GSH-Glo™
Glutathione Assay luminescence kit (cat. no. V6911; Promega
Corporation) was used to analyze intracellular GSH levels,
according to the manufacturer's instructions. Both cell lines were
seeded at 7.5×103 cells/well in white opaque 96-well
plates and incubated for 24 h at 37°C in a humidified incubator
containing 5% CO2 and 95% air. Different TQ-ox
concentrations (2.5–100 µM) were applied followed by another
incubation for 24 h. Subsequently, GSH solution was added to the
cells and after a 5-min incubation, luminescence was measured using
a BioTek, Synergy HTX Multi-Mode Reader. To correct for differences
in cell number, GSH luminescence values were normalized to ATP
levels from parallel wells and compared with the control group.
MMP detection
Both cell lines were treated with different
concentrations of TQ-ox (2.5–100 µM) for 24 h at 37°C in a
humidified incubator containing 5% CO2 and 95% air
followed by medium removal and washing with 1X dPBS. Subsequently,
40 nM 3,3′-dihexyloxacarbocyanine iodide (3) (Molecular Probes; Thermo Fisher
Scientific, Inc.) was added and the cells were incubated for 15 min
at 37°C in a humidified incubator containing 5% CO2 and
95% air. Washing was performed three times using 1X dPBS, and
fluorescence intensity was measured using an excitation of 484 nm
and an emission of 501 nm using a fluorescence plate reader
(BioTek, Synergy HTX Multi-Mode Reader). The results were analyzed
and compared with those of the control group.
Apoptosis analysis via acridine
orange/ethidium bromide (AO/EB) double staining
AO/EB double staining was used to analyze
apoptosis-related nuclear morphological changes, as described in a
study reported by McGahon et al (18). Based on the cytotoxicity assay
results, sub-cytotoxic concentrations of TQ-ox close to the
IC50 value were selected to evaluate mechanistic
apoptotic responses without inducing extensive necrotic cell death.
Therefore, apoptotic experiments were conducted using 5–40 µM
TQ-ox, and both AGS cells and HGEpiCs were incubated with TQ-ox for
24 h at 37°C in a humidified incubator containing 5% CO2
and 95% air prior to staining. The treated cells were stained with
1:1 AO/EB double stain for 5 min at room temperature. A total of
~50 individual cells per TQ-ox concentration were analyzed to
quantify apoptotic morphology and ensure reliable statistical
evaluation using ImageJ V1.54 software (National Institutes of
Health). Apoptotic and necrotic cells were then visualized under a
fluorescence microscope (Nikon Eclipse Ts2; Nikon Corporation) and
images of stained cells were captured.
DNA damage assessment via comet
assay
Based on the cytotoxicity assay results,
sub-cytotoxic concentrations of TQ-ox close to the IC50
value (5–40 µM) were selected to evaluate genotoxic responses
without inducing extensive necrotic cell death. After treatment
with TQ-ox for 24 h at 37°C in a humidified incubator containing 5%
CO2 and 95% air, DNA damage was assessed using the
alkaline single-cell gel electrophoresis method described in a
previous study reported by Singh et al (19). Briefly, both cell lines were
detached with 0.25% trypsin/EDTA, washed twice with 1X dPBS and
resuspended in 0.7% low-melting agarose. The suspension was layered
onto slides that had been pre-coated with 1% normal-melting agarose
and air-dried at room temperature (22–24°C) for 1 h. The slides
were then kept at 4°C for 15 min to allow solidification. Slides
were subsequently incubated for ≥4 h in lysis buffer containing 50
mM Tris-HCl, 150 mM NaCl, and 1 mM EDTA (pH 10). All reagents were
obtained from Sigma-Aldrich (Merck KGaA). After lysis, the slides
were electrophoresed in alkaline buffer (pH 13) at 300 mA for 20
min.
After electrophoresis, the slides were stained with
5 mg/ml EB for 5 min at room temperature and examined under a
fluorescence microscope at an excitation of 546 nm. A total of ~50
individual cells per TQ-ox condition were analyzed using the Comet
Assay IV software (Instem), and images of stained cells were
captured. DNA damage was quantified as the percentage of DNA in the
comet tail.
Ethics statement
The present study involved only in vitro experiments
using commercially available human cell lines and did not include
human participants or animal subjects. Ethics approval was obtained
from the Hamidiye Scientific Research Ethics Committee of the
University of Health Sciences (Istanbul, Türkiye; approval no.
2024/15-15/33-24/760).
