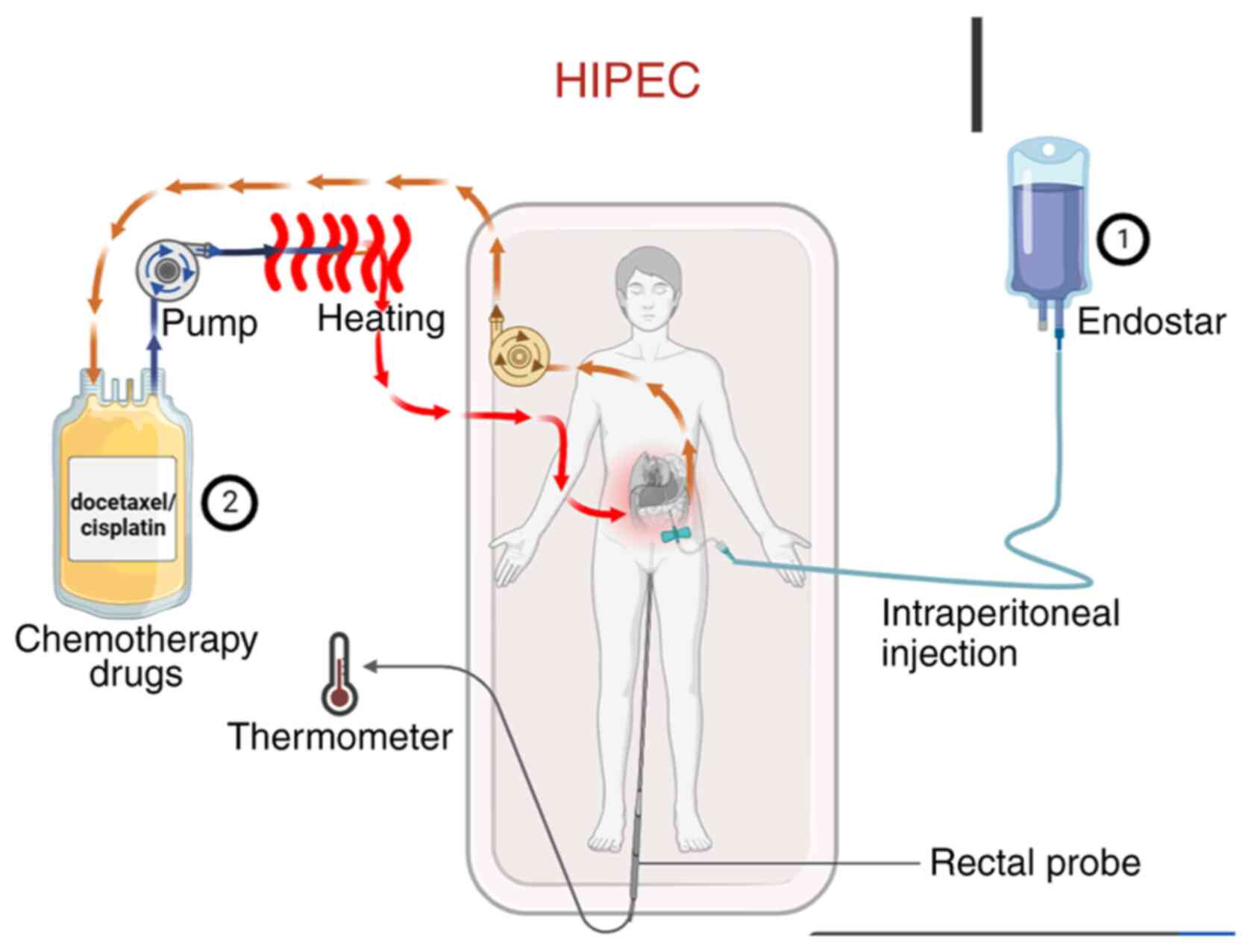
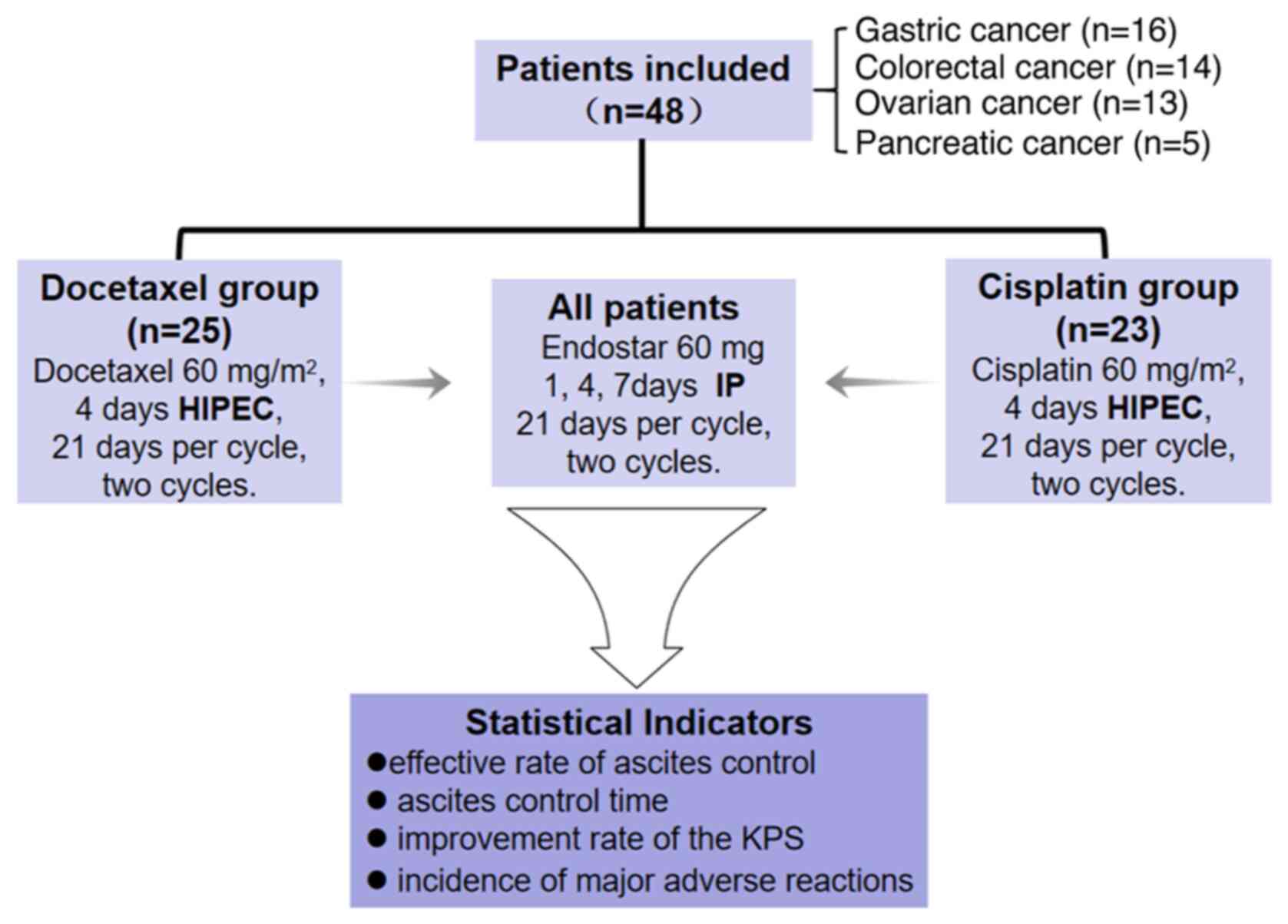
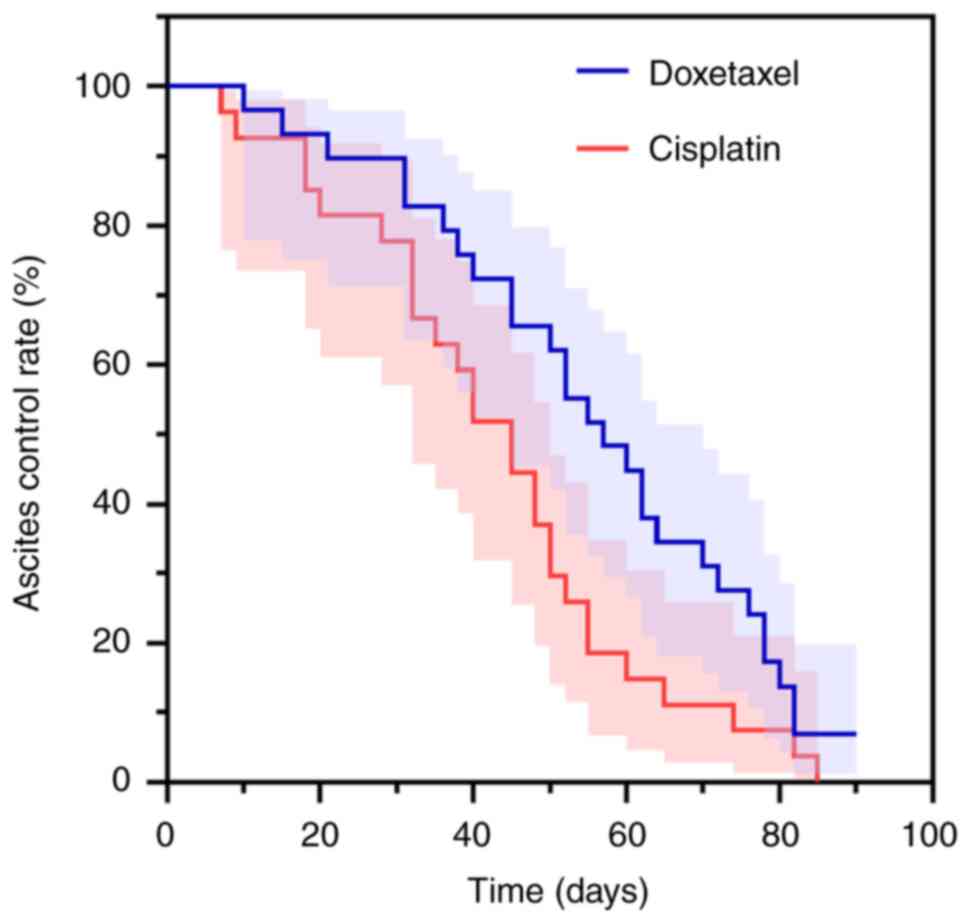
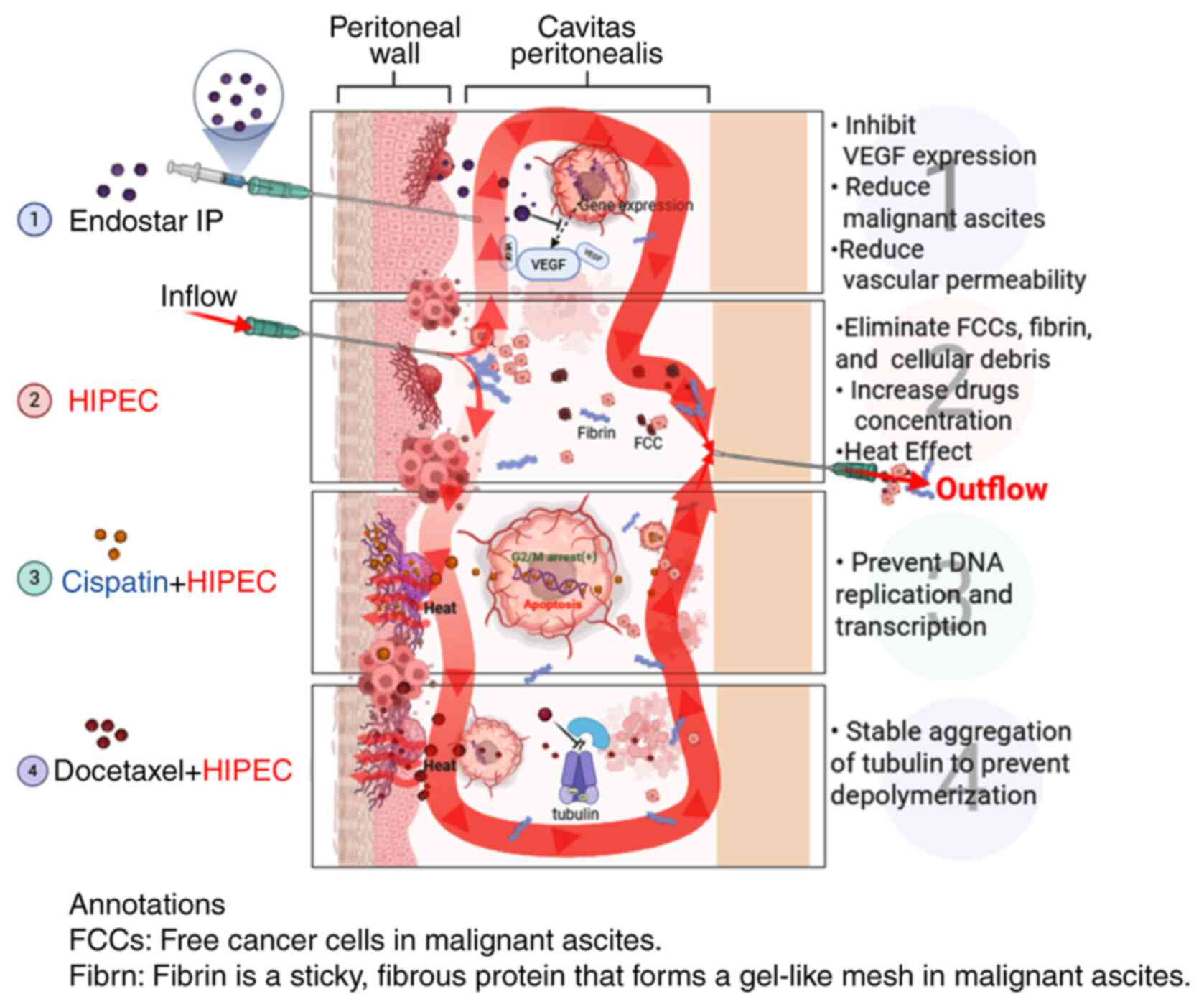
|
1
|
Soliman F, Ye L, Jiang W and Hargest R:
O17: Hyaluronic acid dependent adhesion in colorectal cancer
peritoneal metastasis. Br J Surg. 108 (Suppl 1):znab117.017. 2021.
View Article : Google Scholar
|
|
2
|
Zhu L, Xu Z, Wu Y, Liu P, Qian J, Yu S,
Xia B, Lai J, Ma S and Wu Z: Prophylactic chemotherapeutic
hyperthermic intraperitoneal perfusion reduces peritoneal
