Introduction
Hyperthermia has been employed in cancer treatment for over 5,000 years. The earliest recorded use of heat therapy for tumors is found in the Edwin Smith Egyptian papyrus scroll, which described the treatment of breast tumors through hyperthermia (1,2). The method of heating tumor tissue (41.5–43°C) remains a critical therapeutic approach. In addition to radiotherapy, chemotherapy and surgery, hyperthermia has been used for decades in Europe, the USA and Asia. Its application has improved both local control and overall survival (OS), provided opportunities for surgery in previously inoperable tumors and enabled lower-dose treatments for relapsed cancer that were previously treated with high-dose radiotherapy, all without increasing toxicity (3). Randomized clinical trials (4–6) have demonstrated that combined therapy effectively prolongs disease-free survival and ensures local tumor control without added toxicity. Consequently, hyperthermia offers a versatile therapeutic option in cancer treatment (7). For instance, Yi et al (8) elucidated the mechanisms by which hyperthermia combined with anticancer drugs or natural products enhances antitumor efficacy.
Hyperthermia (41–47°C) can directly induce tumor cell death, alter the tumor microenvironment, stimulate heat shock proteins, activate immune responses, trigger apoptotic cascades, enhance therapeutic efficacy when combined with other treatments and modulate cell cycle regulation signaling pathways (1,8).
Radiation therapy targets visible tumor lesions by disrupting the DNA structure, delivering either a single high dose or multiple low doses over several weeks to eliminate most proliferating cancer cells while preserving normal tissue (7,8). Co-treatment with hyperthermia enhances radiotherapy effectiveness, especially when standard or increased radiation doses cannot be applied. Righini et al (9) primarily elaborates on the synergistic effects of the combination of hyperthermia and radiotherapy, as well as the phenomena of thermal shock and thermotolerance; however, it lacks clinical trial data to validate these concepts.
The distinct effects of hyperthermia have been widely documented, with numerous studies indicating its positive therapeutic outcomes in cancer treatment (10–15). The combined use of hyperthermia and radiotherapy in tumor treatment is biologically well-supported, particularly for common types of cancer such as head and neck, esophageal, lung, breast and cervical cancer, as well as malignant melanoma (16). Ijff et al (17) systematically elaborated the biological principles of hyperthermia therapy, including patient diagnostic-therapeutic workflows and evaluated its clinical efficacy as a radiosensitizer for cervical cancer treatment. The present review incorporated additional tumor types (such as breast, prostate and bladder cancer) and detailed the advantages of combined thermoradiotherapy (TRT) through clinical data. Zhu et al (18) reviewed the current status and development of hyperthermia, outlined potential mechanisms by which hyperthermia inhibits tumors, described clinical trial attempts combining hyperthermia with radiotherapy, chemotherapy and immunotherapy, and discussed the relationship between nanoparticles (NPs) and hyperthermia. The present review incorporated more clinical trial data to analyze the efficacy and toxicity of combined radiotherapy and hyperthermia, provided a deeper elaboration on the current limitations of hyperthermia and offered unique insights, particularly regarding modulated electro-hyperthermia. Clinical endpoints typically include response rates, local control, disease-free survival without increased toxicity and delayed side effects. Local or topical hyperthermia serves as an effective radiosensitizer for both radiotherapy and chemotherapy (19). When applied appropriately, hyperthermia and radiotherapy can synergistically enhance local tumor control. However, achieving optimal multimodal treatment requires more detailed data regarding dosing, sequencing and therapy duration, alongside further exploration of potential synergisms between these modalities.
Hyperthermia techniques can be categorized into three types based on treatment scope: i) Localized methods utilizing microwaves or ultrasound; ii) regional approaches such as intra-abdominal chemotherapy; and iii) whole-body thermal interventions. Temperature parameters further distinguish these techniques, including fever-range temperatures (39–40°C), moderate heating to induce cellular stress (41–43°C) and high-intensity thermal ablation >43°C for cell destruction. Recent innovations have introduced novel thermal modalities, such as nanomagnetic thermal treatments, modulated electro-hyperthermia (mEHT) and light-activated photothermal systems (20,21). These emerging technologies broaden therapeutic possibilities while ensuring precise temperature control across various biological targets. The present review synthesized the molecular mechanisms underlying hyperthermia-induced radiosensitization, examined recent clinical trial data, addressed ongoing translational challenges, particularly limitations in spatiotemporal thermal control and the need for ±0.5°C thermometric accuracy, and explored emerging strategies to overcome dosimetric and technical barriers.
Molecular and biological rationale of hyperthermia as a radiosensitizer
Direct cytotoxic effects of hyperthermia
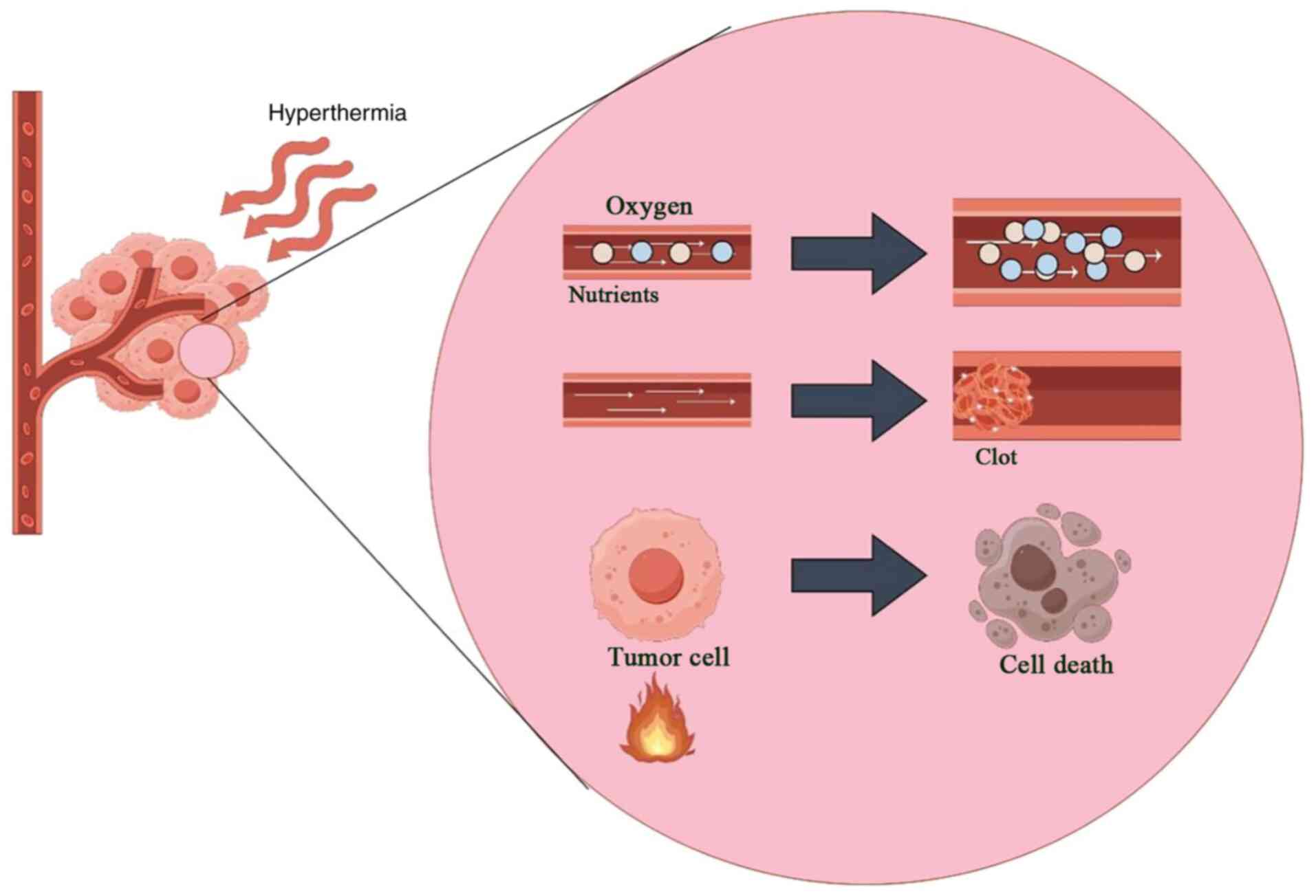
Studies on exponentially proliferating cells indicate that the heat dose, defined by treatment duration and temperature, is a crucial factor in determining the extent of cell death and systemic effects. Temperatures between 41–47°C can directly kill cells both within and outside the body (22–24). The primary physiological response to hyperthermia is alterations in blood flow, oxygen supply and nutrient distribution at the tumor site. Mild hyperthermic temperatures (42°C) increase blood flow and reduce hypoxia, while higher temperatures lead to blood vessel coagulation, intensifying hypoxia and directly causing tumor cell death (Fig. 1) (25). Physiological changes are evident at clinically achievable tumor temperatures (39–42°C) (26). At these lower temperatures (39–42°C), hyperthermia selectively destroys tumor cells in hypoxic and acidic regions of solid tumors, sparing normal tissue (27,28). Hyperthermia is particularly toxic to cells in acidic environments (29), and increased blood flow helps clear acidic metabolites, restore normal extracellular pH and improve the efficacy of combination therapies with radiotherapy (30).