Statistical analysis
Statistical analyses were performed using the
Statistical Package for the Social Sciences version 28.0 (IBM
Corp.). The Shapiro-Wilk test was used to assess the normality of
the data distribution. The results of statistical analyses are
presented as the mean ± standard deviation from at least four
independent experiments. Statistical comparisons were performed
separately for each cell line, with each treatment concentration
compared only to the corresponding vehicle control. Comparisons
among >2 groups were conducted using one-way analysis of
variance followed by Tukey's post hoc test. IC50 values
were calculated via nonlinear regression analysis (GraphPad Prism;
Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
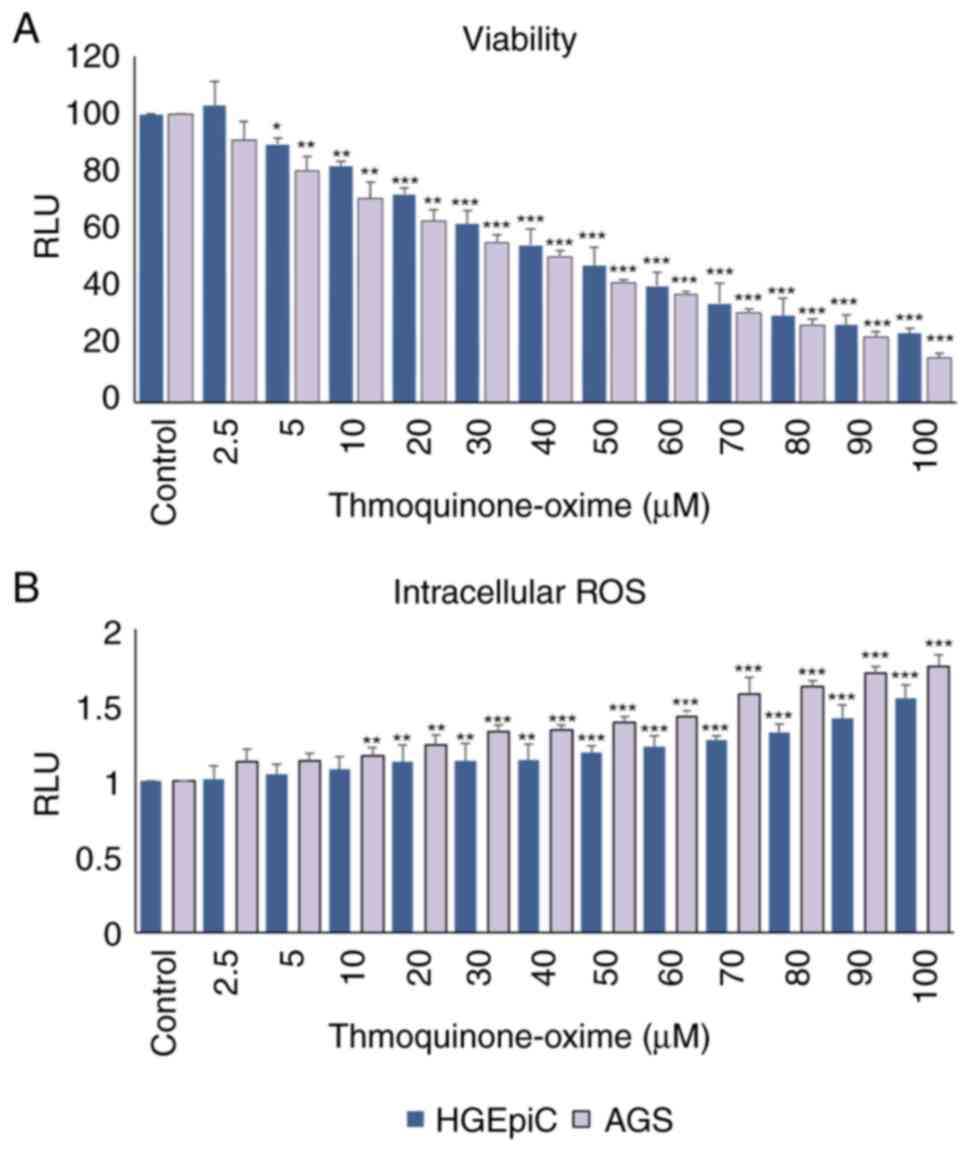
Cytotoxic activity and intracellular
ROS
According to the results of the ATP assay for
detecting cytotoxicity, TQ-ox showed a dose-dependent cytotoxic
effect on AGS cells after 24 h of treatment. When compared with the
0.1% DMSO control group, AGS cell viability showed a statistically
significant reduction beginning at 5 and maintained up to 20 µM
(P<0.01). This effect became more pronounced at 30 µM
(P<0.001), demonstrating a clear dose-dependent effect, with
marked cytotoxicity observed at concentrations ≥40 µM (P<0.001)
(Fig. 1A). A similar dose-dependent
decline was observed in HGEpiCs, where viability started to
significantly decrease at 5 µM (P<0.05), became more pronounced
at 10 µM (P<0.01), and reached strong significance at
concentrations ≥20 µM (P<0.001). At the highest concentrations
(80–100 µM), both cell lines showed a marked reduction in
viability, with viability dropping to 20–30% of control levels
(P<0.001).