metastasis in gastric cancer: A retrospective clinical study. BMC
Cancer. 20:8272020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu L, Xu Y, Shan Y, Zheng R, Wu Z and Ma
S: Intraperitoneal perfusion chemotherapy and whole abdominal
hyperthermia using external radiofrequency following radical D2
resection for treatment of advanced gastric cancer. Int J
Hyperthermia. 36:403–407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mei S, Chen X, Wang K and Chen Y: Tumor
microenvironment in ovarian cancer peritoneal metastasis. Cancer
Cell Int. 23:112023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Avula LR, Hagerty B and Alewine C:
Molecular mediators of peritoneal metastasis in pancreatic cancer.
Cancer Metastasis Rev. 39:1223–1243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roth L, Russo L, Ulugoel S, Freire Dos
Santos R, Breuer E, Gupta A and Lehmann K: Peritoneal metastasis:
Current status and treatment options. Cancers (Basel). 14:602021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu L, Hofmann J, Holash J, Yancopoulos GD,
Sood AK and Jaffe RB: Vascular endothelial growth factor trap
combined with paclitaxel strikingly inhibits tumor and ascites,
prolonging survival in a human ovarian cancer model. Clin Cancer
Res. 11:6966–6971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Wu Y, Wu J and Wu C: Direct and
indirect anticancer effects of hyperthermic intraperitoneal
chemotherapy on peritoneal malignancies (Review). Oncol Rep.
45:232021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karimi M, Shirsalimi N and Sedighi E:
Challenges following CRS and HIPEC surgery in cancer patients with
peritoneal metastasis: A comprehensive review of clinical outcomes.