Solid tumors develop perfusion-compromised regions due to oxygen diffusion limitations and reperfusion insufficiencies, leading to pathological hypoxia, hypoxic acidosis, nutrient deprivation (such as glucose deficiency) and micronutrient scarcity. These metabolically stressed cellular subpopulations exhibit 2 to 3-fold greater radioresistance than normoxic cells. The degree of hypoxia in solid tumors correlates with poor prognosis across various tumor types, including cancer of the breast, rectum and pancreas (28,31). Clinical studies show that targeted hyperthermia enhances tumor blood flow and increases perfusion by modulating temperature. After targeted hyperthermia, significant physiological changes in tumor blood circulation occur, including increased vascular permeability, enhanced oxygenation, reduced interstitial fluid pressure and normalization of pH. These changes can influence tumor areas sensitive to radiation therapy. Although local hyperthermia induces rapid physiological effects, such as changes in perfusion, oxygenation and blood flow, these effects can indirectly enhance radiosensitization through reoxygenation (30,32).
Survival curve analysis reveals distinct cellular responses to hyperthermia exposure. Initial heating phases cause linear growth inhibition, progressing to accelerated cell death over time. Experimental data suggest that exponential cell mortality requires specific thermal thresholds, correlating with observable protein structural changes in laboratory settings. This process is likely linked to heat-induced alterations in cytoplasmic and nuclear proteins, although direct correlations with heat combined with radiation or chemotherapy remain unverified (33–35). Localized thermal treatments improve vascular activity in tumors with immature blood vessels. Enhanced circulation increases oxygen levels, thereby enhancing radiation sensitivity, as documented in several studies (36–41). However, the complex interplay between temperature elevation, blood flow dynamics and oxygenation makes precise clinical predictions challenging, despite the use of advanced modeling techniques (36,42).
Effects of hyperthermia on tumor microenvironment
The characteristics of the tumor microenvironment significantly impacts therapeutic outcomes. Malignant tumors function as autonomous organs with unique microenvironments characterized by reduced blood flow and diminished blood vessel density. Within tumor tissues, the disorganized and complex vasculature creates conditions conducive to acidosis, hypoxia and ATP depletion, making cells, particularly in poorly perfused regions, more sensitive to heat (43).
Hyperthermia induces various physiological responses, primarily regulating tumor blood flow and enhancing tumor oxygenation. However, the extent of tumor hemodynamic changes and oxygenation improvements during and after hyperthermia remains variable and not fully understood. Hyperthermia improves tumor oxygenation and blood flow through two key mechanisms: i) In the short term, by inducing vasodilation in both the upstream normal tissue and tumor; and ii) in the long term, by reducing interstitial fluid pressure, thereby restoring adequate perfusion pressure and activating hypoxia-inducible factor-1α- and VEGF-mediated angiogenesis. These changes not only improve tumor oxygenation but also increase the diffusion rate of oxygen and its dissociation from hemoglobin (44).
The primary mechanism of hyperthermia is immune system activation through danger signals mediated by heat shock proteins, which then regulate the immune status of the tumor microenvironment. The immunomodulatory effects of hyperthermia support its direct anticancer actions and foster a Type I-like tumor microenvironment, characterized by programmed death-ligand 1 upregulation and an enriched population of tumor-infiltrating lymphocytes, thereby enhancing the efficacy of immune checkpoint inhibitors (45). Under heat stress, tumor cells release heat shock protein 70 (HSP70), triggering antitumor immune responses through two mechanisms: Stimulating chemokine secretion within tumor tissues and activating dendritic cell recruitment via TLR4 signaling pathways. As a radio/chemotherapy enhancer, hyperthermia amplifies treatment efficacy through complementary cytotoxic mechanisms (Fig. 2) (2). Emerging evidence positions thermal therapy as a spatially targeted immunomodulatory intervention, demonstrating significant therapeutic potential (46). The therapeutic outcomes of thermal interventions are temperature- and time-dependent, necessitating precise calibration (47).
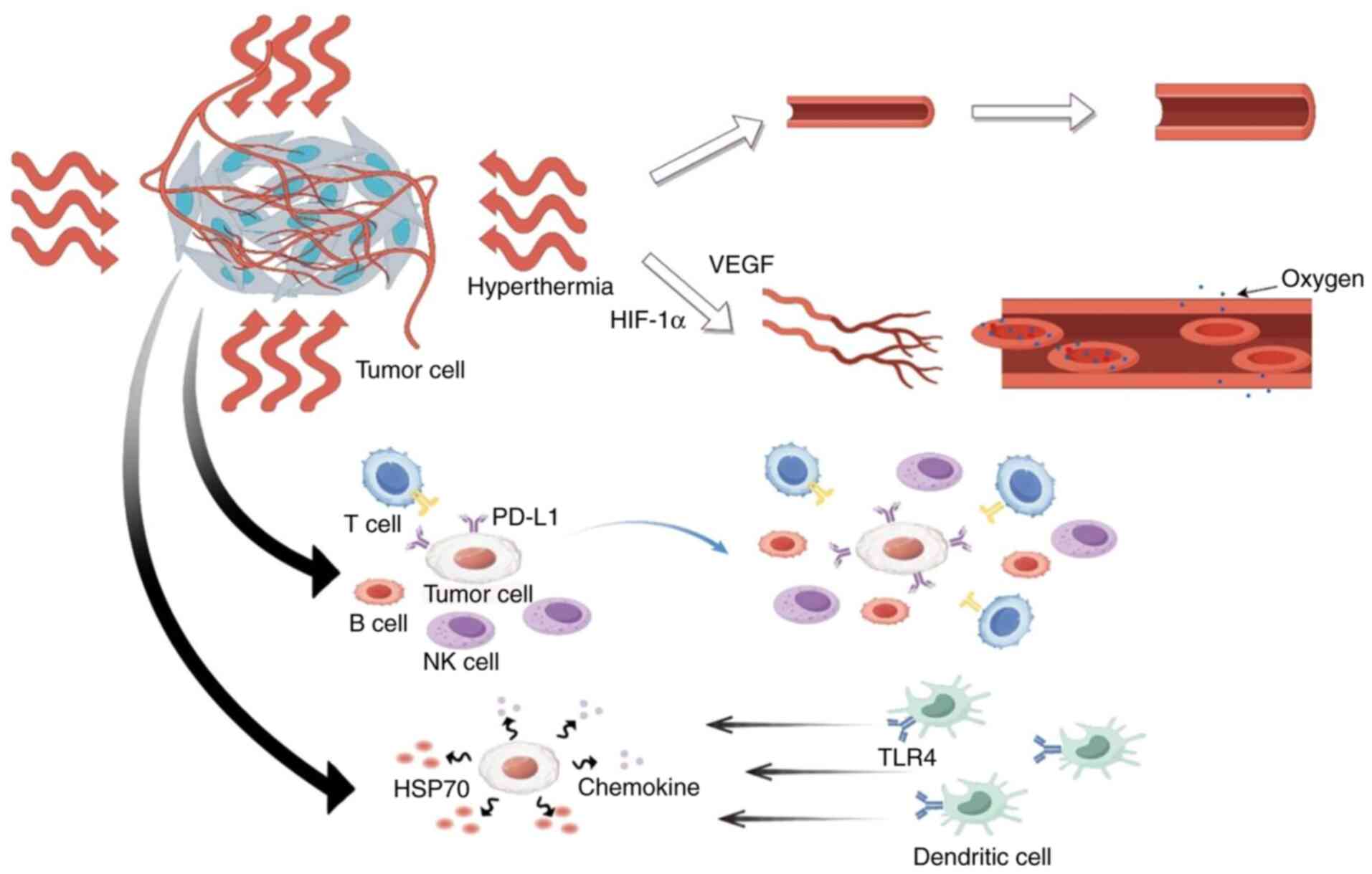 |
Figure 2.