A fluorescent H2DCF-DA probe was used to
assess intracellular ROS levels in each cell line. A dose-dependent
increase in ROS production was observed in AGS cells, becoming
statistically significant at 10 µM (P<0.01) and highly
significant at ≥30 µM (P<0.001) compared with the control group
(Fig. 1B). HGEpiCs showed a similar
but slightly attenuated pattern, ROS levels were significantly
increased at 20 µM (P<0.01), and were strongly elevated at
50–100 µM (P<0.001).
IC50 values were determined via nonlinear
regression analysis. Based on the cytotoxicity assay, AGS cells
demonstrated slightly higher sensitivity to TQ-ox compared with
HGEpiCs (IC50, 40.29 vs. 46.42 µM). Therefore, apoptosis
and comet assays were conducted using sub-cytotoxic concentrations
near the IC50 value (5–40 µM) to evaluate biologically
relevant apoptotic and genotoxic responses rather than non-specific
necrotic cell death.
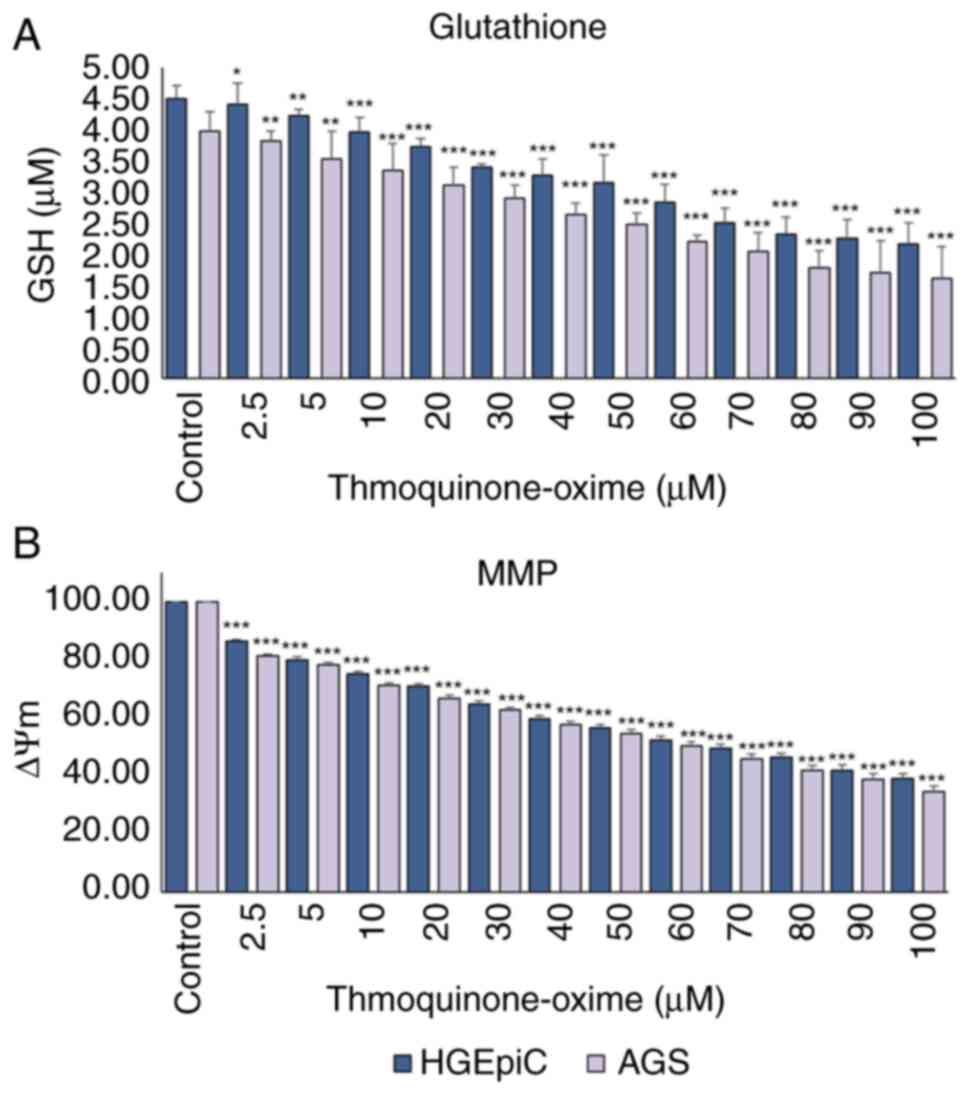
Intracellular GSH levels and MMP
GSH levels were decreased in TQ-ox-treated HGEpiCs
and AGS cells in a dose-dependent manner. In HGEpiCs, GSH levels
showed a modest but significant reduction beginning at 2.5 µM
(P<0.05), followed by a more pronounced decline at 5 µM
(P<0.01), and a highly significant decrease at concentrations
≥10 µM (P<0.001). In AGS cells, a significant decrease in GSH
levels was observed, starting at 2.5 and 5 µM (P<0.01), becoming
stronger at 10 µM (P<0.001) (Fig.