Front Surg. 11:14985292024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung H: Interaction of thermotolerance and
thermosensitization induced in CHO cells by combined hyperthermic
treatments at 40 and 43 degrees C. Radiat Res. 91:433–446. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spratt JS, Adcock RA, Muskovin M, Sherrill
W and McKeown J: Clinical delivery system for intraperitoneal
hyperthermic chemotherapy. Cancer Res. 40:256–260. 1980.PubMed/NCBI
|
|
12
|
Cortes-Guiral D and Glehen O: Expanding
uses of hipec for locally advanced colorectal cancer: A European
perspective. Clin Colon Rectal Surg. 33:253–257. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Braam HJ, Schellens JH, Boot H, van
Sandick JW, Knibbe CA, Boerma D and van Ramshorst B: Selection of
chemotherapy for hyperthermic intraperitoneal use in gastric
cancer. Crit Rev Oncol Hematol. 95:282–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Bree E and Michelakis D: An overview
and update of hyperthermic intraperitoneal chemotherapy in ovarian
cancer. Expert Opin Pharmacother. 21:1479–1492. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding Y, Wang Y, Cui J and Si T: Endostar
blocks the metastasis, invasion and angiogenesis of ovarian cancer
cells. Neoplasma. 67:595–603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaheen RM, Davis DW, Liu W, Zebrowski BK,
Wilson MR, Bucana CD, McConkey DJ, McMahon G and Ellis LM:
Antiangiogenic therapy targeting the tyrosine kinase receptor for
vascular endothelial growth factor receptor inhibits the growth of
colon cancer liver metastasis and induces tumor and endothelial
cell apoptosis. Cancer Res. 59:5412–5416. 1999.PubMed/NCBI
|
|
17
|
Hu Y, Zhou Z and Luo M: Efficacy and
safety of endostar combined with cisplatin in treatment of
non-small cell lung cancer with malignant pleural effusion: A
meta-analysis. Medicine (Baltimore). 101:e322072022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biaoxue R, Xiguang C, Hua L, Wenlong G and
Shuanying Y: Thoracic perfusion of recombinant human endostatin
(Endostar) combined with chemotherapeutic agents versus
chemotherapeutic agents alone for treating malignant pleural
effusions: A systematic evaluation and meta-analysis. BMC Cancer.
16:8882016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guchelaar NAD, Noordman BJ, Koolen SLW,
Mostert B, Madsen EVE, Burger JWA, Brandt-Kerkhof ARM, Creemers GJ,
de Hingh IHJT, Luyer M, et al: Intraperitoneal chemotherapy for
unresectable peritoneal surface malignancies. Drugs. 83:159–180.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chia DKA, Demuytere J, Ernst S, Salavati H
and Ceelen W: Effects of hyperthermia and hyperthermic
intraperitoneal chemoperfusion on the peritoneal and tumor immune
contexture. Cancers (Basel). 15:43142023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chinese Society of Clinical Oncology
Antitumor Drug Safety Management Expert Committee and Chinese
Society of Clinical Oncology Vascular Targeted Therapy Expert
Committee, . Expert consensus on the clinical application of
recombinant human endostatin for the treatment of malignant serous
effusions. Chinese Clinical Oncology. 25:849–856. 2020.(In
Chinese). PubMed/NCBI
|
|
22
|
Ning T, Jiang M, Peng Q, Yan X, Lu ZJ,
Peng YL, Wang HL, Lei N, Zhang H, Lin HJ, et al: Low-dose
endostatin normalizes the structure and function of tumor
vasculature and improves the delivery and anti-tumor efficacy of
cytotoxic drugs in a lung cancer xenograft murine model. Thorac
Cancer. 3:229–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv Y, Jiang R, Ma C, Li J, Wang B, Sun L
and Mu N: Clinical observation of recombinant human vascular
endostatin durative transfusion combined with window period
arterial infusion chemotherapy in the treatment of advanced lung
squamous carcinoma. Zhongguo Fei Ai Za Zhi. 18:500–504. 2015.(In
Chinese). PubMed/NCBI
|
|
24
|
Lin MI and Sessa WC: Antiangiogenic
therapy: Creating a unique ‘window’ of opportunity. Cancer Cell.
6:529–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang XD, Dai P, Qiao Y, Wu J, Song DA and
Li SQ: Clinical study on the recombinant human endostatin regarding
improving the blood perfusion and hypoxia of non-small-cell lung
cancer. Clin Transl Oncol. 14:437–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karnofsky DA and Burchenal JH: The
clinical evaluation of chemotherapeutic agents in cancer. MacLeod
CM: Evaluation of Chemotherapeutic Agents. Columbia University
Press; New York: pp. 191–205. 1949
|
|
28
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
US Department of Health and Human
Services, National Institutes of Health and National Cancer
Institute. Common Terminology Criteria for Adverse Events (CTCAE).