Hyperthermia modulates the tumor microenvironment through vascular and immune mechanisms. The schematic illustrates dual pathways: i) Improved oxygen delivery mediated by HIF-1α/VEGF signaling (upper panel); and ii) immune activation initiated by heat shock protein release, leading to dendritic cell recruitment via TLR4, chemokine secretion and enhanced infiltration of lymphocytes such as T, B and NK cells (lower panel). Concurrently, hyperthermia can induce upregulation of PD-L1 on tumor cells, a hallmark of an adaptive immune response that also defines a targetable axis for combination with checkpoint inhibitors. NK, natural killer; HSP70, heat shock protein 70; Hif-1α, hypoxia-inducible factor 1-α; TLR4, Toll-like receptor 4; PD-L1, programmed death-ligand 1.
|
Recent studies confirm that thermal therapy can modify cancer cell biology and reshape the tumor microenvironment, improving treatment efficacy (48,49). Current clinical protocols (50,51) rarely integrate tumor microenvironment metrics as eligibility criteria. Given the significant inter-patient variability, individual tumor characteristics, such as blood flow patterns, vascular integrity and oxygen saturation, must be considered alongside the patient's thermal response when designing personalized treatment strategies.
Hyperthermia augments effects of radiotherapy
Radiation therapy primarily works by inducing irreparable damage to cellular DNA. It has proven to be an effective cancer treatment, with its therapeutic success largely depending on the tolerance of normal tissues within the radiation field (52). However, some tumor cells exhibit resistance to radiation-induced oxidative stress and DNA damage, triggering cell death via various intracellular pathways. While higher radiation doses are more likely to induce tumor cell death, they also increase the risk of damaging adjacent healthy tissues, leading to associated side effects (53). Therefore, discovering or developing drugs that can enhance tumor cell response without harming normal tissues is crucial. Hyperthermia has been identified as one of the most promising approaches to specifically promote tumor cell death (54).
Impact of heat on the repair of radiation-induced DNA damage
The impact of heat on radiation-induced DNA damage repair may result from a combined mechanism of radiotherapy and hyperthermia (25,55). Heat can directly damage DNA (56) and/or interfere with DNA repair mechanisms (57,58), leading to the accumulation of damaged molecules, which in turn causes cell death and enhances the effectiveness of antitumor treatments.
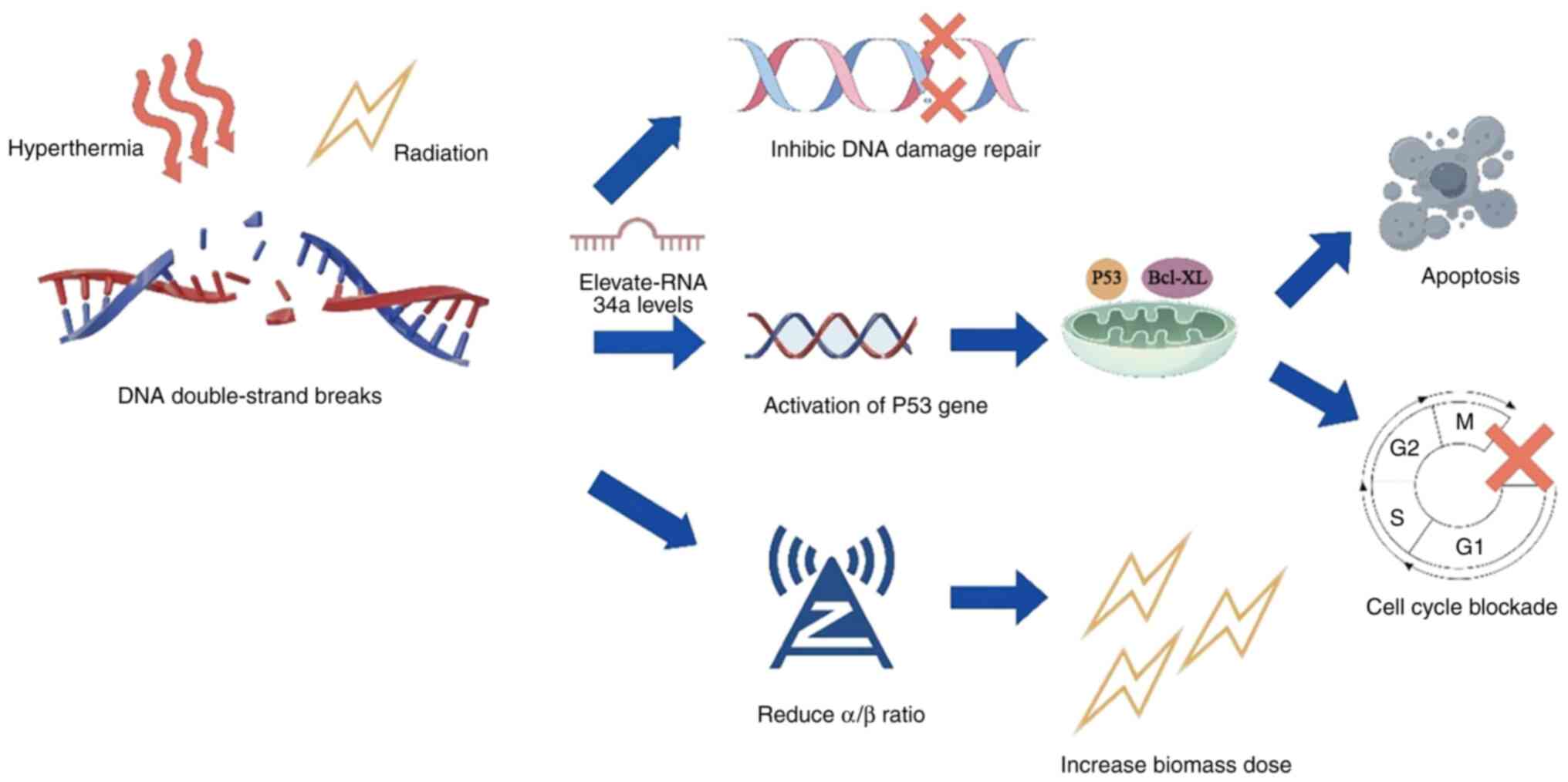
During the cell cycle, cells exhibit peak thermosensitivity in the M-phase, while S-phase cells show significant susceptibility to hyperthermic exposure. In comparison to G0/G1 and G2 phase cells, S-phase cells are less sensitive to ionizing radiation but more sensitive to hyperthermia (59). These cell cycle-specific responses help explain the diversity of molecular mechanisms by which hyperthermia induces cell death (60–62). Ionizing radiation induces DNA double-strand breaks both directly and indirectly. These breaks rapidly activate cellular repair mechanisms, primarily involving homologous recombination and non-homologous end joining pathways (63). Thermal treatment transiently disrupts both repair systems (55), leading to persistent genomic lesions. When combined with radiation therapy, hyperthermia-induced suppression of repair mechanisms leads to an accumulation of DNA damage (64). This synergistic effect raises microRNA-34a levels, which in turn activate p53-mediated transcriptional programs that induce cell cycle arrest and apoptotic signaling (65). Thermal exposure alters nuclear architecture through protein aggregation, which may explain its interference with DNA repair machinery (66,67). Notably, heat-induced radiosensitization appears to be linked to impaired base damage repair processes, where the failure of nucleotide re-polymerization generates secondary DNA strand breaks, resulting in cytotoxic consequences (68).
