Introduction
In vitro pharmacological and pathological
models serve as indispensable tools in antitumor drug discovery and
development. In vitro tumor cell culture not only enables
the investigation of specific tumor cell behaviors such as growth
state, metastasis, differentiation, signal transduction, gene
expression and other key processes (1) but also offers the advantages of high
efficiency and cost-effectiveness. In comparison, tumor animal
models have limitations that restrict their application, including
high costs, lengthy experimental duration, low controllability,
high vulnerability to internal and external environmental
variables, species-specific differences that compromise accurate
prediction of human drug responses and the inability to construct
all human tumor models (2).
Collectively, these attributes establish the in vitro method
as a foundational platform for antitumor drug development and
screening (3). Cell culture models
can generally be categorized into two main categories:
Two-dimensional (2D) and three-dimensional (3D) cell models. As
research on cell culture models progresses, several limitations of
2D cell models have been revealed, including low cell viability,
susceptibility to cell morphology damage and the absence of actual
tissue structure (4). This
structural deficiency restricts cell-cell and cell-extracellular
matrix (ECM) interactions, thus failing to mimic the in vivo
tumor microenvironment. Therefore, 2D models prove inadequate for
accurately replicating the actual growth characteristics of tumors
in vivo (5). By supporting
cell proliferation and interactions within a 3D space, 3D cell
models facilitate the 3D structural formation of cell populations
(6). This system better simulates
and replicates in vivo cell biology, resulting in enhanced
culture efficiency, increased yields of cytokines, antibodies and
other biomolecules, and improved overall cell proliferation
(7). Current 3D models for studying
tumor cells can be classified into two principal categories based
on scaffold utilization: Scaffold and scaffold-free models. To
systematically evaluate these culture modalities, Table I provides a comprehensive framework
that delineates their respective technical boundaries and
translational potential, thereby guiding researchers in selecting
the most appropriate model for specific research objectives. Among
these, scaffold-based models have emerged as a central platform in
cutting-edge translational applications such as 3D bioprinting and
organ-on-a-chip technologies, due to the marked diversity and
tunability of their biomaterial components (8–10).
Therefore, the present review first outlines fundamental research
progress on scaffold-based models, followed by an examination of
the core mechanisms underlying scaffold-free systems. Building upon
this foundation, the present review integrates and discusses the
potential of various in vitro 3D models for drug-screening
and clinical applications. Lastly, the present review offers a
forward-looking perspective on bridging the gap between model
systems and clinical practice in the future.
 | Table I.Scaffold-based vs. scaffold-free
culture methods. |
Table I.
Scaffold-based vs. scaffold-free
culture methods.
| A, Scaffold
model |
|---|
|
|---|
| First author,
year | Subtype | Description | Advantages | Limitations |
Applications/examples | (Refs.) |
|---|
| Alafnan, 2022 | Solid porous
scaffolds | Polymers (for
example, PEGDA) with porous structures for cell support and drug
delivery | Simple fabrication,
high permeability and uniform density | Limited dynamic
microenvironment simulation | Gemcitabine-loaded
microneedles for inflammatory breast cancer | (14) |
| Fu, 2024 | Hydrogel
scaffolds | Natural/artificial
hydrogels (for example, fibrin and agarose) mimicking ECM
properties | High
biocompatibility, nutrient diffusion and structural
flexibility | Variable nutrient
transport depending on material composition | Breast tumor drug
resistance studies | (23) |
| Kim, 2019 | Non-hydrogel
scaffolds | Natural materials
(collagen and chitosan) for controlled drug release | Biodegradable and
supports sustained/controlled drug delivery | Lower mechanical
strength compared with synthetic scaffolds | Glioblastoma drug
resistance screening | (33) |
| Liu, 2021 | Fibrous
scaffolds | Silk/chitosan-based
scaffolds with web-like structures for cell adhesion | High surface area
and promotes gas/nutrient exchange | Complex fabrication
and high cost | Photothermal
therapy for post-surgical tumor ablation | (34) |
| Xu, 2023 | Microfluidic
chips | Nano/microchannels
for cell culture and analysis | Precise fluid
control, mimics vascular dynamics and high-throughput
screening | High manufacturing
complexity | CTC isolation with
90% purity | (40) |
| Hen and Jv,
2023 | Microsphere
scaffolds | Spherical carriers
(for example, alginate) loaded with cytokines/nutrients | Adjustable porosity
and enhances mass transfer | Challenges in
uniformity control | HepG2 spheroid
formation for drug testing | (44) |
| Liu, 2023 | 3D bioprinting | Customized
scaffolds using biocompatible materials | High precision and
tailored mechanical properties | High cost and
technical complexity | 3D-printed
microfluidics concentration gradient chip and a paper chip | (46) |
|
| B, Scaffold-free
models |
|
| First author,
year | Subtype |
Description |
Advantages |
Limitations |
Applications/examples | (Refs.) |
|
| Safari, 2021 | Hanging drop
culture | Cells aggregate in
liquid droplets via surface tension | Simple and limited
equipment is required | Limited droplet
volume (<50 µl) and inappropriate for large-scale use | Prostate cancer
co-culture model | (55) |
| Wang, 2019 | Spheroid
culture | Cells self-assemble
into 3D spheres in non-adherent conditions | Retains stemness
and mimics tumor heterogeneity | Variable
sphere-forming efficiency across cell types | Pancreatic CSC
enrichment | (61) |
| Kumar, 2024 | Rotary cell
culture | Simulates
microgravity via rotational motion | Low shear stress
and promotes tissue-like structures | Equipment-dependent
and parameter optimization required | Pancreatic cancer-β
cell interaction studies | (64) |
| Du, 2022 | Ultra-low
adsorption | Cells form
spheroids on non-adhesive surfaces | Easy operation and
no specialized tools | High cost of
culture plates | Effect of umbilical
cord mesenchymal stem cell supernatant on esophageal squamous cell
carcinoma spheres | (69) |
| Calamak, 2020 | Bioreactor
culture | Controlled
environment (temperature and fluid dynamics) for large-scale
culture | High
reproducibility and scalable | Complex setup and
maintenance | HCT-116 colorectal
cancer cells in a peristaltic continuous flow bioreactor to
simulate physiological hemodynamics | (71) |
| Jaganathan,
2014 | Magnetic
suspension | Magnetic
nanoparticles guide 3D cell assembly | Precise spatial
control and scaffold-free | Requires magnetic
particle integration | Magnetic suspension
coculture system for breast cancer cells and fibroblasts | (78) |
| Zhang, 2020 | Agarose
coating | Agarose gel
prevents cell adhesion and promotes spheroid formation | Non-toxic and
simple protocol | Lacks cell-matrix
interaction | HepG2
spheroid-based drug screening | (80) |
Scaffold models
Scaffolds are the most extensively used materials in
3D cell culture, acting as an artificial matrix that replicates the
complex 3D structure and key characteristics of living tissues. The
primary function of scaffolds encompasses providing structural
support for cells and serving as a medium for the diffusion of
soluble factors, thereby facilitating key cellular processes such
as adhesion, migration, proliferation, differentiation and
long-term survival, which collectively lead to the formation of
tissues and organs (11). The
various types of scaffolds utilized in 3D cell culture include
solid porous scaffolds, hydrogel scaffolds, non-hydrogel scaffolds,
fibrous scaffolds, microfluidic chips, microsphere scaffolds and 3D
printing techniques. Advances in enabling technologies, such as 3D
printing and microfluidic chips, are rapidly expanding the
applications of scaffold-based 3D culture systems, making them
invaluable tools for cancer research, high-throughput drug
screening and clinical translation.
Solid porous scaffolds
Solid porous scaffolds can be prepared using simple
processes (12) with materials
exhibiting good permeability and uniform density (13). Furthermore, high-performance solid
composite materials exist that are suitable in manufacturing
scaffolds that meet the requirements of diverse cell culture
systems.
Alafnan et al (14) fabricated microneedle patches using a
polyethylene glycol diacrylate diphenyl phosphine oxide polymer,
which were then coated with gemcitabine and sodium carboxymethyl
cellulose to establish an in vitro drug delivery system for
inflammatory breast cancer treatment. In a separate study, Bai
et al (15) created a
stimulus-responsive scaffold by incorporating graphene oxide (GO)
into a copolymer of polyacrylium-g-polylactic acid. When integrated
with polycaprolactone (PCL) and gambogic acid (GA), this composite
scaffold demonstrated a selective response to tumors and exhibited
notable accumulation of GO/GA in breast tumor cells under acidic
conditions in vitro, with only minor effects on normal cells
at physiological pH. This previous study suggested that
pH-responsive photothermal combination therapy is more effective in
inhibiting tumor growth compared with independent treatments.
Dettin et al (16)
engineered a novel 3D culture scaffold through the conjugation of
hyaluronic acid with ion-complementary self-assembling peptides,
which effectively promoted the proliferation of HCC1569 and
MDA-MB-231 human breast cancer cell lines. This platform was
successfully implemented for evaluating electroporation efficacy,
demonstrating that the 3D scaffold culture system may advance brain
tumor electroporation research compared with conventional 2D
models, providing a reliable platform for the validation of novel
electroporation-based drug delivery protocols (17).