Version 4.0. 2009.
|
|
30
|
Deraco M, Kusamura S, Virzì S, Puccio F,
Macrì A, Famulari C, Solazzo M, Bonomi S, Iusco DR and Baratti D:
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
as upfront therapy for advanced epithelial ovarian cancer:
Multi-institutional phase-II trial. Gynecol Oncol. 122:215–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsusaki K, Aridome K, Emoto S, Kajiyama
H, Takagaki N, Takahashi T, Tsubamoto H, Nagao S, Watanabe A,
Shimada H and Kitayama J: Clinical practice guideline for the
treatment of malignant ascites: Section summary in clinical
practice guideline for peritoneal dissemination (2021). Int J Clin
Oncol. 27:1–6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horikawa N, Abiko K, Matsumura N,
Hamanishi J, Baba T, Yamaguchi K, Yoshioka Y, Koshiyama M and
Konishi I: Expression of vascular endothelial growth factor in
ovarian cancer inhibits tumor immunity through the accumulation of
myeloid-derived suppressor cells. Clin Cancer Res. 23:587–599.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhan N, Dong WG and Wang J: The clinical
significance of vascular endothelial growth factor in malignant
ascites. Tumour Biol. 37:3719–3725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiva LM and Gonzalez-Martin A: A critical
appraisal of hyperthermic intraperitoneal chemotherapy (HIPEC) in
the treatment of advanced and recurrent ovarian cancer. Gynecol
Oncol. 136:130–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao WY, Chen DY, Chen JH and Ji ZN:
Effects of intracavitary administration of Endostar combined with
cisplatin in malignant pleural effusion and ascites. Cell Biochem
Biophys. 70:623–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei H, Qin S, Yin X, Chen Y, Hua H, Wang
L, Yang N, Chen Y and Liu X: Endostar inhibits ascites formation
and prolongs survival in mouse models of malignant ascites. Oncol
Lett. 9:2694–2700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laplace N, Kepenekian V, Friggeri A,
Vassal O, Ranchon F, Rioufol C, Gertych W, Villeneuve L, Glehen O
and Bakrin N: Sodium thiosulfate protects from renal impairement
following hyperthermic intraperitoneal chemotherapy (HIPEC) with
cisplatin. Int J Hyperthermia. 37:897–902. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goodman MD, McPartland S, Detelich D and
Saif MW: Chemotherapy for intraperitoneal use: A review of
hyperthermic intraperitoneal chemotherapy and early post-operative
intraperitoneal chemotherapy. J Gastrointest Oncol. 7:45–57.
2016.PubMed/NCBI
|
|
39
|
Liu P, Wu Y, Xu X, Fan X, Sun C, Chen X,
Xia J, Bai S, Qu L, Lu H, et al: Microwave triggered
multifunctional nanoplatform for targeted photothermal-chemotherapy
in castration-resistant prostate cancer. Nano Res. 16:9688–9700.
2023. View Article : Google Scholar
|
|
40
|
Li Y, Köpper F and Dobbelstein M:
Inhibition of MAPKAPK2/ MK2 facilitates DNA replication upon cancer
cell treatment with gemcitabine but not cisplatin. Cancer Lett.
428:45–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pu YS, Huang CY, Wu HL, Wu JH, Su YF, Yu
CTR, Lu CY, Wu WJ, Huang SP, Huang YT and Hour TC: EGFR-mediated
hyperacetylation of tubulin induced docetaxel resistance by
downregulation of HDAC6 and upregulation of MCAK and PLK1 in
prostate cancer cells. Kaohsiung J Med Sci. 40:23–34. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Van Driel WJ, Koole SN, Sikorska K,
Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT,
van der Velden J, Arts HJ, Massuger LFAG, et al: Hyperthermic
intraperitoneal chemotherapy in ovarian cancer. N Engl J Med.
378:230–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhan ZW, Wang XJ, Yu JM, Zheng JX, Zeng Y,
Sun MY, Peng L, Guo ZQ and Chen BJ: Intraperitoneal Infusion of
Recombinant Human Endostatin Improves Prognosis in Gastric Cancer
Ascites. Future Oncol. 18:1259–1271. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fu YB, Yang R, Su YD, Ma R, Wei T, Yu Y,
Li B and Li Y: Cisplatin + docetaxel improves survival over
cisplatin + mitomycin C in hyperthermic intraperitoneal
chemotherapy for pseudomyxoma peritonei: A retrospective study
based on propensity score matching. Int J Hyperthermia.