The mechanistic basis underlying hyperthermia-mediated DNA repair inhibition remains incompletely defined. However, radiation-induced cell lethality and thermal radiosensitization are significantly potentiated. The radiobiological characteristics of hyperthermia share notable similarities with high linear energy transfer (LET) radiation, such as 12C ion therapy (69). In clinical practice, radiotherapy dose fractionation schedules are typically derived from the linear-quadratic model, which considers the ratio of the linear and quadratic components of cell death (70). The a/β value, where the linear and quadratic components are equal, is a key determinant for optimizing fractionation schemes in radiation oncology (α, irreparable, single-hit lethal damage; β, reparable, multi-hit sublethal damage) (71). Tumor radiosensitivity profiles, quantified through α/β measurements relative to healthy tissue thresholds, fundamentally guide radiation protocol development. Consequently, future research should focus on the impact of thermal intervention on the α/β ratio, as it may contribute to the refinement of existing treatment systems (72). Studies involving 259 patients with breast cancer, 338 patients with head and neck cancer and 267 patients with locally advanced cervical cancer (LACC) have demonstrated that combining hyperthermia with radiotherapy significantly reduces α/β values (73–77). This reduction corresponds with a substantial increase in the biomass dose, which may be a key mechanism behind hyperthermia-induced radiosensitization (69). Consequently, it has been considered that hyperthermia may be conceptually likened to a cost-effective strategy for simulating the effects of high-LET radiotherapy (Fig. 3) (78).
Hyperthermia increases radiation-induced cytotoxicity in tumor cells
Growing evidence supports hyperthermia as a potent selective radiosensitizer, demonstrating enhanced thermal effects in malignant tissues while sparing adjacent normal stroma (44,79). Typically, hyperthermia targets localized tumor tissue, akin to confining the radiation field to the tumor site. It also improves blood flow and oxygenation within the tumor, prioritizing the destruction of hypoxic cells and enhancing tumor sensitivity to radiotherapy (36). While hyperthermia disrupts DNA repair in both tumor and normal tissues via elevated temperatures, radiation therapy targets all proliferating cells, necessitating precise control of the treatment area to prevent normal tissue damage. However, this approach may miss some cancer cells infiltrating surrounding normal tissue, potentially causing heat-induced damage to the edges of normal tissues (2,42,55).
Clinical trials involving fractionated radiotherapy often employ this approach, as normal cells typically repair DNA damage faster than malignant cells. This biological difference implies a critical timing window: Applying hyperthermia post-radiation significantly enhances tumor radiosensitivity. A pivotal in vivo study demonstrated that the sequence and interval between hyperthermia and radiation are critical. The optimal therapeutic gain is achieved not by simultaneous treatment, but by administering radiation at least 4 h before heat, selectively targeting radioresistant tumor cells while sparing normal tissue (80). Hyperthermia potentiates the thermo-radiosensitization effect on tumors without significantly increasing normal tissue toxicity in the short term (81,82). The heat dose applied at the edge of the tumor is typically lower than that used for the tumor tissue itself (83), effectively damaging tumor cells while minimizing the risk of normal tissue damage. These findings offer an effective strategy for enhancing tumor cytotoxicity. During the interval between radiotherapy and hyperthermia, the normal tissue surrounding the tumor is also subjected to heat (84). Tumor hypoxia further augments the therapeutic impact of hyperthermia, especially when radiation and heat are applied separately, fostering synergies between the two modalities. With advancements in medical technology, the tumor dose of heat and radiation will significantly differ from that of normal tissues.
Activation of the immune response by hyperthermia
Hyperthermia-induced immune modulation enhances tumor cell sensitivity to radiotherapy (85). This radiosensitization is clinically significant, likely due to the enhanced immunogenicity of tumor cells (86). The abscopal effect, where tumor regression occurs at sites distant from the irradiated area, is now understood as a systemic immune response (Fig. 4). Both hyperthermia and radiotherapy produce synergistic immunological effects by releasing damage-associated molecular patterns (87), which are danger signals from stressed or dying cells that promote cytokine and chemokine secretion, increase lymphocyte infiltration and facilitate apoptosis. Furthermore, these cytokines and chemokines enhance the recognition capabilities of macrophages and dendritic cells, resulting in stronger tumor antigen presentation and cytotoxic T lymphocyte-mediated immune responses through the upregulation of extracellular tumor protein HSP70 (88). Enhanced hemodynamics and the secretion of cell adhesion molecules facilitate lymphocyte transport, thereby amplifying systemic antitumor effects (89). While the mechanisms of hyperthermia warrant further exploration, current research indicates that it serves a significant therapeutic role in tumor treatment, helping to improve the effectiveness of other therapies.
TRT against cancer
Accumulating clinical evidence demonstrates that TRT enhances oncological outcomes across various malignancies, including locoregionally recurrent breast carcinoma, cervical cancer and squamous cell carcinoma of the head and neck. Numerous studies indicate that combining hyperthermia with radiotherapy improves local tumor control rates and reduces patient prognostic risks (90,91). Ongoing phase III trials are actively enrolling participants to expand validated indications, with preliminary data suggesting therapeutic benefits across diverse disease sites (92,93). Based on a comprehensive review of level 1 evidence supporting TRT efficacy, the present study proposes evidence-based recommendations for future translational research. A systematic assessment of the toxicity profile associated with TRT was performed based on the published studies. Consequently, disease-specific clinical outcomes were categorized by tumor subsite in the subsequent sections.
Oncological response outcomes
Clinical results in cervical carcinoma
Radiotherapy alone offers limited local control rates for advanced pelvic tumors. A multicenter study was conducted to assess whether integrating hyperthermia with radiotherapy improves localized disease management and long-term outcomes compared with that of conventional radiation monotherapy. Between 1990 and 1996, Dutch cancer centers recruited 358 patients with histologically confirmed bladder carcinoma (T2-T4, N0 and M0), cervical cancer (IIB-IV) or rectal adenocarcinoma (M0-1). Participants were stratified randomly into two treatment arms: Radiotherapy alone (median total dose 65 Gy; n=176) or radiotherapy combined with hyperthermia (n=182). Clinical records revealed a 39% complete response (CR) rate for radiotherapy alone compared with 55% for the combination therapy (P<0.001), with significantly extended local tumor control in the hyperthermia cohort (P=0.04). While there were no significant differences in therapeutic effects across different tumor sites, hyperthermia was particularly effective in cervical cancer. Specifically, the CR rate in the radiotherapy + hyperthermia group was 83%, compared with 57% with radiotherapy alone (P=0.003). Furthermore, the 3-year OS rate was 27% in the radiotherapy group and 51% in the radiotherapy + hyperthermia group (12).