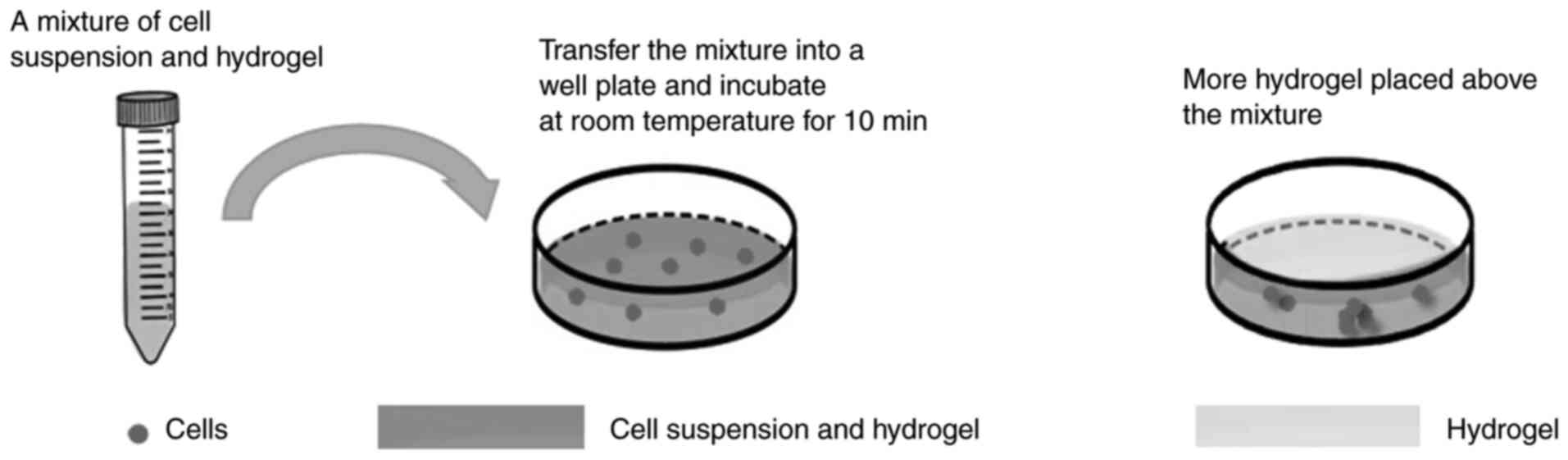
Hydrogel scaffolds
Hydrogel scaffolds are porous 3D structures that
support cell adhesion, proliferation and migration. As shown in
Fig. 1, the process of hydrogel
scaffold-based 3D cell culture involves mixing the cell suspension
with hydrogel followed by incubation for in vitro 3D
cultivation. In tumor cell culture, naturally derived hydrogel
scaffolds sourced from the ECM have demonstrated notable support,
plasticity and biocompatibility (18,19),
whereas hydrogels derived from artificial tissue culture have also
been extensively implemented (18).
The selection of appropriate scaffold materials is key to 3D
culture and materials optimal for cell viability, proliferation,
observation, detection and other pertinent aspects should be used
(20). Prompted by these
considerations, researchers have developed a series of 3D hydrogel
scaffold materials through systematic comparison of diverse culture
methodologies, which are now extensively employed in 3D cultivation
of tumor cells (11,21). Hydrogels exhibit physical properties
resembling human tissues, including softness, elasticity and
permeability, which enable them to provide structural support for
cells, facilitate nutrient diffusion and assist in metabolite
transport. However, hydrogel scaffolds have certain limitations;
for example, variations in material composition may impede nutrient
influx and distribution, adversely affecting cell culture (22). Commonly used gel-forming materials
include fibrin, agarose, polyethylene and ethylene glycol. Fu et
al (23) developed a
fiber-hydrogel composite scaffold loaded with platelet-rich plasma,
which combined efficient material transport with enhanced cell
adhesion and exhibited an elastic modulus similar to that of breast
tumor tissue, thereby promoting cell aggregation and reducing
resistance to chemotherapeutic agents. Therefore, this composite
scaffold provides a valuable platform for in vitro oncology
research and antitumor drug efficacy prediction. Quazi et al
(24) constructed a polypeptide
drug delivery carrier using stepwise functionalization of a
nano-DNA hydrogel scaffold, which was loaded with a
cell-penetrating anticancer peptide via electrostatic adsorption to
achieve light-triggered drug delivery. This delivery system
demonstrated safety, specificity and high efficiency, establishing
an ideal platform for anticancer peptide administration and
offering a promising strategy for future cancer treatment. By
integrating a microfluidic system with a soft hydrogel, Jiang et
al (25) established a 3D in
vitro model mimicking the in vivo lung gland
microenvironment. Analysis of cancer cell morphology, proliferation
and invasion within this model provided key insights into the
invasive mechanisms of in vivo lung cancer cells.
Engineering of PCL into nano-porous PCL (NP-PCL)
yields an emerging biomaterial characterized by an extensive
nanoporous network structure, which provides chondrocytes with an
improved growth environment and markedly enlarged adsorption
surface area. Furthermore, NP-PCL exhibits notable mechanical
properties and closely mimics the structural characteristics of
native bone, thereby markedly enhancing chondrocyte adhesion and
proliferation (26).
Non-hydrogel scaffolds
Natural scaffolds, fabricated from biological
substances including collagen, chitosan, polysaccharides, seaweed
salts, ECM components and other materials (27), exhibit high biocompatibility and
biodegradability. However, their structural strength is typically
inferior to that of synthetic alternatives. The development of
novel drug delivery systems has facilitated the engineering of
chitosan-based targeted formulations for sustained and controlled
release, and targeted delivery, which offer notable advantages for
enhanced drug absorption, improved bioavailability and reduced
adverse effects. A key example is folic acid-modified
nanoparticles, synthesized by conjugating folic acid onto chitosan
nanoparticles (28). Upon loading
with chemotherapeutic agents, such as paclitaxel and fluorouracil
(29), they can be internalized
into tumor cells via endocytosis, thereby enabling the recognition
and elimination of an extensive range of cancer cells (30). Paclitaxel demonstrates marked
efficacy in the treatment of colon cancer. It exerts its
therapeutic effect by interfering with microtubule dynamics, which
are essential for cancer cell division, thereby inhibiting cell
proliferation and ultimately inducing apoptosis (31).
Chaicharoenaudomrung et al (32) demonstrated that human glioblastoma
cells cultured in 3D calcium-alginate scaffolds exhibited reduced
proliferation, enhanced tumor sphere formation, upregulation of
stemness-associated genes (CD133, Sox2, Nestin and Musashi-1) and
increased expression of differentiation-related markers (glial
fibrillary acidic protein and β-tubulin III) compared with those
under 2D conditions. Further investigation into the cellular
response to anticancer agents, including doxorubicin and
cordycepin, revealed that cells in the 3D scaffold system developed
markedly stronger drug resistance. These findings support the
utility of 3D calcium-alginate cultures as a valuable platform for
anticancer drug-screening and resistance mechanism analysis. In a
related study, Kim et al (33) employed 3D matrix scaffolds to
culture two bladder cancer cell lines, SBT31A and T24, both
expressing cyclin D1b mRNA, and evaluated the antitumor effects of
cyclin D1b knockdown via small interfering RNA. Their results
indicated that suppression of cyclin D1b expression promoted
apoptosis, attenuated cancer stemness and epithelial-mesenchymal
transition (EMT) and therefore, suppressed malignant phenotypes in
bladder cancer cells.
Fibrous scaffolds
Fibrous scaffold materials offer notable advantages
due to their fibrillar architecture, which provides extensive
surface area for cell attachment, proliferation and
differentiation. These scaffolds feature a dense, spiderweb-like
pore network within a pliable 3D structure, facilitating nutrient
exchange and gas transport. They are primarily categorized into
natural fiber scaffolds (for example, silk fibroin and chitosan)
and synthetic fiber scaffolds (for example, PCL).
Liu et al (34) constructed a core-shell fibrous
scaffold by uniformly coating 3D-printed alginate-gelatin scaffolds
first with PCL and subsequently with polydopamine to impart a
pronounced photothermal effect, thereby achieving on-demand drug
release triggered by near-infrared irradiation. The released
doxorubicin, in combination with photothermal therapy, demonstrated
effective liver cancer suppression and ablation both in
vitro and in vivo. This scaffold presents a promising
strategy for localized tumor therapy and tissue regeneration,
particularly in patients with cancer post-surgery. It can be
implanted at the resection site to eliminate residual or recurrent
cancer cells while promoting the repair of surgically induced
tissue defects.
Microfluidic chips
Microfluidic chips are technological platforms
fabricated by etching microscale to even nanoscale channels and
analytical detection units on substrates such as glass and silicon
(35). Functioning as a miniature
technological system, these devices enable diverse applications
including microscale sample analysis, cell culture and signal
transduction (36). Their internal
architecture resembles a miniaturized chemical laboratory,
incorporating fluidic channels, reaction platforms, analytical
detection modules and separation components, with dimensional
characteristics comparable to those of human capillaries (37). This technology demonstrates multiple
advantages, including precise fluid flow control, minimized reagent
consumption, enhanced detection efficiency and reduced processing
times, while simultaneously enabling comprehensive simulation of
in vivo microenvironments and supporting 3D tumor cell
culture (38).
Microfluidics technology demonstrates unique
advantages in modulating the physicochemical properties that govern
drug release behavior. A previous study by Matsuura-Sawada et
al (39) revealed that both
lipid concentration and flow rate ratio markedly influenced
liposome structure and drug release profiles. When the lipid
concentration was maintained at ≥50 mmol/l with a flow rate ratio
of 3, multilamellar liposomes were predominantly formed. The
barrier effect conferred by their bilayer lipid membranes markedly
reduced the drug release rate, resulting in a cumulative release of
<58% over 72 h. By contrast, under conditions of low lipid
concentration (10 mmol/l) or a high flow rate ratio (flow rate
ratio=9), unilamellar liposomes became the dominant structure,
leading to a comparatively faster drug release. By flexibly
adjusting these two key parameters, liposomes with varying
structures can be engineered and their proportions controlled,
thereby enabling precise regulation of drug release kinetics. Xu
et al (40) developed an
integrated microfluidics chip for online labeling, separation and
enrichment of rare circulating tumor cells (CTCs) from blood
samples, followed by analysis via inductively coupled plasma mass
spectrometry (ICP-MS). Using HepG2 cells as a model, the team
combined single-cell ICP-MS to quantitatively analyze
asialoglycoprotein receptors on individual cells.
Lanthanide-labeled anti-asialoglycoprotein monoclonal antibodies
and anti-epithelial cell adhesion molecule-modified magnetic beads
were prepared as signal probes and magnetic probes, respectively,
enabling specific cellular recognition. Using the application of
targeted magnetic separation techniques for aggregation and sorting
within a designated separation zone, both the average cell recovery
rate and purity of HepG2 cells were observed to be notably high.
This methodology was thus established as a viable strategy for the
absolute quantification of asialoglycoprotein on individual liver
cancer cells, providing an efficient analytical platform to
investigate targeted drug delivery in cancer therapeutics.
Microsphere scaffolds
Microsphere scaffolds, characterized by spherical
structures with particle sizes ranging between 10 and 100 µm,
contain cytokines and nutrients key to stimulating cell
proliferation and division (41).
By adjusting preparation parameters or incorporating bioactive
components such as hepatic decellularized matrix (42), the mass transfer efficiency of these
scaffolds can be markedly enhanced, thereby further mimicking the
in vivo microenvironment, and promoting the proliferation
and invasion of tumor cells.