2A). These results indicated that TQ-ox weakened the
intracellular antioxidant defense system and disrupted GSH redox
balance.
TQ-ox also induced a significant dose-dependent
decrease in MMP in HGEpiCs and AGS cells. From a low TQ-ox
concentration of 2.5 µM, MMP was significantly decreased compared
with that in the control group (P<0.001). This decrease became
more pronounced as the dose increased; MMP levels decreased by ~60%
of the initial values after treatment with 100 µM TQ-ox (Fig. 2B).
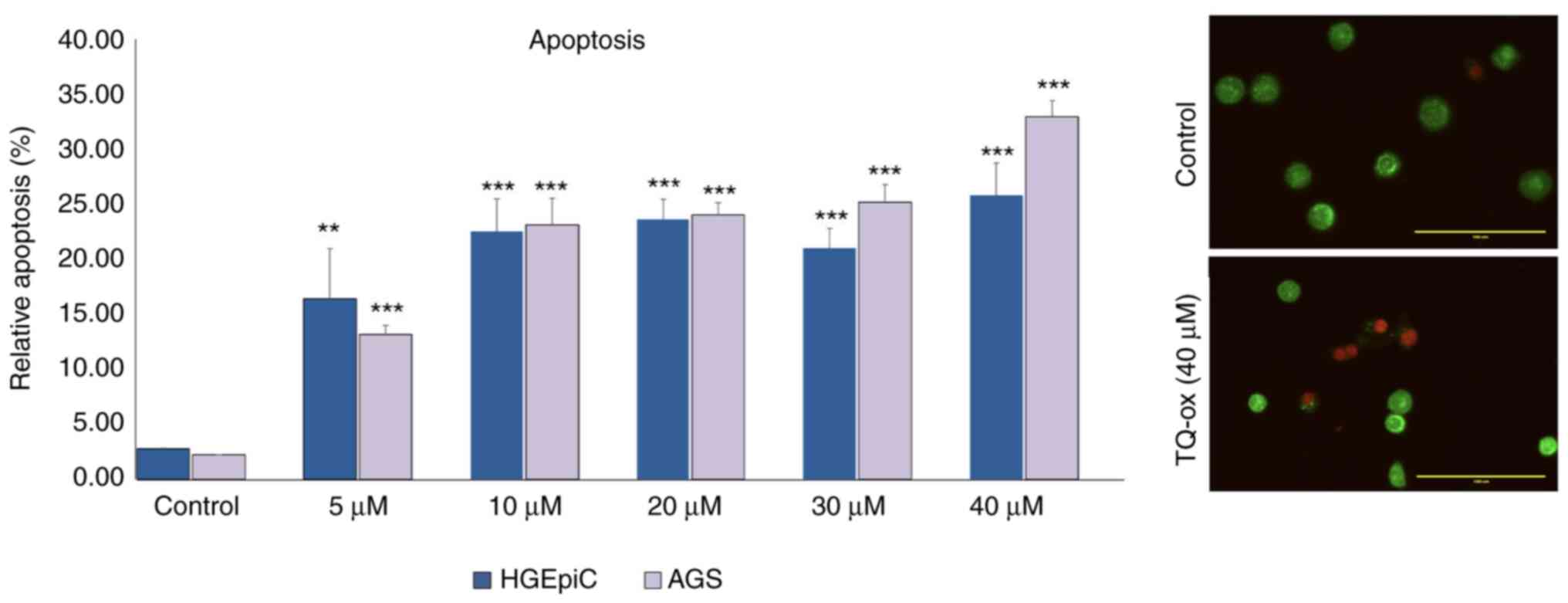
Apoptosis analysis via AO/EB double
staining
The potential of TQ-ox to induce apoptosis in cancer
cells was evaluated using the AO/EB double staining assay. The
apoptotic effects of TQ-ox doses below IC50 were
detected by incubating cancer cells with TQ-ox for 24 h followed by
fluorescence microscopy. In AGS cells, the data obtained showed
that apoptosis significantly increased in a dose-dependent manner
compared with that the control group (P<0.001). In HGEpiCs,
apoptosis percentage levels showed a modest but significant
increase at 5 µM (P<0.01), and a highly significant increase at
concentrations ≥10 µM (P<0.001). Representative images of AGS
cells and HGEpiCs revealed distinct morphological differences among
the experimental groups (Figs. S1
and S2). After staining with
AO/EB, viable and healthy cells fluoresced green, whereas apoptotic
cells appeared orange and necrotic cells appeared red due to
chromatin density and nuclear fragmentation. Fig. 3 shows the apoptotic values as the
mean percentage of the ratio of apoptotic cells to total cells at
each dose point.