Hyperthermia has a significant impact on locally advanced cervical tumors, with the synergistic effects of hyperthermia and radiotherapy in cervical cancer treatment being well-documented in previous clinical trials. The aforementioned Dutch hyperthermia research group reported that combining hyperthermia with radiotherapy increased the 3-year local control rate from 41 to 61%, and OS from 27 to 51% (94). In this trial, 62% of patients presented with FIGO stage III disease, and the combination therapy demonstrated favorable tolerability (Common Terminology Criteria for Adverse Events Grade ≤2), with no clinically significant increase in thermal-specific toxicities observed in the hyperthermia-radiotherapy group compared with that of radiation alone. A 12-year longitudinal analysis demonstrated sustained improvements in local control and survival with hyperthermia integration for locoregionally advanced cervical cancer (76), indicating durable therapeutic effects rooted in lasting biological alterations. Another study found that the CR rate in the hyperthermia plus radiotherapy group was significantly higher compared with that in the radiotherapy-only group (80 vs. 50%) (77). Although trends toward improved disease-free survival (64 vs. 45%) and OS (58 vs. 48%) were observed in the hyperthermia-radiotherapy group, these differences were not statistically significant, likely due to sample size limitations. A separate trial of 435 patients with locally advanced cervical cancer demonstrated significant improvement in OS in the triple therapy group, with 5-year OS rates of 82% for chemoradiotherapy combined with hyperthermia and 72% for chemoradiotherapy alone (5,77). However, no significant difference in relapse-free survival was found between the two groups, and clinical data indicated comparable relapse-free survival durations across treatment groups. Additionally, there were no significant differences in acute or long-term toxicity profiles among the therapeutic approaches. Building on these findings, Servayge et al (95) explored whether combining neoadjuvant chemotherapy with TRT outperforms conventional chemoradiation (CRT) in LACC. In the multicenter trial, 370 patients were stratified into three cohorts: 213 receiving standard CRT, 66 undergoing lymph node debulking before CRT (LND-CRT) and 91 treated with the triple-modality (TT) protocol. A 5-year survival analysis revealed similar outcomes: 53% for CRT, 45% for LND-CRT and 53% for TT groups. Statistical analysis confirmed no meaningful survival advantage between regimens, with all groups showing comparable treatment-related adverse effects. In conclusion, radiotherapy combined with hyperthermia following chemotherapy may represent a promising new option for the treatment of LACC.
A meta-analysis demonstrated that adding hyperthermia to chemoradiotherapy significantly improved OS, though it did not lead to a notable improvement in local relapse-free survival (5). Yea et al (96) compared the therapeutic effects of chemoradiotherapy combined with hyperthermia and standard chemoradiotherapy in the local treatment of advanced cervical cancer. The study involved 536 patients, evenly split between two treatment arms: 268 receiving concurrent CRT with hyperthermia (CCRT + H) and 268 undergoing standard CCRT. Clinical trial data revealed that 55% (n=295) of participants had early-stage disease (FIGO I–II), while 45% (n=241) had advanced-stage malignancies (FIGO III–IV). Survival analysis showed that the CCRT + H group had superior 5-year OS rates compared with that of the control group (HR=0.67; 95% CI, 0.47–0.96; P=0.027), aligning with current epidemiological models that suggest thermal enhancement improves treatment penetration in solid tumors. Although the local recurrence-free survival in the CCRT + H group was numerically higher compared with the CCRT-only group, the difference did not reach statistical significance (HR=0.74; 95% CI, 0.49–1.12; P>0.05).
As previously noted, hyperthermia and radiotherapy work synergistically. However, the optimal sequence of these modalities remains controversial. To address this gap, Mei et al (97) conducted comparative analyses using cervical cancer cell cultures, patient-derived xenografts and human organoid models, systematically evaluating treatment sequencing and temporal coordination. Clinical validation involved 58 patients with LACC undergoing combined thermal-radiation treatment. Across all experimental systems, enhanced therapeutic outcomes were observed when the time intervals between treatments were minimized. Mechanistically, applying hyperthermia immediately after radiation led to a 2 to 8-fold increase in DNA fragmentation and a 10 to 100-fold reduction in clonogenic survival, while simultaneously suppressing tumor growth kinetics. Clinically, this approach resulted in a significant improvement in the 5-year survival rate when treatments were administered within 80 min. Notably, the treatment sequence had minimal impact on therapeutic efficacy across all platforms evaluated.
Human papillomavirus (HPV) infections are commonly associated with carcinomas of the head, neck and cervix (98). Previous oncology studies indicate that HPV-positive malignancies exhibit 40–60% greater radiation sensitivity than their HPV-negative counterparts (99). Thermal therapy induces the breakdown of the cancer-promoting E6 proteins of HPV, which is particularly important as E6 proteins typically inhibit the p53 tumor suppressor (100). By reducing E6 levels, hyperthermia can reactivate the apoptotic functions of p53, providing a biological rationale for combining thermal and radiotherapy approaches (101). This synergistic effect may explain why combined modality therapies achieve 25–30% higher CR rates in HPV-associated cancer. Current data strongly support incorporating HPV testing into therapeutic protocols for more precise patient stratification (102). Future clinical trials will further investigate the combination of radiotherapy dose reduction and enhanced hyperthermia in HPV-positive patients (103).
Clinical results in prostate cancer
Prostate cancer ranks as the second most prevalent malignancy in men globally (104). Given the high long-term survival rate in affected patients, research has increasingly focused on addressing the long-term toxicities resulting from radiotherapy, such as radiation-induced erectile dysfunction, and intestinal and urinary impairments (105). In this context, hyperthermia has emerged as a promising adjunct or alternative to systemic radiosensitizers.
Numerous phase I/II trials have explored the combination of hyperthermia and radiotherapy in prostate cancer treatment, yielding improved survival outcomes (106,107). A 42-month follow-up study involving 37 patients with prostate cancer compared external beam radiation (with or without androgen suppression) in combination with transrectal ultrasound-induced hyperthermia. Clinical data indicated that >85% of participants experienced only mild gastrointestinal effects (Common Terminology Criteria for Adverse Events Grade, ≤2), affirming the safety of this thermal approach for localized advanced tumors. These results suggest that incorporating standardized toxicity assessments could broaden the clinical applicability of hyperthermia.
Deger et al (108) report of the 1997–2000 hyperthermia trial, organized by the German Research Foundation, utilized cobalt-palladium thermoseeds and conformal radiation in interstitial hyperthermia for 57 patients with localized prostate cancer. Hyperthermia was applied via a magnetic field in 6 weekly sessions of 1 h each, while 3D-conformal radiotherapy (68.4 Gy) was administered daily in 1.8 Gy fractions. Intraprostatic temperatures ranged from 42 to 46°C, and a marked decline in prostate-specific antigen levels was observed without significant complications. Although novel thermal ablation techniques for prostate cancer treatment hold great potential, challenges remain in achieving complete prostate ablation without damaging surrounding structures, such as the urethra, bladder, rectum and neurovascular tracts (106). Early-phase trials suggest that intracavitary hyperthermia, particularly when combined with image-guided targeting, demonstrates promising tumor-suppressing effects, paving the way for personalized thermal dosing strategies (109).