Qiu et al (43) achieved efficient separation of
CTC-like particles using an acoustic fluidic chip coupling system,
utilizing carboxylic acid-functionalized polystyrene microspheres
encapsulating aminated mesoporous acoustic particles to simulate
CTCs. Hen et al (44)
prepared uniformly sized methacrylated alginate microspheres via
microfluidic technology combined with online UV-induced
crosslinking and subsequently fabricated large-pore microspheres
using freeze-drying techniques. Evaluation using HepG2 cells as a
model system demonstrated that these microspheres supported cell
aggregation into spheroids with viability >85% and promoted
cellular proliferation, indicating their suitability as 3D cell
culture scaffolds. The results confirmed that these scaffolds
sustained anticancer drug release and effectively suppressed cancer
cell proliferation in vitro, suggesting their potential
applications in cancer research and drug screening in the
future.
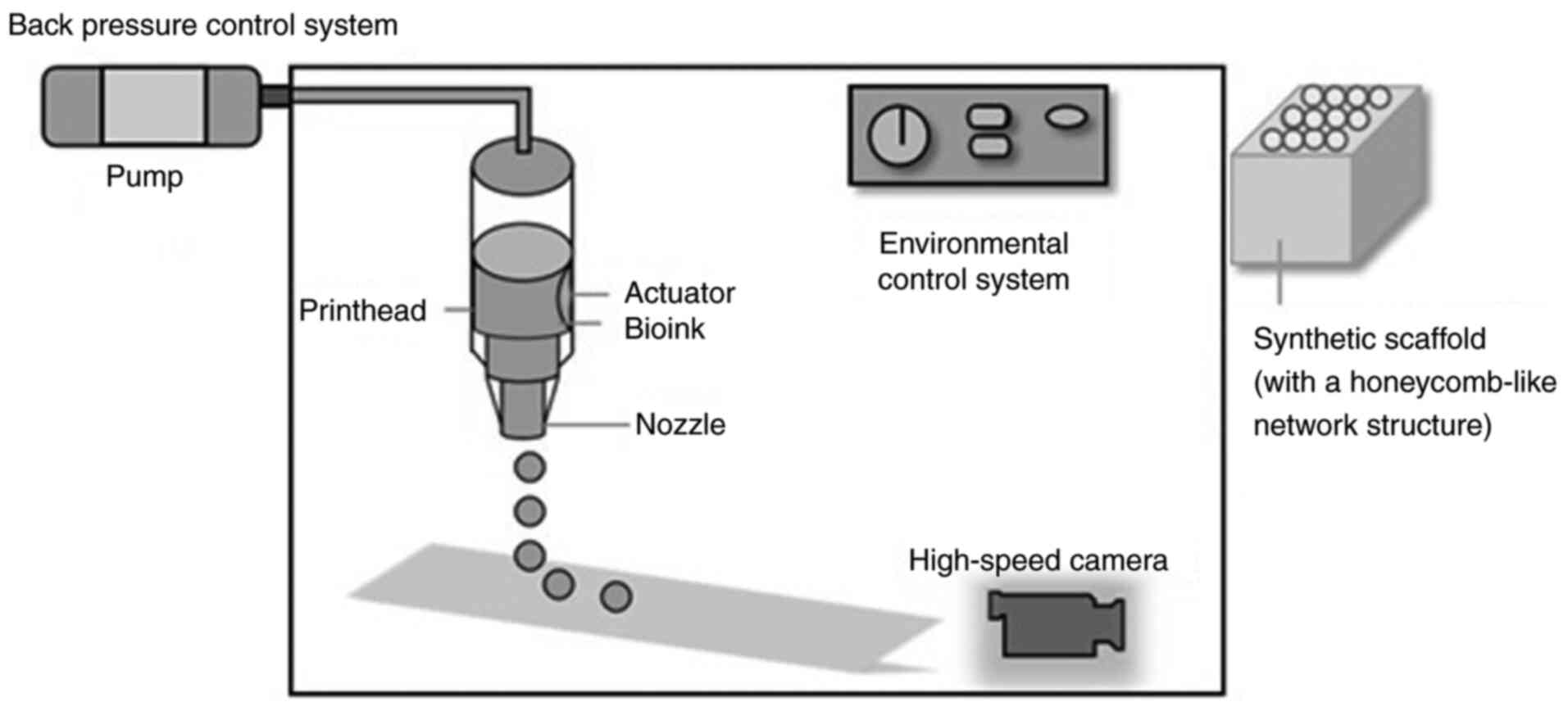
3D bioprinting
3D bioprinting is an advanced manufacturing
technique that fabricates solid structures through the integration
of computer-aided design, systematic analysis of target scaffold
characteristics (including material properties, geometry and
dimensions) and programmed processing procedures. Fig. 2 illustrates the control system
components for 3D bioprinting, including the pump, printhead,
actuator, bioink, nozzle, environmental control system and
high-speed camera, along with the resulting honeycomb-like scaffold
model produced after printing. The integration of 3D bioprinting
with alternative scaffold materials for tumor cell culture and
viability assessment offers distinct advantages, particularly in
achieving enhanced mechanical strength and toughness along with
demonstrated non-cytotoxic properties. However, this special
culture method imposes strict requirements on both computers and
printers, and its high cost combined with these equipment demands
currently limits its application (45).
Liu et al (46) developed an integrated platform
comprising a 3D-printed microfluidics concentration gradient chip
and a paper chip to investigate the effects of hydrogen sulfide on
tumor cells and intracellular signaling molecules, demonstrating
that sustained exposure to low concentrations effectively induced
apoptosis and suppressed proliferation in malignant cells. Jin
et al (47) constructed a
concentric cylindrical tetra-culture model containing gallbladder
carcinoma (GBC) and endothelial cells, fibroblasts and macrophages
using 3D bioprinting technology, and validated the model
characteristics by combining hematoxylin and eosin staining,
immunofluorescence labeling and single-cell RNA sequencing. Using
comparative transcriptomics analysis, this model was reported to
effectively recapitulate key features of the tumor microenvironment
and heterogeneity, induce more aggressive tumor cell phenotypes and
provide a high-fidelity platform for GBC biological studies and
antitumor drug development.
Scaffold-free culture
Scaffold-free cell culture approaches fundamentally
promote the self-assembly of tumor cells into 3D spheroid-like
aggregates through autonomous cellular processes (48). Established methodologies encompass
hanging drop cultures, spheroid formation techniques, the rotary
cell culture system (RCCS), ultra-low adsorption cell culture,
bioreactor cultures, magnetic suspension culture and agarose
coating protocols (49). Cellular
models derived from these platforms demonstrate advantages of
reduced experimental costs, operational simplicity and suitability
for industrial production (50),
rendering them particularly suitable for high-throughput
drug-screening applications. However, its extensive implementation
faces challenges including prolonged culture durations, high
equipment investment and limited imaging penetration, which makes
it difficult to ensure the uniformity of experimental results
(51).
Hanging drop culture
The hanging drop method involves depositing cell
suspension onto the bottom surface of a culture dish or plate,
forming hanging drops via liquid surface tension. The vessel is
then inverted, enabling cells to aggregate at the bottom of the
drops under gravity through intercellular adhesion, thereby forming
3D structures (52). While this
technique offers notable advantages in generating homogeneous
spheres with controllable size and shape (53), it presents technical challenges for
subsequent drug treatment procedures and morphological analysis,
thereby limiting its practical applicability for large-scale
cultivation.
Rodoplu et al (54) demonstrated a notable increase in
both the percentage area and total length of tumor
angiogenesis-associated blood vessels following a 6-day co-culture
of embryonic bodies and tumor spheroids on a microfluidic
hanging-drop platform, which establishes this methodology as a
straightforward and efficient approach for generating co-cultured
cell spheroids. Safari et al (55) employed the hanging drop technique to
establish a 3D co-culture model of prostate cancer cells, revealing
that conditioned medium derived from human amniotic mesenchymal
stromal cells exhibited potent anticancer activity, a finding that
provides compelling evidence supporting the therapeutic potential
of stem cell-based strategies in suppressing prostate cancer
progression.
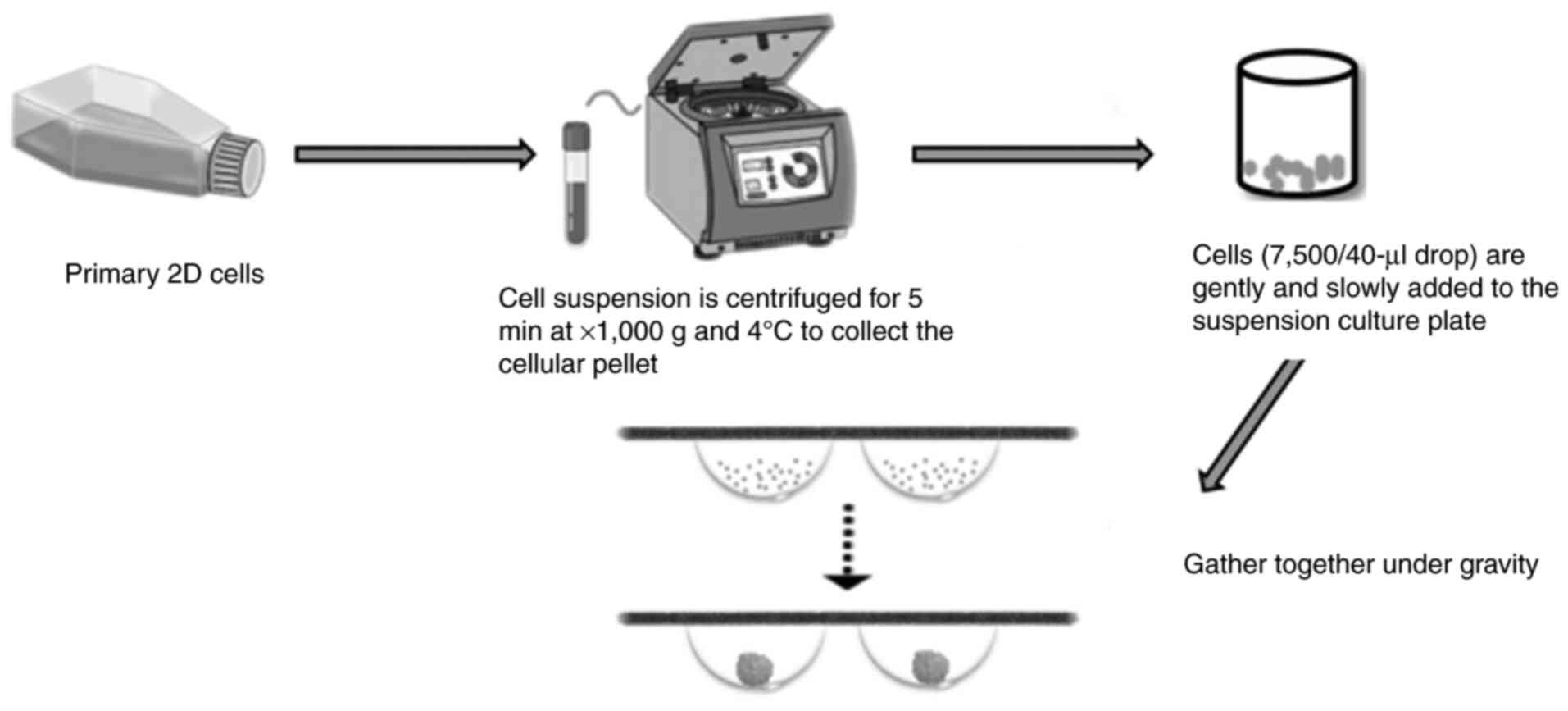
Spheroid culture
The spheroid culture method refers to a technique
wherein cells aggregate to form clump-like cellular assemblages
that subsequently develop into spherical structures through 3D
cultivation (56). Fig. 3 illustrates the process of forming
3D cell spheroids: 2D cultured cells are first grown to the
logarithmic phase, then centrifuged. The harvested cells are
diluted to create a cell suspension, which is uniformly dispensed
into multi-well plates. Under gravity, the cells aggregate to form
3D spheroids. For cancer stem cells (CSCs), their sphere-forming
capacity serves as a key diagnostic parameter in evaluating the
self-renewal potential of individual cells under specific growth
conditions (57). While tumor cells
such as glioma and breast carcinoma cells demonstrate relatively
high sphere-forming rates, epithelial-derived tumor cells including
hepatocellular and colorectal carcinoma cells tend to exhibit
comparatively lower sphere-forming rates (58). Therefore, when investigating the
growth potential of these tumor cell types, culture conditions
should prevent adherent growth while allowing tumor cells to form
spherical aggregates in specialized media. While this method is
relatively low-cost, the thickness of the formed spheroids and
their inherent light scattering/absorption properties markedly
affect imaging quality, thus requiring more sophisticated optical
instruments and advanced analytical algorithms to achieve accurate
data interpretation.