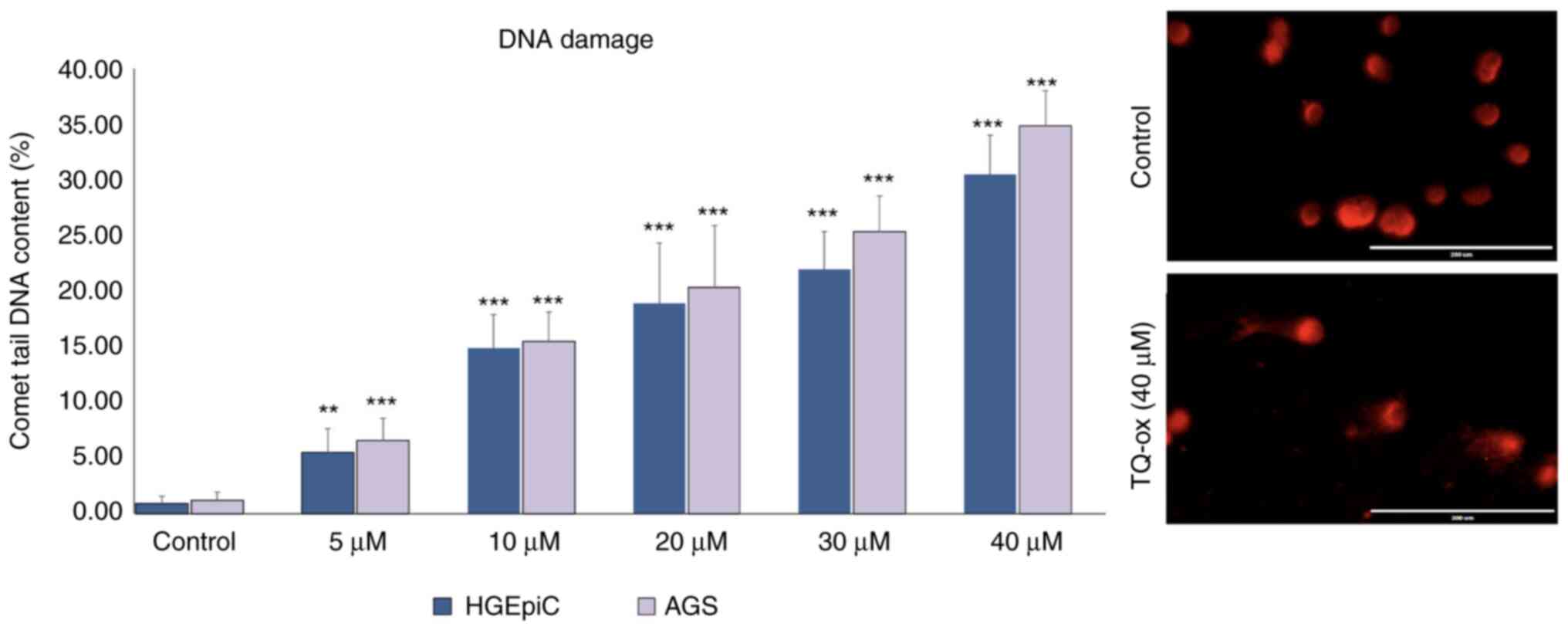
DNA damage analysis via comet
assay
AGS cells and HGEpiCs were incubated with TQ-ox
doses below its IC50 values to assess genotoxic damage.
In the comet assay, damaged DNA appears as comet-shaped structures
with bright, fragmented nuclei, whereas undamaged DNA appears as
large, intact and round nuclei. In the current study, TQ-ox
treatment increased the proportion of cells exhibiting comet-like
tails, indicating enhanced DNA fragmentation, whereas the nuclei of
untreated control cells predominantly remained intact and round.