42:24672962025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harlev C, Bue M, Petersen EK, Jørgensen
AR, Bibby BM, Hanberg P, Schmedes AV, Petersen LK and Stilling M:
Dynamic assessment of local abdominal tissue concentrations of
cisplatin during a HIPEC procedure: Insights from a porcine model.
Ann Surg Oncol. 32:3804–3813. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y and Ren H: Multi-omics sequencing
revealed endostar combined with cisplatin treated non small cell
lung cancer via anti-angiogenesis. BMC Cancer. 24:1872024.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hakeam HA, Breakiet M, Azzam A, Nadeem A
and Amin T: The incidence of cisplatin nephrotoxicity post
hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive
surgery. Ren Fail. 36:1486–1491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gómez-Ruiz ÁJ, González-Gil A, Gil J,
Alconchel F, Navarro-Barrios Á, Gil-Gómez E, Martínez J, Nieto A,
García-Palenciano C and Cascales-Campos PA: Acute renal disease in
patients with ovarian peritoneal carcinomatosis treated with
cytoreduction and HIPEC: The influence of surgery and the
cytostatic agent used. Langenbecks Arch Surg. 406:2449–2456. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ye J, Ren Y, Wei Z, Peng J, Chen C, Song
W, Tan M, He Y and Yuan Y: Nephrotoxicity and long-term survival
investigations for patients with peritoneal carcinomatosis using
hyperthermic intraperitoneal chemotherapy with cisplatin: A
retrospective cohort study. Surg Oncol. 27:456–461. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sin EI, Chia CS, Tan GHC, Soo KC and Teo
MC: Acute kidney injury in ovarian cancer patients undergoing
cytoreductive surgery and hyperthermic intra-peritoneal
chemotherapy. Int J Hyperthermia. 33:690–695. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Koemans WJ, van der Kaaij RT, Wassenaar
ECE, Grootscholten C, Boot H, Boerma D, Los M, Imhof O, Schellens
JHM, Rosing H, et al: Systemic exposure of oxaliplatin and
docetaxel in gastric cancer patients with peritonitis
carcinomatosis treated with intraperitoneal hyperthermic
chemotherapy. Eur J Surg Oncol. 47:486–489. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Morgan RJ Jr, Doroshow JH, Synold T, Lim
D, Shibata S, Margolin K, Schwarz R, Leong L, Somlo G, Twardowski
P, et al: Phase I trial of intraperitoneal docetaxel in the
treatment of advanced malignancies primarily confined to the
peritoneal cavity: Dose-limiting toxicity and pharmacokinetics.
Clin Cancer Res. 9:5896–5901. 2003.PubMed/NCBI
|
|
53
|
Marchettini P, Stuart OA, Mohamed F, Yoo D
and Sugarbaker PH: Docetaxel: Pharmacokinetics and tissue levels
after intraperitoneal and intravenous administration in a rat
model. Cancer Chemother Pharmacol. 49:499–503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Miller RP, Tadagavadi RK, Ramesh G and
Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins (Basel).
2:2490–2518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Clarke SJ and Rivory LP: Clinical
pharmacokinetics of docetaxel. Clin Pharmacokinet. 36:99–114. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kurokawa Y, Matsuyama J, Nishikawa K,
Takeno A, Kimura Y, Fujitani K, Kawabata R, Makari Y, Terazawa T,
Kawakami H, et al: Docetaxel plus S-1 versus cisplatin plus S-1 in
unresectable gastric cancer without measurable lesions: A
randomized phase II trial (HERBIS-3). Gastric Cancer. 24:428–434.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alyami MM and Alasmari AA: Exploratory and
confirmatory factor analysis of the arabic medical outcomes
study-social support Survey-6 among Saudi adults. Saudi J Med Med
Sci. 13:197–204. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Derogatis LR, Rickels K and Rock AF: The
SCL-90 and the MMPI: A step in the validation of a new self-report
scale. Br J Psychiatry. 128:280–289. 1976. View Article : Google Scholar : PubMed/NCBI
|