Xue et al (59) cultivated spheroids in specialized
culture plates and demonstrated that silica stimulation induced an
anti-apoptotic phenotype in myofibroblasts through activation of
the nuclear factor erythroid 2-related factor 2/Bax pathway. Sun
et al (60) conducted
comparative assessment of 22 liver injury-positive and 5 liver
injury-negative compounds using lung cancer cells cultured in
spheroid or 2D systems, revealing that the spheroid culture
markedly enhanced model sensitivity in detecting compound
cytotoxicity compared with conventional 2D culture. Wang et
al (61) demonstrated that
pancreatic CSCs enriched via spheroid culture methods exhibited
higher co-expression levels of stemness-related genes CD24 and CD44
compared with conventional 2D cell culture systems, thus
establishing spheroid culture as a suitable platform in maintaining
pancreatic CSCs under in vitro conditions. Raggi et
al (62) employed spheroid
culture to enrich stem-like subpopulations in human intrahepatic
cholangiocarcinoma and via extracellular flux analysis using
Seahorse technology coupled with high-resolution respiratory
measurements, the study established that the respiratory phenotype
of cholangiocarcinoma cells in the spheroid culture was markedly
more efficient compared with that in the monolayer culture. The
spheroid culture platform enabled direct microscopic visualization
of morphological characteristics and growth status within
individual wells, while simultaneously facilitating relatively
straightforward environmental control during experimental
manipulations, such as reagent addition and medium replacement,
thereby offering particular advantages for implementation in
small-scale laboratory settings.
RCCS
The RCCS simulates microgravity conditions by
inducing rotational motion of cells, tissues and culture medium in
a state approximating free fall (63), which effectively promotes cellular
proliferation and differentiation while facilitating intercellular
signaling transduction. Operating without propellers or mechanical
agitators, the system generates minimal shear stress that poses
negligible cellular damage, with additionally adjustable rotation
speeds enabling controlled reduction of sedimentation rates as
cellular aggregates develop.
Utilizing a 785 nm semiconductor laser for cellular
stimulation and Raman spectra acquisition, Kumar et al
(64) established a 3D RCCS to
co-culture multiple pancreatic ductal adenocarcinoma (PDAC) cell
lines with MIN6 pancreatic β-cells, while systematically
investigating morphological characteristics and viability using
integrated time-lapse imaging, confocal and scanning electron
microscopy, and immunohistochemical analysis. This methodology
successfully generated a co-culture platform capable of forming 3D
PDAC spheroids and β-cell aggregates (pseudo-islets), with cellular
surface morphology and growth patterns closely recapitulating the
in vivo microenvironment, while further demonstrating the
propensity of PDAC cells to surround and invade the pseudo-islet
structures. Belloni et al (65) cultured bone marrow stromal cells
with tumor cells in a RCCS, revealing that this 3D culture model
effectively recapitulated tumor-mesenchymal transition processes
and provided a physiologically relevant platform for drug-screening
applications.
Ultra-low adsorption cell culture
The ultra-low adsorption cell culture method offers
operational simplicity without requiring additional equipment,
employing only round-bottomed vessels coated with inert,
non-adhesive surfaces to prevent cellular attachment to vessel
walls, thereby promoting cell aggregation and adhesion into 3D
spheroids. However, the high cost of these specialized culture
dishes or plates restricts their widespread implementation
(66).
Using ultra-low adsorption plates, Malhão et
al (67) generated
multicellular aggregates from four breast cell lines and
characterized them using morphometric analysis, qualitative
cytology and quantitative immunohistochemistry, revealing that
while each cell line formed homogeneous multicellular aggregates,
distinct structural heterogeneity existed between different lines.
In another experimental study (68), the team employed this ultra-low
adsorption approach to cultivate 3D spheroids, observing compact
structural integrity, robust cellular viability and straightforward
procedural implementation. In a separate study, Du et al
(69) demonstrated that culture
supernatants from umbilical cord mesenchymal stem cells markedly
enhanced spheroid formation in esophageal squamous cell carcinoma
cultures prepared using ultra-low adsorption plates.
Bioreactor culture
Bioreactors represent specialized cultivation
systems engineered to accommodate specific culture methodologies,
with key parameters, including temperature, humidity, pressure,
nutrient supply, CO2 concentration, and physical or
chemical stimuli, mimicking in vivo conditions with high
fidelity. This configuration enables more precise regulation and
enhanced controllability compared with alternative culture
environments while facilitating streamlined tumor cell cultivation,
thereby finding extensive applications in biomedical research
(70).
Calamak et al (71) cultured HCT-116 colorectal cancer
cells in a peristaltic continuous flow bioreactor to simulate
physiological hemodynamics, and demonstrated that this system
induces reprogramming of cancer cells toward a mesenchymal niche
while accurately replicating circulatory conditions. These findings
established that hemodynamic forces alter membrane composition and
morphological characteristics in malignant cells, providing notable
insights in the development of novel cancer therapeutics. Huo et
al (72) investigated a 3D
perfusion bioreactor that maintained neuroblastoma tissue
architecture and cellular matrix integrity for 7 days, enabling
continuous drug response monitoring using isothermal
microcalorimetry. This platform additionally incorporated 56
metabolic assessment methods with rapid detection and high
sensitivity to advance personalized treatment for neuroblastoma.
Bober et al (73) designed a
bioreactor-based 3D culture system combining non-invasive proton
magnetic resonance (MR) relaxation time measurements at 1.5 Tesla
with immunohistochemical analysis to evaluate trastuzumab delivery
efficiency in breast cancer cells (CRL2314) vs. normal controls
(HTB-125). The results revealed notably reduced relaxation times in
both treated and untreated CRL2314 cells compared with HTB-125
cells, validating MR relaxation time analysis as an effective
approach in assessing drug responses and cellular viability in 3D
culture models.
Magnetic suspension culture
Magnetic suspension culture employs a hydrogel
medium composed of bacteriophages and magnetic iron oxide particles
to establish 3D cultures, enabling spatial control over cellular
aggregates through magnetic manipulation to achieve multicellular
organization in coculture systems (74). Nevertheless, this technique presents
several limitations: The magnetic beads are costly, potentially
cytotoxic at elevated concentrations and enable limited aggregate
yield (75). By integrating
quantitative mass spectrometry-based proteomics with magnetic
suspension culture, Vu et al (76) characterized proteomic alterations in
squamous cell carcinoma cells, demonstrating that the absence of
xenogenic protein scaffolds permits integrated analysis of cells
with their endogenous ECM. Magnetic suspension has thus been
demonstrated as a valuable methodology in elucidating proteomic
dynamics underlying 3D tissue architecture. Qin et al
(77) identified circadian rhythms
in tumor cells maintained in suspension culture, advancing drug
delivery strategies through stage-specific pharmacological
interventions. Jaganathan et al (78) developed a magnetic suspension
coculture system for breast cancer cells and fibroblasts,
quantifying tumor size and cellular density while comparing
phenotypic characteristics with in vivo tumors and examining
matrix protein composition. Their results confirmed that this
approach can facilitate precise control over tumor cell composition
and density, rapidly generating large-scale breast tumor models
within 24 h that markedly recapitulate the in vivo tumor
microenvironment for antitumor drug evaluation.
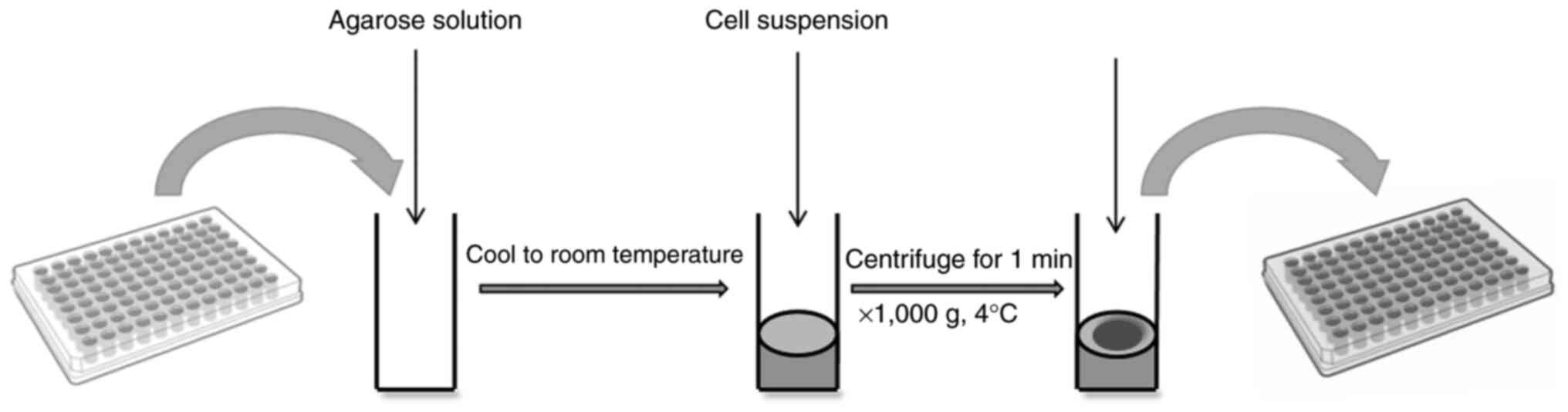
Agarose coating
Agarose, a polysaccharide extracted from marine
algae, dissolves in boiling aqueous solution and solidifies into
non-cytotoxic gels upon cooling. These resultant gels inherently
lack cell adhesion motifs, thereby effectively preventing the
attachment of human and animal cells (79). Fig.