Clinical results in breast cancer
Breast cancer has been the subject of numerous studies among malignant tumors. Vernon et al (74) conducted a randomized trial between 1981 and 1991, involving 306 patients with inoperable primary or recurrent disease. Patients were allocated to receive either radiotherapy alone or radiotherapy combined with hyperthermia. The results showed significantly improved outcomes (odds ratio=2.3, P<0.001) in the combined treatment arm, although there was no substantial change in survival benefit. Notable changes in the patient group over the study period were particularly evident in preradiation-relapsed lesions. Radiation therapy can also induce skin toxicities, such as blisters, ulcers and necrosis, which are more prevalent in the radiotherapy and hyperthermia groups. However, these side effects can be managed with conservative treatment and clinical care. A study involving 151 patients, enrolled between 1987 and 1993, also demonstrated that the combination of radiotherapy and hyperthermia improved the 5-year tumor control rate and extended OS for patients with locally advanced breast cancer (90). These findings suggest that hyperthermia, along with tumor size, influences both disease-free survival and OS, highlighting its clinical relevance in tumor treatment (90).
In the Netherlands, the standard treatment for patients with locally recurrent inoperable breast cancer is combined irradiation and hyperthermia (110). Datta et al (14) conducted 8 two-arm and 24 single-arm studies, involving 2,110 patients with local recurrent disease. CR rates were similar (63.4%) across both groups, which were both significantly higher than in the control groups (OR=2.6, P<0.001). In a separate study of 779 pre-radiotherapy patients, the CR rate reached 66.4% (with a mean repeated radiotherapy dose of 36.7 Gy). Notably, no increase in associated toxicity was observed following hyperthermia treatment, with the mean acute grade 3/4 toxicity rate at 14.4%. Previous research has identified significant variations in radiotherapy doses, hyperthermia fraction schedules, total hyperthermia fraction, duration of hyperthermia, and the mean reached temperature across all groups. However, subgroup and meta-regression analyses found no prognostic treatment variables. This result aligns with the 70% CR rate reported by Linthorst et al (111) in a cohort of 248 patients treated with re-irradiation and hyperthermia for unresectable recurrence. A study by Thomsen et al (112), which focused on patients with local or (local) regional recurrent breast cancer with gross tumors, employed low-fractionation re-irradiation hyperthermia, delivering a total dose of 20–24 Gy with a weekly dose of 4 Gy. The authors aimed to uncover key mechanisms and markers that trigger therapeutic responses, providing a foundation for future treatment decision-making (112).
In resectable cases, a treatment regimen combining surgery, radiotherapy, hyperthermia, and partial chemotherapy or hormone therapy achieved a local control rate of 78% after 5 years (113). Notter et al (82) applied a 5×4 Gy low-fractionation radiotherapy scheme combined with hyperthermia once a week for patients with locally recurrent breast cancer. The CR rate reached 61%, with no treatment-related toxicity observed. This evidence supports the use of hyperthermia as an adjunctive therapy in the treatment of locally recurrent breast cancer.
Radiation-associated angiosarcoma of the breast (RAASB) is primarily treated surgically, though this approach can present challenges for clinicians. The treatment of RAASB remains largely surgical due to the unpredictable local recurrence rates, and the prognosis for RAASB with local recurrence or in cases that are unresectable is poor. Notter et al (114) studied 10 patients with RAASB, some of whom had locally relapsed or unresectable tumors, treated with thermoimager-controlled water-filtered infrared-A superficial hyperthermia followed by reirradiation. Tumors were graded by size, with local control rates varying depending on the extent of the tumor. The control rate was 100% in two patients who underwent R0 or R2 resection, while it was 33% in three patients with inoperable macroscopic lesions. In the future, combining hyperthermia with reirradiation may be considered as: i) Adjunctive therapy for RAASB, particularly in patients with positive excision margins or those who have undergone surgery for local recurrence; and ii) as a primary treatment option for unresectable RAASB.
Clinical results in bladder cancer
Hyperthermia has become a widely utilized treatment modality for bladder cancer (113–115). The procedure typically involves injecting a warm saline solution containing bleomycin into the bladder. Research has demonstrated that the therapeutic efficacy of the combined radiotherapy and hyperthermia group (84%) was significantly higher compared with that of the radiotherapy-only group (56%; P<0.001), and the toxicity rate was notably lower in the combined treatment group (115).
In another study, 49 patients with T-stage node-negative bladder cancer were treated with low-fractionation radiotherapy (24 Gy, 4 Gy per fraction). The patients were divided into two groups, one receiving adjuvant hyperthermia and the other receiving no hyperthermia. The results revealed that the radiotherapy and hyperthermia combined group was further categorized into high-temperature (>41.5°C) and low-temperature (<41.5°C) subgroups. The high-temperature group demonstrated significantly improved outcomes, suggesting that a moderate increase in temperature enhances therapeutic efficacy (116). A trial conducted in Germany involving high-risk T1 and T2 bladder cancer utilized a treatment regimen combining transurethral resection with radiotherapy, hyperthermia and chemotherapy (50.4 Gy + 5.4–9 Gy; Cisplatin and 5-FU). Following a 6-week follow-up, a pathological CR rate of 96%, an OS rate of 89% after 34 months and 80% patient satisfaction with postoperative bladder function was reported (117). In a comprehensive evaluation of chemoradiotherapy combined with local deep hyperthermia for high-risk bladder cancer after transurethral resection of bladder tumor (TURBT) (118), 369 patients with pTa, pTis, pT1 or pT2 cN0-1 cM0 bladder cancer were included between 1982 and 2016. Of these, 215 patients received chemoradiotherapy (RCT); 79 in RCT combined with radiofrequency hyperthermia (RHT) and 75 in a radiotherapy-only group. All patients received radiotherapy targeting the bladder and local lymph nodes, and therapeutic efficacy was evaluated 4–6 weeks after TURBT. The results showed a total CR rate of 83% (290/351), with 68% (45/66) in the radiotherapy-only group, 86% (178/208) in the RCT group and 87% (67/77) in the RCT + RHT group. The RCT group exhibited significantly improved CR rates compared with that of radiotherapy alone, though the effect of hyperthermia was less pronounced. The OS after RCT was increased compared with that following radiotherapy alone (118). While bladder preservation strategies have demonstrated good long-term outcomes, radical cystectomy with appropriate lymph node dissection remains the standard of care for patients with muscle-invasive bladder cancer. A comparison of various bladder preservation treatments indicates that multimodal therapy, including maximal transurethral bladder tumor resection followed by radiotherapy combined with platinum-based chemotherapy and RHT, significantly improves bladder retention and survival in patients with Ta, Tis and T1-2 bladder cancer (118). These findings present a promising alternative to radical cystectomy (118). The National Comprehensive Cancer Network Guidelines establish radiotherapy with concurrent chemotherapy following TURBT as a standard organ-preserving treatment for muscle-invasive bladder cancer (119). Riesterer et al (120) conducted a multicenter Phase IIB study to assess whether adding hyperthermia to radiotherapy and chemotherapy after transurethral resection could improve disease-free survival in patients with muscle-invasive bladder cancer. The study, which included 27 patients (21 evaluable), reported a CR rate of 93%, a 2-year cystectomy-free rate of 95%, progression-free survival of 76%, locoregional recurrence-free survival of 81% and OS of 86%. The acute and chronic toxicities were 10 and 13%, respectively, with the four-modal treatment regimen being well tolerated.