This demonstrated that TQ-ox may induce measurable DNA damage. In
addition, a significant increase in percentage tail values was
observed with increasing concentrations of TQ-ox in both cell lines
(Fig. 4). While DNA damage was
relatively low in the control group, the application of TQ-ox
starting at 5 µM resulted in a statistically significant increase
in DNA damage (P<0.01 and P<0.001), with the highest effect
observed at 40 µM. AGS cells exhibited notably greater DNA damage
than HGEpiCs, particularly at high doses. Representative comet
assay images of AGS cells and HGEpiCs demonstrated distinct
differences in DNA damage (tail formation) among the experimental
groups (Figs. S3 and S4).
Discussion
The therapeutic uses of oxime compounds for various
diseases are currently under investigation (20). The search for synthetic
modifications of steroid compounds is a promising strategy for
finding new drug candidates. Even subtle changes in the
substitution pattern of steroid chemical frameworks can have marked
effects on their specific biological activities. In particular,
some steroidal oxime compounds and oxime ethers have exhibited
notable antioxidant, antibacterial and antitumor properties,
highlighting their potential as therapeutic agents (21,22).
The cytotoxic, genotoxic and apoptotic effects of
ox-modified TQ were investigated in AGS cells and HGEpiCs. TQ-ox
significantly reduced cell viability in a dose-dependent manner,
increased intracellular ROS levels, disrupted MMP and induced DNA
damage. These results suggested that TQ-ox exerted its anticancer
effects through OS-mediated apoptotic mechanisms and mitochondrial
dysfunction.
OS is caused by an imbalance between the antioxidant
capacity of the cell and pro-oxidant molecules (18). This imbalance can lead to DNA
damage, protein aggregation and digestive system membrane
dysfunction (23). By interacting
with macromolecules, such as DNA, ROS can disrupt important
cellular functions. Oxidative DNA damage may lead to the
development of cancer by increasing the risk of mutagenesis
(24). Oxime compounds effectively
modulate ROS and mediate cellular OS levels. These compounds have
been shown to exhibit therapeutic potential against OS-related
diseases by regulating ROS production and maintaining redox balance
(25).
The addition of oxime groups may enhance the
biological effects and therapeutic applications of therapeutic
agents by optimizing their interactions with biomolecules (26). A study by Bozali et al
(17) reported that cytotoxicity,
ROS elevation, GSH depletion, mitochondrial dysfunction, DNA damage
and apoptosis were notably induced in B16F10 melanoma cells at
concentrations of 10–20 µM TQ-ox. Similarly, Güler and Bozali
(11) found that TQ-ox induced
dose-dependent increases in ROS and Ca2+ levels, as well
as notable genotoxic and apoptotic responses in HepG2 liver cancer
cells. Similar patterns of OS, MMP collapse and DNA damage have
been observed in the ovarian carcinoma SKOV-3 cell line (8).
A recent study on A549 lung cancer cells
demonstrated that TQ-ox induces dose-dependent cytotoxicity through
OS, GSH depletion, calcium elevation and MMP loss, leading to
apoptosis (27). These findings
support the consistent pro-oxidant and pro-apoptotic effects of
TQ-ox observed across cancer types and therefore its potential as
an anticancer agent. Collectively, the findings of these previous
studies underscore that oxime modification of TQ maintains or
enhances its pro-oxidant and genotoxic mechanisms, possibly by
facilitating increased interactions with mitochondrial and
redox-sensitive targets. Consistent with these studies, the
findings of the present study demonstrated that TQ-ox exerted
similar pro-oxidant and cytotoxic effects in AGS cells and HGEpiCs.
The observed dose-dependent increase in intracellular ROS levels,
GSH depletion and MMP loss suggested that TQ-ox disrupted redox
homeostasis and mitochondrial integrity, ultimately leading to
apoptosis. The slightly higher sensitivity of AGS cells compared
with normal HGEpiCs reinforced the potential selectivity of TQ-ox
toward malignant cells. DNA damage analysis via comet assay
revealed a dose-related increase in tail fluorescence intensity,
suggesting that TQ-ox exerted genotoxic effects in AGS cells. The
observed DNA fragmentation further supported the role of OS and
mitochondrial impairment in TQ-ox-induced cytotoxicity. These
combined results suggested that TQ-ox triggered cell death via
multiple converging mechanisms, including oxidative imbalance,
mitochondrial collapse, apoptosis and genotoxic stress.