4 illustrates the following procedure: A quantified agarose
solution is dispensed into a 96-well plate, cooled to room
temperature and subsequently supplemented with a quantified cell
suspension for culture. Ultimately, the formation of clustered cell
spheroids is observed.
Zhang et al (80) established a 3D HepG2 cell model
using 96-well flat-bottom plates combined with agarose gel,
observing that well-defined cellular spheroids formed on the
agarose surface when seeding densities were maintained between
1.975×103 and 1×104 cells/well. With
increasing cell inoculation numbers, spheroid volumes expanded
progressively, demonstrating a strong linear association within the
density range of 1.975–6.667×103 cells per well. Chen
et al (81) systematically
investigated the effects of primary components of tea polyphenols
on breast CSCs cultured via the agarose-coating method. Using
integrated experimental approaches including cell migration assays,
scratch tests and cellular repair assessments among other
techniques, the research team established that transcriptional
downregulation of key EMT genes effectively suppressed invasive
phenotypes in breast CSCs, diminished transcriptional activation of
breast cancer marker genes, thus preventing manifestation of
self-renewal characteristics. These findings markedly expand the
potential pharmacological applications of primary tea polyphenol
components in anticancer therapeutic development. Capitalizing on
the unique thermally-responsive properties of agarose that undergo
physical cross-linking at 35–40°C, Gong et al (82) employed agarose as a gelatin
substitute for 3D bioprinting at both 10 and 24°C. Comparative
evaluation revealed that structures fabricated using agarose
pre-gelation methods exhibited notably enhanced dimensional
precision, structural stability and mechanical rigidity compared
with those produced using gelatin pre-gelation approaches.
Application of 3D culture technique for
tumor cells
3D cell culture models have demonstrated marked
potential in both assessing drug safety and efficacy, and modeling
diverse pathological conditions (83). This 3D culture technology serves as
a versatile platform for culturing tumor cells in vitro,
enabling systematic investigation of oncogenesis, metastatic
progression, invasive behavior, recurrence patterns and therapeutic
strategies, while simultaneously facilitating drug discovery and
screening applications (84).
Biological behavior of tumors
3D tumor cell culture technology enables more
accurate simulation of the authentic in vivo growth
environment under in vitro conditions (85), demonstrating unique advantages and
profound implications for investigating diverse tumor biological
behaviors, therapeutic interventions and drug-screening processes
(86). For example,
CD271+ uveal melanoma stem cells may undergo
vasculogenic mimicry in 3D Matrigel culture (87). Another previous study revealed that
nicastrin, a novel type I transmembrane glycoprotein, is associated
with breast cancer stem cell properties, as determined using
Matrigel culture (88).
Tumor angiogenesis
Vascular endothelial growth factor serves as a key
regulator of pathological angiogenesis in tumors, activating
endothelial cells to migrate, develop tip cells and ultimately
anastomose into nascent blood vessels (89). To access the circulatory system,
malignant cells must reside in proximity to the vasculature, due to
their fundamental dependence on oxygen and nutrient supply for
survival and proliferation. This complex process necessitates
coordinated interactions among cells, extracellular matrices and
signaling networks. 3D tumor cell culture systems can generate
neovascular networks that closely mirror native vascular
architecture, thereby establishing an advanced platform for
investigating tumor migration and invasion mechanisms in
vitro (90). Methods to
vascularize various tissue/organ types using several synthetic and
naturally occurring biomaterials have already been established. For
example, Lazzari et al (91)
constructed a poly-HEMA-based 3D tumor model by co-culturing
PANC-1, MRC-5 and human umbilical vein endothelial cells to
synthesize vascularized tumor spheroids of pancreatic cancer
cells.
Tumor microenvironment
The tumor microenvironment comprises multiple
cellular components, including endothelial cells, fibroblasts,
pericytes, adipocytes and immune cells, which collectively
contribute to tumor initiation, progression and metastasis
(92). Through dynamic
intercellular interactions, signal transduction and cellular
communication, it provides key support for tumor survival and
growth, while simultaneously modulating key processes such as
immune responses, angiogenesis and drug resistance (93). Furthermore, the tumor
microenvironment facilitates proto-oncogene expression and
tumor-promoting protein production while impairing immune cell
functionality. Due to the bidirectional regulatory interplay
between tumors and their microenvironment, elucidating these
complex mechanisms carries notable importance in understanding
tumor biology, and advancing diagnostic and therapeutic strategies
(94). Amaral et al
(95) employed two different 3D
cell culture techniques, the hanging drop and the forced floating
with ultra-low attachment plates, to form human bladder cancer RT4
spheroids. These models are gaining popularity, given their ability
to reproduce key aspects of the tumor microenvironment, concerning
the 3D tumor architecture, as well as the interactions of tumor
cells with the extracellular matrix and surrounding non-tumor cells
(86).
CSCs
CSCs, which are characterized by self-renewal
capacity, differentiation potential, high tumorigenicity and
enhanced drug resistance, can withstand non-specific treatments
including radiotherapy and chemotherapy, while serving key roles in
tumor initiation, metastatic progression, drug resistance
development and disease recurrence (96). The self-renewal capability and
unlimited proliferative potential of these cells represent
fundamental mechanisms sustaining tumor cell population viability,
and their migratory activity may initiate tumor cell dissemination
(97). When maintained in prolonged
dormant states, CSCs harbor diverse drug-resistant molecules and
demonstrate reduced sensitivity to exogenous physicochemical agents
that typically eliminate tumor cells, such as anthracyclines,
taxanes, anti-metabolites and alkylating agents (98) In recent years, the identification of
novel targets specifically present in CSCs has emerged as a
promising direction in the development of innovative antitumor
therapeutics (99–101). CSCs grown in 3D model assays offer
marked potential in understanding therapy response rates. Such
cells have already been successfully isolated from a 3D model of
human osteosarcoma treated with epirubicin. In this manner, 3D CSC
models have provided novel insights into tumor drug resistance
(102).
Development and screening of antitumor
drugs
The field of anticancer drug development continues
to grow with increasing demand for specifically targeted
therapeutics (103). However,
conventional 2D culture-based drug-screening platforms exhibit
notable limitations, as antineoplastic efficacy observed in
vitro frequently fails to translate to clinical settings
(104). This translational gap
primarily originates from the inherent inability of 2D systems to
replicate key features of the native tumor microenvironment
(105), which serves a key role in
driving tumor progression, metastatic dissemination and drug
resistance mechanisms. Therefore, developing 3D culture models that
more accurately mimic tumor cell interactions with ECM components
is of key importance. Such advanced models provide vital platforms
for precisely evaluating chemotherapeutic performance and cytotoxic
responses, thereby markedly enhancing the predictive capacity of
high-throughput screening for anticancer compounds.
To address this challenge, researchers have
developed a 3D ex vivo tumor model system utilizing the
AXTEX-4D™ platform (106). This
system is characterized by its capacity to generate physiologically
relevant microenvironments through self-assembly of endogenously
secreted matrix components without requiring exogenous scaffolding
materials, thereby spontaneously establishing biochemical gradients
that better approximate in vivo conditions. In
immuno-oncology applications, the platform has demonstrated
efficacy in evaluating therapeutic outcomes through monitoring key
parameters, including immune cell proliferation, migration,
infiltration, cytokine secretion and tumor-specific cytotoxicity
(107). As a highly integrated
physiomimetic system, it serves as a robust platform for
high-throughput screening of immunotherapeutic agents, markedly
enhancing the predictive accuracy and translational relevance of
preclinical drug evaluation (105).
Rosendahl et al (108) established 3D coculture models of
MCF7 and MDA-MB-231 human breast cancer cell lines within
2,2,6,6-tetramethylpiperidine 1-oxyl cellulose nanofibril
(TEMPO-CNF) scaffolds, observing multilayered tumor growth with
distinct morphological patterns, while demonstrating that these
TEMPO-CNF scaffolds upregulated the expression of stem cell marker
CD44 and migration markers Vimentin/SNAI1 in MCF7 cells compared
with 2D culture systems, thereby establishing TEMPO-CNF as a
promising biomaterial in developing 3D culture platforms applicable
to anticancer drug screening.
Significance and prospects
Technical challenges requiring
resolution
Notwithstanding their enhanced physiological
relevance, the broad implementation of 3D models faces constraints
from several key technical hurdles.
First, operational expenses markedly exceed those of
conventional 2D systems, primarily due to dependence on specialized
and costly components such as commercial basement membrane extracts
and sophisticated bioreactor systems designed for extended culture
maintenance and perfusion requirements. Furthermore,
high-resolution imaging of 3D specimens typically necessitates
advanced instrumentation including confocal or light sheet
microscopy, representing considerable additional investment. These
cumulative costs restrict accessibility for resource-constrained
laboratories and thereby reduce implementation scalability in
high-throughput screening campaigns.
Second, reproducibility and standardization issues
present notable obstacles. Batch-to-batch variations in scaffold
materials introduce considerable experimental variables that
compromise result reliability (109), while manual production methods in
scaffold-free models frequently generate spheroids with inadequate
size uniformity that consequently demonstrate elevated data
variability (110). The field
currently lacks unified culture protocols, standardized analytical
criteria and clearly defined efficacy endpoints, thus preventing
direct comparison and integration of experimental data across
different laboratories.