Clinical results in rectal carcinoma
In a meta-analysis by De Haas-Kock et al (121), 520 patients with advanced rectal cancer received a regimen of hyperthermia with or without radiotherapy. The study results revealed a higher CR rate at the 2-year follow-up in the combination group (risk ratio=2.81; P=0.01) and an improved OS (HR=2.06; P=0.001). However, no survival benefit was observed, and no significant differences in acute toxicity were noted. Hyperthermia may reduce the stage of the primary tumor and involved lymph nodes (122), and it is generally well tolerated without impairing quality of life (123). This demonstrated the positive effects of adding adjuvant hyperthermia, though further trials are required to evaluate the practical value and effectiveness of combining neoadjuvant radiotherapy, hyperthermia and chemotherapy, particularly for recurrent disease.
Hyperthermia is also utilized as an adjunct in colorectal cancer treatment, often in combination with preoperative chemotherapy for patients with locally advanced non-metastatic or metastatic colorectal cancer. This combined approach increases OS rates while maintaining sustained tumor suppression. Compared with that of exclusive chemotherapy regimens, the thermal-chemical integrated strategy results in reduced adverse effects (124–126). Similar therapeutic benefits have been observed in pancreatic cancer treatments where hyperthermia serves dual purposes, palliative symptom relief and curative intent. Current oncology practices confirm that thermal-based protocols not only extend survival but also improve quality of life in patients with locally aggressive pancreatic tumors.
Clinical results in squamous cell carcinoma of the esophagus
Current clinical trials confirm the feasibility of integrating hyperthermia with CRT for preoperative cancer treatment (127–130). A study involving patients with esophageal squamous cell carcinoma reported that 27% achieved complete tumor regression, while 45% maintained stable disease. Survival analysis indicated 1-, 2- and 5-year survival rates of 72.7, 54.5 and 9.1%, respectively, following combined therapy (131). In metastatic esophageal cancer, 3-year survival rates showed 34.9% RFS and 42.5% OS with thermo-chemotherapy protocols. Thermal treatments caused discomfort (38% reported pain) and fatigue (40% experienced tiredness), but these side effects were manageable (130–132). Compared with that of conventional chemotherapy, the thermal-enhanced approach demonstrated superior survival and disease control in esophageal cancer, with fewer treatment-related toxicities.
Clinical results in head and neck cancer
In patients with head and neck cancer, combining hyperthermia and chemoradiotherapy has resulted in increased complete remission rates, prolonged progression-free and disease-free intervals and improved OS (133–135). Across the three studies, the safety profiles were comparable between the TRT and RT groups; however, patients in the TRT group demonstrated superior patient-reported quality of life outcomes. These results suggest that hyperthermia in conjunction with chemoradiotherapy is a viable treatment for head and neck tumors. Quantitative analysis from six comparative studies, involving 451 participants (five randomized), confirmed these findings. The combination of radiation and thermal therapy resulted in a 62.5% CR rate, significantly higher than the 39.6% observed in radiation-only groups (OR=2.92; P<0.0001) (102). The safety profiles of both treatments were consistent, with no significant differences in severe acute or late complications (102). Of the five studies, three randomized trials involving 417 patients assessed the impact of radiotherapy, chemotherapy and hyperthermia in nasopharyngeal carcinoma (133–135); two trials showed advantages in CR rates, with survival benefits observed in both. All three studies confirmed improved progression-free and disease-free intervals in the multimodal treatment groups. Higher tumor temperatures and more frequent hyperthermia sessions correlated with improved outcomes. Importantly, all study groups maintained therapeutic safety, with no increased toxicity risks. These findings suggest that hyperthermia is an effective treatment for advanced head and neck tumors.
Clinical results in superficial tumors
Hyperthermia can enhance the response of superficial tumors to radiotherapy. Jones et al (136) compared the efficacy of radiotherapy alone vs. radiotherapy combined with hyperthermia for tumors ≤3 cm in depth. A thermal dosing metric was employed, defined as the cumulative duration >43°C at 90% of intratumoral measurement points. Of the 122 eligible participants, 109 (89%) met the criteria for thermal intervention and were randomized into two treatment cohorts. The combined therapy group demonstrated a 66.1% CR rate, compared with 42.3% for radiation monotherapy (OR=2.7; 95% CI, 1.2–5.8; P=0.02). Notably, patients with prior radiation exposure showed significant therapeutic differences, with 68.2% achieving partial responses in the combination therapy group, vs. 23.5% in the control group. Survival analysis revealed no significant differences in OS between the groups. Final analysis confirmed that adjuvant thermal exposure (>43°C) significantly improved localized tumor control in irradiated superficial malignancies.
Recurrent pediatric tumors present a challenge for treatment, particularly after radiotherapy. While positive results of chemotherapy combined with hyperthermia in adults have been reported, its efficacy in children has not been well characterized (137). The feasibility of combining radiotherapy with hyperthermia for treating infield recurrent pediatric sarcomas in the pelvic region and extremities has been recently examined; four patients received a regimen of 2 Gy/23 radiotherapy and weekly hyperthermia, with biological models used to quantify the radiosensitization effect of hyperthermia. Equivalent radiotherapy dose calculations indicated that the equivalent biological dose D95% for hyperthermia was 10 Gy. In summary, irradiation heating therapy is theoretically an effective treatment for children with recurrent pelvic and limb sarcomas, but its actual clinical efficacy requires further study (137).
Toxicity profiles
Integrating thermal therapy with radiation offers superior analgesic efficacy and prolonged therapeutic durability compared with that of standard radiation monotherapy in managing metastatic bone pain (92). Pain recurrence post-radiation is common in palliative bone metastasis care. A phase III clinical trial reported by Chi et al (92) systematically evaluated multiple therapeutic parameters, including onset velocity, response sustainability and complete pain remission rates between conventional radiation (30 Gy/10 fractions) and combinatorial thermo-radiation protocols. The trial, involving 57 participants from 2013 to 2016, was terminated early after an interim evaluation. The final cohort included 29 patients receiving thermal-enhanced radiotherapy and 28 undergoing standard radiation. After three months, the combined treatment group showed a 37.9% CR rate, significantly higher than the 7.1% observed in the radiation-only group (P=0.006). Cumulative response analysis revealed 58.6% vs. 32.1% CR rates favoring the thermal group (P=0.045). Radiation monotherapy patients who achieved CR (n=9) had a median pain progression time of 55 days, while the thermal group maintained pain control throughout the 24-week observation period (P<0.01). This combination therapy not only enhanced pain management and improved bone regeneration but also maintains therapeutic effectiveness, confirming its clinical applicability in metastatic bone disease. Furthermore, it has been reported that heat exposure can activate osteoblast function, promote bone formation and reduce the risk of pathological fractures (138).
Hyperthermia also enhances the efficacy of radiation in patients with recurrent disease. A retrospective analysis by Dharmaiah et al (139) assessed 36 patients with breast cancer receiving TRT for disease recurrence. Of these, 83.3% (n=30) had prior radiation exposure; overall response rate of 61.1%, with complete tumor regression in 47.2% (n=17), partial response in 13.9% (n=5) and disease stabilization in 30.6% (n=11). The safety profile revealed 72.2% acute toxicities, primarily pain and erythema, and equivalent chronic effects, including hyperpigmentation and lymphedema. In total, three cases developed transient ulcers, which resolved with standard care and one patient experienced exercise-induced dyspnea due to pulmonary fibrosis. These findings support TRT as a viable salvage therapy for recurrent breast lesions.
Chemoradiotherapy improves regional control in locally advanced colorectal cancer, and the combination of chemoradiotherapy and hyperthermia enhances therapeutic effects in locally advanced non-metastatic colorectal cancer. This combination allows for prolonged tumor control and extends patient survival (125). No significant differences were observed in adverse events between the two groups, with both experiencing mild skin reactions (5.2% transient burns), gastrointestinal disturbances and mucosal inflammation (140–142). An analysis showed 88% 5-year survival rates for thermal-chemoradiation, compared with 76% with standard protocols (P<0.08). Radiotherapy plus hyperthermia proved effective and well-tolerated in treating chemorefractory liver metastases from colorectal cancer, yielding a 30% objective response with mild to moderate toxicity (126). In summary, the toxicity of preoperative chemoradiotherapy combined with hyperthermia is acceptable, and this combined treatment improves the treatment response rate and prolongs patient survival. However, the long-term effects of this combination therapy on locally advanced colorectal cancer and liver metastasis require further investigation.