However, the present study also had some
limitations. Primarily, experiments were conducted in vitro,
and in vivo validation is needed to fully characterize the
pharmacodynamic and pharmacokinetic properties of TQ-ox. Future
studies including additional GC subtypes and animal models will be
valuable to further generalize these findings.
In conclusion, the present study revealed that
increasing TQ-ox concentrations induced cytotoxicity, genotoxicity
and apoptosis in AGS cells and HGEpiCs by increasing intracellular
ROS and decreasing cell viability, intracellular GSH levels and
MMP. TQ-ox exerted pro-oxidant activity on cancer cells at higher
concentrations, triggering cell death mechanisms. While TQ-ox
exerted strong pro-oxidant, cytotoxic, apoptotic and genotoxic
effects in both AGS cancer cells and HGEpiCs at the same
concentrations, the magnitude of these responses differed between
the two cell types. AGS cells exhibited a more pronounced and
earlier response, characterized by a significant reduction in cell
viability and increased oxidative stress at lower concentrations,
followed by marked induction of apoptosis and DNA damage at higher
doses. By contrast, HGEpiCs displayed a comparatively attenuated
response, with significant effects occurring predominantly at
higher concentrations of TQ-ox. Overall, these findings indicate
that although TQ-ox exerts cytotoxic and genotoxic effects in both
normal and cancerous gastric epithelial cells, AGS cells are more
susceptible to TQ-ox-induced oxidative stress-mediated damage,
suggesting a differential cellular sensitivity between malignant
and non-malignant cells. These findings support the therapeutic
potential of TQ-ox in GC but highlight the need for further
research into its dose-selectivity, molecular mechanisms and in
vivo efficacy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KB, Zİ, NK, TG, MK, YMK, ME, MDa, EA, FT, CA, OS,
MDu and EMG were responsible for conceptualization, methodology,
formal analysis and investigation of the present study, as well as
contributions towards composing the original draft of the
manuscript, reviewing and editing the manuscript, and the
acquisition of resources and funding. EMG was also responsible for
supervision of the present study. KB, Zİ and EMG confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the principles of The Declaration of Helsinki and was approved by
the Hamidiye Scientific Research Ethics Committee of the University
of Health Sciences, Türkiye (approval no.
2024/15-15/33-24/760).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript
[Grammarly (app.grammarly.com) and Trinka AI (trinka.ai)] and to
translate the manuscript into English [DeepL Translator
(deepl.com/en/translator)], and subsequently, the authors revised
and edited the content produced by the AI tools as necessary,
taking full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Guan WL, He Y and Xu RH: Gastric cancer
treatment: Recent progress and future perspectives. J Hematol
Oncol. 16:572023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burz C, Pop V, Silaghi C, Lupan I and
Samasca G: Prognosis and treatment of gastric cancer: A 2024
update. Cancers (Basel). 16:17082024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Gastric cancer, : ESMO clinical practice guideline for
diagnosis, treatment and follow-up. Ann Oncol. 33:1005–1020. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imran M, Rauf A, Khan IA, Shahbaz M,
Qaisrani TB, Fatmawati S, Abu-Izneid T, Imran A, Rahman KU and
Gondal TA: Thymoquinone: A novel strategy to combat cancer: A
review. Biomed Pharmacother. 106:390–402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabassum S, Thakur V, Rosli N, Ichwan SJA,
Mishra P and Suriyah WH: Therapeutic implications of thymoquinone
and its molecular and functional mechanisms against oral and lung
cancer. Gene Rep. 27:1–9. 2022.
|
|
7
|
Butnariu M, Quispe C, Herrera-Bravo J,
Helon P, Kukula-Koch W, López V, Les F, Vergara CV, Alarcón-Zapata
P, Alarcón-Zapata B, et al: The effects of thymoquinone on
pancreatic cancer: Evidence from preclinical studies. Biomed
Pharmacother. 153:1133642022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kale E, Kale A, Bozali K, Gulgec AS,
Ozdemir M, Yalcin B and Guler EM: TQ-Ox, a novel synthetic
derivative of thymoquinone on ovarian cancer cells in vitro. Nat
Prod Res. 37:3015–3024. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang HM, Zhao YL, Sun Q, Ouyang XH and Li
JH: Recent advances in N-O bond cleavage of oximes and
hydroxylamines to construct N-heterocycle. Molecules. 28:17752023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dudchak R, Podolak M, Holota S,
Szewczyk-Roszczenko O, Roszczenko P, Bielawska A, Lesyk R and
Bielawski K: Click chemistry in the synthesis of antibody-drug
conjugates. Bioorg Chem. 143:1069822024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guler EM and Bozali K: Synthesised
thymoquinone-oxime induces cytotoxicity, genotoxicity and apoptosis
in hepatocellular cancer cells: In vitro study. Nat Prod Res.