Furthermore, the intrinsic 3D architecture of these
models creates notable challenges for imaging procedures and
subsequent data interpretation. From an optical perspective, light
scattering and absorption phenomena restrict both imaging depth and
resolution, particularly in specimens >200 µm in thickness,
often necessitating specialized methodologies such as tissue
clearing methods or light sheet fluorescence microscopy (111). From an analytical standpoint,
extracting quantitative parameters from 3D images, including
cellular viability, morphological characteristics and spatial
heterogeneity in protein expression, proves markedly more complex
compared with corresponding 2D analyses (112). This process requires sophisticated
computational algorithms and artificial intelligence capabilities
to execute tasks involving 3D cell segmentation, structural
reconstruction and phenotypic characterization (113), thereby demanding enhanced
computational resources and specialized technical expertise.
Future directions and innovation
To address these challenges and facilitate clinical
translation of 3D tumor models, future investigations should
concentrate on the following key domains.
Technological integration and
automation
i) 3D bioprinting. Bioprinting technology serves as
a robust methodology to address standardization challenges by
enabling precise and reproducible deposition of cellular components
and biomaterials. Through the layered assembly of multiple cell
types with gradient biofactors, this approach facilitates the
construction of highly biomimetic and structurally controllable
tumor microenvironment models (114). These advanced systems prove
particularly valuable for investigating complex pathological
processes including tumor invasion and metastatic progression
(114). Long-term perspectives
encompass applications in vascular regeneration and cartilage
repair, with potential clinical translation toward surgical
reconstruction of blood vessels and cartilage tissues, alongside
the development of personalized drug delivery platforms (115).
ii) Organ-on-a-chip platforms. The integration of 3D
tumor models with microfluidics technology enables the development
of organ-on-a-chip systems. These platforms dynamically simulate
physiological parameters including hemodynamic flows, mechanical
stresses and inter-tissue interactions, thereby achieving more
accurate recapitulation of in vivo drug distribution and
metabolic processing. Such technological synergy provides an
innovative framework for enhancing the physiological relevance of
preclinical drug evaluation methodologies (116).
Data integration and intelligent
analysis
i) Multi-omics data integration. Integrating
phenotypic readouts from 3D models with subsequent multi-omics
analyses enables elucidation of underlying molecular mechanisms
(117), where this model-to-omics
strategy establishes a key bridge connecting observed phenotypic
responses with their mechanistic drivers, while facilitating
identification of novel therapeutic targets and predictive
biomarkers.
ii) Artificial intelligence and machine learning.
Targeting artificial intelligence to process complex 3D imaging and
multi-omics datasets allows identification of patterns
imperceptible through conventional analysis (118), enabling automated high-content
phenotypic screening and supporting development of robust
predictive models in evaluating therapeutic efficacy and patient
prognosis.
Defining clinical translation
pathways
Future research endeavors should prioritize
systematic validation of 3D models by rigorously establishing
associations between their predictive accuracy and clinical
outcomes. Advancing standardization of technologies, such as 3D
bioprinting and organ-on-a-chip systems, while integrating them
comprehensively with multi-omics analyses and artificial
intelligence will enable the development of more predictive tumor
models. These advancements will not only improve the fundamental
understanding of cancer biology but also expedite the development
of innovative anticancer therapies, ultimately propelling the field
of precision oncology toward novel frontiers.
Acknowledgements
Not applicable.
Funding
The present review was funded by the Scientific Research Project
of the Department of Education of Liaoning Province (grant no.
LQ2020021).
Availability of data and materials
Not applicable.
Authors' contributions
SD and HL conceptualized and designed the present
review. SD, HW, DX, JY and SM drafted the manuscript, and prepared
the tables and figures. Data authentication is not applicable. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Madrid MF, Mendoza EN, Padilla AL,
Choquenaira-Quispe C, de Jesus Guimarães C, de Melo Pereira JV,
Barros-Nepomuceno FWA, Lopes Dos Santos I, Pessoa C, de Moraes
Filho MO, et al: In vitro models to evaluate multidrug resistance
in cancer cells: Biochemical and morphological techniques and
pharmacological strategies. J Toxicol Environ Health B Crit Rev.
28:1–27. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Faber MN, Sojan JM, Saraiva M, van West P
and Secombes CJ: Development of a 3D spheroid cell culture system
from fish cell lines for in vitro infection studies: Evaluation
with Saprolegnia parasitica. J Fish Dis. 44:701–710. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Langhans SA: Using 3D in vitro cell
culture models in Anti-cancer drug discovery. Expert Opin Drug
Discov. 16:841–850. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu X, Liu X, Zhong H, Zhang W, Yu S and
Guan R: Progress on Three-dimensional cell culture technology and
their application. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi.
40:602–608. 2023.(In Chinese). PubMed/NCBI
|
|
5
|
Sugimoto M, Kitagawa Y, Yamada M, Yajima
Y, Utoh R and Seki M: Micropassage-embedding composite hydrogel
fibers enable quantitative evaluation of cancer cell invasion under
3D coculture conditions. Lab Chip. 18:1378–1387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang T, Zhang KN and Ke CX: Research
progress of three-dimensional cell culture in bladder cancer. J Med
Res. 50:16–18. 2021.
|
|
7
|
Zhang C, Yang Z, Dong DL, Jang TS, Knowles
JC, Kim HW, Jin GZ and Xuan Y: 3D culture technologies of cancer
stem cells: Promising ex vivo tumor models. J Tissue Eng.
11:20417314209334072020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jovanović Stojanov S, Grozdanić M, Ljujić
M, Dragičević S, Dragoj M and Dinić J: Cancer 3D Models: Essential
tools for understanding and overcoming drug resistance. Oncol Res.
33:2741–2785. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lonkwic KM, Zajdel R and Kaczka K:
Unlocking the potential of spheroids in personalized medicine: A
systematic review of seeding methodologies. Int J Mol Sci.
26:64782025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaur N, Savitsky MJ, Scutte A, Mehta R,
Dridi N, Sogbesan T, Savannah A, Lopez D, Yang D and Ali J:
Navigating cancer treatment: A journey from 2D to 3D cancer models
and nanoscale therapies. ACS Pharmacol Transl Sci. 8:3773–3800.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abuwatfa WH, Pitt WG and Husseini GA:
Scaffold-based 3D cell culture models in cancer research. J Biomed
Sci. 31:72024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Kawazoe N and Chen G: Interaction
of immune cells and tumor cells in gold nanorod-gelatin composite
porous scaffolds. Nanomaterials (Basel). 9:13672019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Zhang J, Li J, Chen Y, Kawazoe N
and Chen G: Bifunctional scaffolds for the photothermal therapy of
breast tumor cells and adipose tissue regeneration. J Mater Chem B.
6:7728–7736. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alafnan A, Seetharam AA, Hussain T, Gupta
MS, Rizvi SMD, Moin A, Alamri A, Unnisa A, Awadelkareem AM,
Elkhalifa AO, et al: Development and characterization of PEGDA
microneedles for localized drug delivery of gemcitabine to treat
inflammatory breast cancer. Materials (Basel). 15:76932022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai G, Yuan P, Cai B, Qiu X, Jin R, Liu S,
Li Y and Chen X: Stimuli-responsive scaffold for breast cancer
treatment combining accurate photothermal therapy and adipose
tissue regeneration. Adv Funct Mater. 29:19044012019. View Article : Google Scholar
|
|
16
|
Dettin M, Sieni E, Zamuner A, Marino R,
Sgarbossa P, Lucibello M, Tosi AL, Keller F, Campana LG and Signori
E: A Novel 3D scaffold for cell growth to asses electroporation
efficacy. Cells. 8:14702019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Z, Chen L, Moser MA, Zhang W, Qin Z
and Zhang B: Electroporation-based therapy for brain tumors: A
review. J Biomech Eng. 143:1008022021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lou J and Mooney DJ: Chemical strategies
to engineer hydrogels for cell culture. Nat Rev Chem. 6:726–744.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radulescu DM, Neacsu IA, Grumezescu AM and
Andronescu E: New insights of scaffolds based on hydrogels in
tissue engineering. Polymers (Basel). 14:7992022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Ye X, Zhao Y, Bai L, He Z, Tong Q,
Xie X, Zhu H, Cai D, Zhou Y, et al: Cryogenic 3D printing of porous
scaffolds for in situ delivery of 2D black phosphorus nanosheets,
doxorubicin hydrochloride and osteogenic peptide for treating tumor
resection-induced bone defects. Biofabrication. 12:0350042020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang X, Wang Y, Mao T, Wang Y, Liu R, Yu L
and Ding J: An oxygen-enriched thermosensitive hydrogel for the
relief of a hypoxic tumor microenvironment and enhancement of
radiotherapy. Biomater Sci. 9:7471–7482. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang A, Bai Y, Dong X, Ma T, Zhu D, Mei L
and Lv F: Hydrogel/nanoadjuvant-mediated combined cell vaccines for
cancer immunotherapy. Acta Biomater. 133:257–267. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu SJ, Liu XX, Hu MB, Li CJ, Wang FJ and
Wang L: Preparation and characterization of fiber-hydrogel
composite scaffolds for three-dimensional culture of breast
tumorcells. J Donghua Univ (Nat Sci). 50:11–19. 2024.
|
|
24
|
Quazi MZ and Park N: DNA Hydrogel-based
nanocomplexes with Cancer-targeted delivery and Light-triggered
peptide drug release for Cancer-specific therapeutics.
Biomacromolecules. 24:2127–2137. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang R, Huang J, Sun X, Chu X, Wang F,
Zhou J, Fan Q and Pang L: Construction of in vitro 3-D model for
lung cancer-cell metastasis study. BMC Cancer. 22:4382022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao J, Li J, Du G and Zhao JM: Effect of
nano-microporous polycaprolactone combined with bone marrow
mesenchymal stem cells on cartilage injury in rabbits. Guangdong
Med J. 35:3307–3309. 2014.
|
|
27
|
Mahendiran B, Muthusamy S, Selvakumar R,
Rajeswaran N, Sampath S, Jaisankar SN and Krishnakumar GS:
Decellularized natural 3D cellulose scaffold derived from Borassus
flabellifer (Linn.) as extracellular matrix for tissue engineering
applications. Carbohydr Polym. 272:1184942021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nemati M, Bani F, Sepasi T, Zamiri RE,
Rasmi Y, Kahroba H, Rahbarghazi R, Sadeghi MR, Wang Y, Zarebkohan A
and Gao H: Unraveling the effect of breast cancer patients' plasma
on the targeting ability of folic acid-modified chitosan
nanoparticles. Mol Pharm. 18:4341–4353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian XH: Preparation of pH-responsive
Chitosan Anticancer Drug Carriers and Their Application. Jinzhou
Medical University; 2019
|
|
30
|
De Araújo JT, Tavares Junior AG, Di
Filippo LD, Duarte JL, Ribeiro TD and Chorilli M: Overview of
chitosan-based nanosystems for prostate cancer therapy. Eur Polymer
J. 160:1108122021. View Article : Google Scholar
|
|
31
|
Pham DT, Saelim N and Tiyaboonchai W:
Paclitaxel loaded EDC-crosslinked fibroin nanoparticles: A
potential approach for colon cancer treatment. Drug Deliv Transl
Res. 10:413–424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chaicharoenaudomrung N, Kunhorm P,
Promjantuek W, Heebkaew N, Rujanapun N and Noisa P: Fabrication of
3D calcium-alginate scaffolds for human glioblastoma modeling and
anticancer drug response evaluation. J Cell Physiol.
234:20085–20097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim CJ, Terado T, Tambe Y, Mukaisho KI,
Sugihara H, Kawauchi A and Inoue H: Anti-oncogenic activities of
cyclin D1b siRNA on human bladder cancer cells via induction of
apoptosis and suppression of cancer cell stemness and invasiveness.
Int J Oncol. 52:231–240. 2018.PubMed/NCBI
|
|
34
|
Liu C, Wang Z, Wei X, Chen B and Luo Y: 3D
printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered
drug release for cancer therapy and wound healing. Acta Biomater.
131:314–325. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niculescu AG, Chircov C, Bîrcă AC and
Grumezescu AM: Fabrication and applications of microfluidic
devices: A review. Int J Mol Sci. 22:20112021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Modena MM, Chawla K, Misun PM and
Hierlemann A: Smart cell culture systems: Integration of sensors
and actuators into microphysiological systems. ACS Chem Biol.
13:1767–1784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parihar A and Mehta PP: Lab-on-a-chip
devices for advanced biomedicines: Laboratory scale engineering to
clinical ecosystem. Royal Society of Chemistry; 2024, View Article : Google Scholar
|
|
38
|
Hakim M, Khorasheh F, Alemzadeh I and
Vossoughi M: A new insight to deformability correlation of
circulating tumor cells with metastatic behavior by application of
a new deformability-based microfluidic chip. Anal Chim Acta.
1186:3391152021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuura-Sawada Y, Maeki M, Uno S, Wada K
and Tokeshi M: Controlling lamellarity and physicochemical
properties of liposomes prepared using a microfluidic device.
Biomater Sci. 11:2419–2426. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu Y, Chen B, He M, Cui Z and Hu B:
All-in-One microfluidic chip for online labeling, separating, and
focusing rare circulating tumor cells from blood samples followed
by inductively coupled plasma mass spectrometry detection. Anal
Chem. 95:14061–14067. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu YH, Chen S, Zhang CF, Ikoma T, Guo HM,
Zhang XY, Li X and Chen W: Novel microsphere-packing synthesis,
microstructure, formation mechanism and in vitro biocompatibility
of porous gelatin/hydroxyapatite microsphere scaffolds. Ceramics
Int. 47:32187–32194. 2021. View Article : Google Scholar
|
|
42
|
Allu I, Sahi AK, Koppadi M, Gundu S and
Sionkowska A: Decellularization techniques for tissue engineering:
Towards replicating native extracellular matrix architecture in
liver regeneration. J Funct Biomater. 14:5182023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qiu H, Wang H, Yang X and Huo F: High
performance isolation of circulating tumor cells by acoustofluidic
chip coupled with ultrasonic concentrated energy transducer.
Colloids Surf B Biointerfaces. 222:1131382023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hen SK and Jv XJ: Preparation of open
macroporous alginate microspheres for Three-dimensional cell
culture. Polymer Materials Sci Engine. 39:126–133. 2023.
|
|
45
|
Song JQ, Chen HL and Yang FW: Research and
Application of 3D Bioprinting Technology in Medical Field. Chin Med
Equipment. 36:151–165. 2021.
|
|
46
|
Liu P: Construction and Application of
3D-printed microfluidic chip cell analysis platform. Shandong
Normal University; 2023
|
|
47
|
Jin Y, Zhang J, Xing J, Li Y, Yang H,
Ouyang L, Fang ZY, Sun LJ, Jin B, et al: Multicellular 3D
bioprinted human gallbladder carcinoma forin vitromimicry of tumor
microenvironment and intratumoral heterogeneity. Biofabrication.
16:0450282024. View Article : Google Scholar
|
|
48
|
Mai P, Hampl J, Baca M, Brauer D, Singh S,
Weise F, Borowiec J, Schmidt A, Küstner JM, Klett M, et al:
MatriGrid® based biological morphologies: Tools for 3D
cell culturing. Bioengineering (Basel). 9:2202022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jubelin C, Muñoz-Garcia J, Griscom L,
Cochonneau D, Ollivier E, Heymann MF, Vallette FM, Oliver L and
Heymann D: Three-dimensional in vitro culture models in oncology
research. Cell Biosci. 12:1552022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ayvaz I, Sunay D, Sariyar E, Erdal E and
Karagonlar ZF: Three-dimensional cell culture models of
hepatocellular carcinoma-a review. J Gastrointest Cancer.
52:1294–1308. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
De Pieri A, Rochev Y and Zeugolis DI:
Scaffold-free cell-based tissue engineering therapies: Advances,
shortfalls and forecast. NPJ Regen Med. 6:182021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Czaplinska D, Elingaard-Larsen LO, Rolver
MG, Severin M and Pedersen SF: 3D multicellular models to study the
regulation and roles of acid-base transporters in breast cancer.
Biochem Soc Trans. 47:1689–1700. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kelm JM, Timmins NE, Brown CJ, Fussenegger
M and Nielsen LK: Method for generation of homogeneous
multicellular tumor spheroids applicable to a wide variety of cell
types. Biotechnol Bioeng. 83:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rodoplu D, Matahum JS and Hsu CH: A
microfluidic hanging drop-based spheroid co-culture platform for
probing tumor angiogenesis. Lab Chip. 22:1275–1285. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Safari F, Shakery T and Sayadamin N:
Evaluating the effect of secretome of human amniotic mesenchymal
stromal cells on apoptosis induction and epithelial-mesenchymal
transition inhibition in LNCaP prostate cancer cells based on 2D
and 3D cell culture models. Cell Biochem Funct. 39:813–820. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Heinrich MA, Huynh NT and Heinrich Prakash
J: Understanding glioblastoma stromal barriers against NK cell
attack using tri-culture 3D spheroid model. Heliyon. 10:e248082024.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bahmad HF, Cheaito K, Chalhoub RM, Hadadeh
O, Monzer A, Ballout F, EL-Hajj A, Mukherji D, Liu YN, Daoud G and
Abou-Kheir W: Sphere-formation assay: Three-dimensional in vitro
culturing of prostate cancer stem/progenitor sphere-forming cells.
Front Oncol. 8:3472018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ferreira SM: Effect of normal and tumor
extracellular matrix in cancer stem Cell-Like properties.
Universidade do Porto (Portugal); 2019
|
|
59
|
Xue W, Wang J, Hou Y, Wu D, Wang H, Jia Q,
Jiang Q, Wang Y, Song C, Wang Y, et al: Lung decellularized
matrix-derived 3D spheroids: Exploring silicosis through the impact
of the Nrf2/Bax pathway on myofibroblast dynamics. Heliyon.
10:e335852024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun B, Liang Z, Wang Y, Yu Y, Zhou X, Geng
X and Li B: A 3D spheroid model of quadruple cell co-culture with
improved liver functions for hepatotoxicity prediction. Toxicology.
505:1538292024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang J, Kong Y and Deng FS: Enrichment of
pancreatic cancer stem cells by sphere-forming culture. Anhui Med
J. 40:1303–1305. 2019.
|
|
62
|
Raggi C, Taddei ML, Sacco E, Navari N,
Correnti M, Piombanti B, Pastore M, Campani C, Pranzini E, Iorio J,
et al: Mitochondrial oxidative metabolism contributes to a cancer
stem cell phenotype in cholangiocarcinoma. J Hepatol. 74:1373–1385.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Topal U and Zamur C: Microgravity, stem
cells, and cancer: A new hope for cancer treatment. Stem Cells Int.
2021:55668722021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kumar V, Sethi B, Staller DW, Shrestha P
and Mahato RI: Gemcitabine elaidate and ONC201 combination therapy
for inhibiting pancreatic cancer in a KRAS mutated syngeneic mouse
model. Cell Death Discov. 10:1582024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Belloni D, Ferrarini M, Ferrero E,
Guzzeloni V, Barbaglio F, Ghia P and Scielzo C: Protocol for
generation of 3D bone marrow surrogate microenvironments in a
rotary cell culture system. STAR Protoc. 3:1016012022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Naser Al Deen N, Atallah Lanman N,
Chittiboyina S, Lelièvre S, Nasr R, Nassar F, Dohna H, AbouHaidar M
and Talhouk R: A risk progression breast epithelial 3D culture
model reveals Cx43/hsa_circ_0077755/miR-182 as a biomarker axis for
heightened risk of breast cancer initiation. Sci Rep. 11:26262021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Malhão F, Macedo AC, Ramos AA and Rocha E:
Morphometrical, morphological, and immunocytochemical
characterization of a tool for cytotoxicity research: 3D cultures
of breast cell lines grown in Ultra-low attachment plates. Toxics.
10:4152022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang JY, Liu D, Li L, Li DH and Wang HF:
Effect of propofol on pyroptosis of lung cancer A549 cells by
NLRP3/ASC/caspase-1 pathway. J China Med Univ. 53:132–141.
2024.
|
|
69
|
Du M, Liu ZJ, Zou F, Cai HH, Li JY and
Zhou BMJ: Effect of umbilical cord mesenchymal stem cell
supernatant on esophageal squamous cell carcinoma spheres. Chin J
Gastroenterol Hepatol. 31:634–637. 2022.
|
|
70
|
Liu B, Han S, Modarres-Sadeghi Y and Lynch
ME: Multiphysics simulation of a compression-perfusion combined
bioreactor to predict the mechanical microenvironment during bone
metastatic breast cancer loading experiments. Biotechnol Bioeng.
118:1779–1792. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Calamak S, Ermis M, Sun H, Islam S, Sikora
M, Nguyen M, Hasirci V and Steinmetz LM: A circulating bioreactor
reprograms cancer cells toward a more mesenchymal niche. Adv
Biosyst. 4:e19001392020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huo Z, Bilang R, Supuran CT, von der Weid
N, Bruder E, Holland-Cunz S, Martin I, Muraro MG and Gros SJ:
Perfusion-based bioreactor culture and isothermal microcalorimetry
for preclinical drug testing with the carbonic anhydrase inhibitor
SLC-0111 in Patient-derived neuroblastoma. Int J Mol Sci.