38:1695–1703. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng LM, Wang XF and Huang QX:
Thymoquinone induces cytotoxicity and reprogramming of EMT in
gastric cancer cells by targeting PI3K/Akt/mTOR pathway. J Biosci.
42:547–554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei X, Lv X, Liu M, Yang Z, Ji M, Guo X
and Dong W: Thymoquinone inhibits growth and augments
5-fluorouracil-induced apoptosis in gastric cancer cells both in
vitro and in vivo. Biochem Biophys Res Commun. 417:864–868. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu WQ, Wang J, Guo XF, Liu Z and Dong WG:
Thymoquinone inhibits proliferation in gastric cancer via the STAT3
pathway in vivo and in vitro. World J Gastroenterol. 22:4149–4159.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J, Hu X, Li J, Wu D, Lan Q, Wang Q,
Tian S and Dong W: Enhancing conventional chemotherapy drug
cisplatin-induced anti-tumor effects on human gastric cancer cells
both in vitro and in vivo by Thymoquinone targeting PTEN gene.
Oncotarget. 8:85926–85939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He P, He Y, Ma J, Liu Y, Liu C, Baoping Y
and Dong W: Thymoquinone induces apoptosis and protective autophagy
in gastric cancer cells by inhibiting the PI3K/Akt/mTOR pathway.
Phytother Res. 37:3467–3480. 2023. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bozali K, Koc S, Beyaztas H, Ozdemir M,
Ozkan BN, Dumlu FS, Yalcin B and Guler EM: Thymoquinone oxime
synthesis and its effects on melanoma cells: Cytotoxic, genotoxic,
and apoptotic evaluation. Nat Prod Res. 39:5768–5776. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McGahon AJ, Martin SJ, Bissonnette RP,
Mahboubi A, Shi Y, Mogil RJ, Nishioka WK and Green DR: The end of
the (cell) line: Methods for the study of apoptosis in vitro.
Methods Cell Biol. 46:153–185. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh NP, Danner DB, Tice RR, Brant L and
Schneider EL: DNA damage and repair with age in individual human
lymphocytes. Mutat Res. 237:123–130. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gomes AR, Pires AS, Abrantes AM, Gonçalves
AC, Costa SC, Varela CL, Silva ET, Botelho MF and Roleira FMF:
Design, synthesis, and antitumor activity evaluation of steroidal
oximes. Bioorg Med Chem. 46:1163602021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vágvölgyi M, Laczkó D, Santa-Maria AR,
Vigh JP, Walter FR, Berkecz R, Deli MA, Tóth G and Hunyadi A:
17-Oxime ethers of oxidized ecdysteroid derivatives modulate
oxidative stress in human brain endothelial cells and
dose-dependently might protect or damage the blood-brain barrier.
PLoS One. 19:e02905262024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gomes AR, Pires AS, Roleira FMF and
Tavares-da-Silva EJ: The structural diversity and biological
activity of steroid oximes. Molecules. 28:16902023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zińczuk J, Zaręba K, Kamińska J,
Koper-Lenkiewicz OM, Dymicka-Piekarska V, Pryczynicz A,
Guzińska-Ustymowicz K, Kędra B, Matowicka-Karna J,
Żendzian-Piotrowska M, et al: Association of tumour
microenvironment with protein glycooxidation, DNA damage, and
nitrosative stress in colorectal cancer. Cancer Manag Res.
13:6329–6348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chavda V, Chaurasia B, Garg K, Deora H,
Umana GE, Palmisciano P, Scalia G and Lu B: Molecular mechanisms of
oxidative stress in stroke and cancer. Brain Disorders.
5:1000292022. View Article : Google Scholar
|
|
25
|
Kolsi LE, Leal AS, Yli-Kauhaluoma J, Liby
KT and Moreira VM: Dehydroabietic oximes halt pancreatic cancer
cell growth in the G1 phase through induction of p27 and
downregulation of cyclin D1. Sci Rep. 8:159232018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schepetkin IA, Plotnikov MB, Khlebnikov
AI, Plotnikova TM and Quinn MT: Oximes: Novel therapeutics with
anticancer and anti-inflammatory potential. Biomolecules.
11:7772021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beyaztas H, Babaoglu B, Demirkol B,
Cetinkaya E and Metin Guler E: Synthesis and characterization of
thymoquinone-oxime (TQ-Ox) from thymoquinone and evaluation of its
cytotoxic, genotoxic, and apoptotic potential in lung cancer cells
(A549) in vitro. ChemistrySelect:. 9:e2023049402024. View Article : Google Scholar
|