23:31282022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bober Z, Podgórski R, Aebisher D, Cieślar
G, Kawczyk-Krupka A and Bartusik-Aebisher D: Cellular 1H MR
relaxation times in healthy and cancer Three-dimensional (3D)
breast cell culture. Int J Mol Sci. 24:47352023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Anil-Inevi M, Yilmaz E, Sarigil O, Tekin
HC and Ozcivici E: Single cell densitometry and weightlessness
culture of mesenchymal stem cells using magnetic levitation.
Methods Mol Biol. 2125:15–25. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hoarau-Vechot J, Rafii A, Touboul C and
Pasquier J: Halfway between 2D and animal models: Are 3D cultures
the ideal tool to study cancer-microenvironment interactions? Int J
Mol Sci. 19:1812018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Vu B, Souza GR and Dengjel J:
Scaffold-free 3D cell culture of primary skin fibroblasts induces
profound changes of the matrisome. Matrix Biol Plus. 11:1000662021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Qin WJ, Pan MP, Su ZJ, Hou RR, Lu HJ and
Le ZC: Biological clock synchronization of cancer cells basedw on
suspension culturedand its application in the study of timing drug
administration. J Xiamen Univ (Nat Sci). 138–143. 2021.
|
|
78
|
Jaganathan H, Gage J, Leonard F,
Srinivasan S, Souza GR, Dave B and Godin B: Three-dimensional in
vitro co-culture model of breast tumor using magnetic levitation.
Sci Rep. 4:64682014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
He YY, Li LJ, Zhang SQ, Li YZ, Yang S and
Ji P: Method of constructing cell spheroids based on agarose and
polyacrylic molds. Chin J Tissue Eng Res. 26:553–559. 2022.(In
Chinese).
|
|
80
|
Zhang J, Fan X, Li M, Dou MJ and Wang HF:
Establishment of 3-dimensional culture model of HepG2 cells and
viability detection. Central South Pharmacy. 18:1111–1114.
2020.
|
|
81
|
Chen L, Li KM, Ma HJ, Wang F, Zeng SX and
Song XQ: The inhibitory effect of EGCG on breast cancer stem cell
migration and Self-renewal properties in 3D culture system. J
Xinyang Normal Univ Nat Sci Ed. 32:437–442. 2019.
|
|
82
|
Gong C, Kong Z and Wang X: The effect of
agarose on 3D bioprinting. Polymers (Basel). 13:40282021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang H, Brown PC, Chow ECY, Ewart L,
Ferguson SS, Fitzpatrick S, Freedman BS, Guo GL, Hedrich W, Heyward
S, et al: 3D cell culture models: Drug pharmacokinetics, safety
assessment, and regulatory consideration. Clin Transl Sci.
14:1659–1680. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zuo DQ and Cai ZD: Progress in
three-dimensional cell culture and its application in tumor
research. Tumor. 38:502–507. 2023.
|
|
85
|
Rauner G, Gupta PB and Kuperwasser C: From
2D to 3D and beyond: The evolution and impact of in vitro tumor
models in cancer research. Nat Methods. 22:1776–1787. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Barbosa MA, Xavier CP, Pereira RF,
Petrikaitė V and Vasconcelos MH: 3D cell culture models as
recapitulators of the tumor microenvironment for the screening of
Anti-cancer drugs. Cancers (Basel). 14:1902021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Valyi-Nagy K, Kormos B, Ali M, Shukla D
and Valyi-Nagy T: Stem cell marker CD271 is expressed by
vasculogenic mimicry-forming uveal melanoma cells in
three-dimensional cultures. Mol Vis. 18:588–592. 2012.PubMed/NCBI
|
|
88
|
Lombardo Y, Filipović A, Molyneux G,
Periyasamy M, Giamas G, Hu Y, Trivedi PS, Wang J, Yagüe E, Michel L
and Coombes RC: Nicastrin regulates breast cancer stem cell
properties and tumor growth in vitro and in vivo. Proc Natl Acad
Sci USA. 109:16558–16563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Eelen G, Treps L, Li X and Carmeliet P:
Basic and therapeutic aspects of angiogenesis updated. Circ Res.
127:310–329. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lazzari G, Nicolas V, Matsusaki M, Akashi
M, Couvreur P and Mura S: Multicellular spheroid based on a triple
co-culture: A novel 3D model to mimic pancreatic tumor complexity.
Acta Biomater. 78:296–307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kumari S, Advani D, Sharma S, Ambasta RK
and Kumar P: Combinatorial therapy in tumor microenvironment: Where
do we stand? Biochim Biophys Acta Rev Cancer. 1876:1885852021.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gao QD, Duan XF, Li Y, Xu T, Yu YY and Bai
GD: Research on the role of resveratrol against breast cancer.
China Pharmacy. 35:1408–1412. 2024.
|
|
94
|
Ren X, Zhang L, Zhang Y, Li Z, Siemers N
and Zhang Z: Insights gained from Single-Cell analysis of immune
cells i91n the tumor microenvironment. Annu Rev Immunol. 9:583–609.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Amaral RLF, Miranda M, Marcato PD and
Swiech K: Comparative analysis of 3D bladder tumor spheroids
obtained by forced floating and hanging drop methods for drug
screening. Front Physiol. 8:6052017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang W, Zhao K, Zhang K, Chen X, Hao M
and Yang Z: Advances in the biological characteristics and stem
maintenance mechanisms of tumor stem cells. Chin J Biotech.
40:391–418. 2024.
|
|
97
|
Li XY, Yang X and Huang M: Advances in
cancer therapeutic targets-cancer stem cells. J Logistics Univ PAP
(Med Sci). 3:65–70. 2021.
|
|
98
|
Zhang LS, Shi HY, Zhang T, Wang J, Mao H
and Wang XL: Research progress of biomarkers of endometrial cancer
stemc cells. Med Recapitulate. 934–939. 2022.
|
|
99
|
Lee H, Kim B, Park J, Park S, Yoo G, Yum
S, Kang W, Lee JM, Youn H and Youn B: Cancer stem cells: Landscape,
challenges and emerging therapeutic innovations. Signal Transduct
Target Ther. 10:2482025. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Guo S, Zheng S, Liu M and Wang G: Novel
anti-cancer stem cell Compounds: A Comprehensive review.
Pharmaceutics. 16:10242024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li YR, Fang Y, Lyu Z, Zhu Y and Yang L:
Exploring the dynamic interplay between cancer stem cells and the
tumor microenvironment: implications for novel therapeutic
strategies. J Transl Med. 21:6862023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kimlin LC, Casagrande G and Virador VM: In
vitro three-dimensional (3D) models in cancer research: An update.
Mol Carcinog. 52:167–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chunduri V and Maddi S: Role of in vitro
two-dimensional (2D) and three-dimensional (3D) cell culture
systems for ADME-Tox screening in drug discovery and development: A
comprehensive review. ADMET DMPK. 11:1–32. 2023.PubMed/NCBI
|
|
104
|
Crouigneau R, Li YF, Auxillos J,
Goncalves-Alves E, Marie R, Sandelin A and Pedersen SF: Mimicking
and analyzing the tumor microenvironment. Cell Reports Methods.
4:1008662024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Honkala A, Malhotra SV, Kummar S and
Junttila MR: Harnessing the predictive power of preclinical models
for oncology drug development. Nat Rev Drug Discov. 21:99–114.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Baru A, Mazumder S, Kundu P, Sharma S,
Purakayastha BPD, Khan S, Gupta R and Arora NM: Establishment of a
three-dimensional triculture model on the novel AXTEX-4D™ platform.
Oncol Rep. 49:22023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Goenka A, Khan F, Verma B, Sinha P, Dmello
CC, Jogalekar MP, Gangadaran P and Ahn BC: Tumor microenvironment
signaling and therapeutics in cancer progression. Cancer Commun
(Lond). 43:525–561. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Rosendahl J, Svanström A, Berglin M,
Petronis S, Bogestål Y, Stenlund P, Standoft S, Ståhlberg A,
Landberg G, Chinga-Carrasco G and Håkansson J: 3D printed
nanocellulose scaffolds as a cancer cell culture model system.
Bioengineering (Basel). 8:972021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rafieyan S, Ansari E and
Vasheghani-Farahani E: A practical machine learning approach for
predicting the quality of 3D (bio) printed scaffolds.
Biofabrication. 162024.doi: 10.1088/1758-5090/ad6374. PubMed/NCBI
|
|
110
|
Anthon SG and Valente KPL: Vascularization
strategies in 3D cell culture models: From scaffold-free models to
3D bioprinting. Int J Mol Sci. 23:145822022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yoon S, Cheon SY, Park S, Lee D, Lee Y,
Han S, Kim M and Koo H: Recent advances in optical imaging through
deep tissue: Imaging probes and techniques. Biomater Res.
26:572022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Xu X, Su J, Zhu R, Li K, Zhao X, Fan J and
Mao F: From morphology to single-cell molecules: High-resolution 3D
histology in biomedicine. Mol Cancer. 24:632025. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ali M, Benfante V, Basirinia G, Alongi P,
Sperandeo A, Quattrocchi A, Giannone AG, Cabibi D and Yezzi A:
Applications of artificial intelligence, deep learning, and machine
learning to support the analysis of microscopic images of cells and
tissues. J Imaging. 11:592025. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li Y and Cui H and Cui H: Precision
spatial control of Tumor-stroma interactions in cancer models via
3D bioprinting for advanced research and therapy. Adv Funct
Materials. 35:25033912025. View Article : Google Scholar
|
|
115
|
Hamilton C, Collins V, Butala S, Lee K,
Panse N, Pierce A, Borole A, Gupta S, Rahimi S, Truong H and
Beckerman W: Current trends and outlook of 3D printing in vascular
surgery. JVS-Vascular Insights. 2:1001142024. View Article : Google Scholar
|
|
116
|
Li Z, Hui J, Yang P and Mao H:
Microfluidic organ-on-a-chip system for disease modeling and drug
development. Biosensors. 12:3702022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Nevedomskaya E and Haendler B: From omics
to multi-omics approaches for in-depth analysis of the molecular
mechanisms of prostate cancer. Int J Mol Sci. 23:62812022.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yetgin A: Revolutionizing multi-omics
analysis with artificial intelligence and data processing.
Quantitative Biol. 13:e700022025. View Article : Google Scholar
|