Technical considerations in hyperthermia
Hyperthermia is employed as an adjuvant therapy to enhance the radiosensitivity of cancer cells. Compared with that of radiotherapy alone, the combination of hyperthermia and low-dose radiotherapy effectively controls tumor growth. As mentioned earlier, various factors influence the efficacy of hyperthermia in both in vivo and in vitro trials, such as heating temperature, heating duration, the interval between radiation and heating and the sequence of their application (143). Hyperthermia systems utilize different energy transfer methods, classified based on tissue penetration and thermal field precision. Local hyperthermia, primarily used to enhance radiotherapy or chemotherapy, employs technologies such as capacitors, radiation, infrared A and ultrasound, all of which have demonstrated value in cancer treatment (144).
Early experiments focused on the cell-killing effects of temperatures between 43 and 45°C. Clinical practice has shown that heat treatments >42.5°C affect only localized tumor areas and prolonged high temperatures can limit treatment duration and cause adverse reactions (145). As a result, clinicians adjust treatment plans by lowering the temperature or shortening the treatment course. Clinical adoption of hyperthermia declined in the 1990s due to challenges in controlling thermal doses (2). However, research has since highlighted the therapeutic benefits of moderate heating (39.5–43°C), which has led to a shift in treatment paradigms (26). Modern thermal therapy systems incorporate optimized heating protocols that reduce the risk of local overheating while maintaining treatment efficacy across multiple medical modalities (146,147).
For effective hyperthermia treatment, clinicians require precise tissue temperature mapping. Accurate thermal monitoring not only guides treatment adjustments but also predicts therapeutic success. Research confirms a direct correlation between thermal dosage and patient outcomes, emphasizing the need for dynamic temperature tracking systems (2). Establishing visual thermal distribution patterns enables clinicians to regulate heat in healthy areas and compensate for insufficient heating in tumors. This dual approach ensures treatment consistency while identifying optimal temperature thresholds for maximizing therapeutic benefits (148). For superficial tumors, skin-contact sensors remain a practical solution. Infrared imaging devices offer spatial thermal data for surface lesions, although they require calibration with physical probes for accurate measurements (82). Emerging techniques now enable noninvasive 3D temperature mapping in deeper tissues. T1-weighted imaging, diffusion-sensitive protocols and proton resonance tracking provide reliable thermometric data (148). Among these, proton resonance frequency analysis stands out for its superior clinical accuracy. Integrating real-time MRI thermometry with hyperthermia systems allows for immediate temperature adjustments, optimizing heat distribution and minimizing overheating risks during treatment sessions (149).
The interval between hyperthermia and radiotherapy administration significantly impacts the effectiveness of the combined treatment. DNA repair is most effectively inhibited when hyperthermia is administered simultaneously with radiotherapy; ~4 h after radiotherapy, DNA repair mechanisms gradually recover, reducing the efficacy of the combination therapy. Furthermore, DNA repair inhibition mechanisms are observed in both tumor and normal tissues (80,150). Tumor tissues may undergo DNA repair over longer intervals, making hyperthermia less effective. Therefore, the interval between hyperthermia and ionizing radiation should be minimized, with the optimal time frame being within 1 h (80,81). Although another study (151) found no significant differences in efficacy when hyperthermia is administered 1–4 h after radiotherapy, and a further analysis suggested that the interval should not exceed 1 h for optimal therapeutic benefit (152). In some breast cancer treatments, hyperthermia and radiotherapy are almost simultaneous, with a 5-min interval before and after treatment. However, short intervals are less effective for cervical cancer, possibly due to the heat resistance of cells and blood vessels. The underlying mechanisms of this phenomenon remain unclear (153–155). Researchers have measured the temperature distribution in tumors and adjacent normal tissues, the duration of heating and the time interval between hyperthermia and radiotherapy. These measurements enable systematic exploration of fundamental questions in thermal radiobiology and the calculation of α/β ratios to evaluate the efficacy of combined therapy, helping identify the optimal treatment parameters (156).
In addition to improving therapeutic outcomes, thermal intervention can reduce radiation exposure while maintaining efficacy. Notter et al (82) implemented an abbreviated radiation schedule (5 weekly fractions of 4 Gy) for managing locally recurrent breast carcinoma, demonstrating the feasibility of dose reduction through thermal synergism. This approach achieved a 61% complete tumor regression rate, matching results from studies using higher radiation doses averaging 38.2 Gy. The reduced radiation intensity enabled safe retreatment opportunities. A subsequent trial involving 17 patients with recurring carcinomatous lymphangitis receiving the same retreatment schedule showed tumor disappearance in 31% of cases, demonstrating partial efficacy even under low-dose conditions (157). These findings suggest that thermal interventions may facilitate radiation de-escalation strategies without compromising therapeutic outcomes in certain cancer subtypes.
Current limitations and future directions
Despite extensive empirical evidence supporting hyperthermia as a synergistic therapy with radiotherapy, its clinical adoption remains limited due to a range of multifaceted barriers, which can be broadly categorized into technical limitations, economic constraints and knowledge gaps. These factors are interdependent, collectively hindering implementation. Technical challenges represent the primary impediment, manifested across four key domains: i) Thermal delivery optimization; ii) thermometric precision; iii) dosimetric standardization; and iv) management of biological heterogeneity.
Current heating techniques primarily use isothermal heating protocols, but ensuring uniform temperature distribution (41–43°C) within neoplastic tissues remains technically unfeasible due to inherent tumor heterogeneity and dynamic thermoregulatory responses. The thermodynamic principle of heat flux equilibration exacerbates this issue, as thermal dissipation occurs unpredictably through convection and conduction. A second technological barrier is the lack of non-invasive thermometric techniques with real-time spatial resolution. Conventional practices, such as using natural orifice probes (such as rectal temperature monitoring), have limited correlation with intratumoral thermal profiles. This discrepancy arises from differential thermal buffering effects; tumor microenvironments experience enhanced heat dissipation due to vasodilation and increased blood flow, while natural luminal temperatures remain more stable. Dosimetric standardization is another critical challenge. Unlike the well-established quantification of absorbed dose in radiotherapy (measured in Gray units), hyperthermia uses thermal dose equivalence (CEM43), based on time-temperature integration (158). However, cumulative errors from imprecise thermometry and inter-patient variations in thermal sensitivity create significant uncertainty in dose-response predictions.
Beyond technical limitations, significant economic and evidentiary barriers hinder the widespread clinical adoption of hyperthermia. Modern hyperthermia systems require substantial capital investment, high operational costs and demanding maintenance, imposing financial burdens, particularly in resource-limited settings where comprehensive health coverage may be absent. In such environments, the cost of devices such as the BSD-2000 (Pyrexar Medical), which is often not covered by insurance, can lead to prohibitive financial toxicity for patients.
A survey of 16 tertiary hyperthermia centers in Europe highlighted the challenges of adoption; the aggregate annual treatment capacity was only 637 patients receiving TRT or thermochemotherapy regimens (159). Notably, 34% of these treatments were part of clinical trials, reflecting the investigational nature of the therapy. Treatment sequencing varied widely, with half of the centers administering hyperthermia before radiotherapy or chemotherapy, while the other half reversed the order, highlighting the absence of standardized chronomodulation guidelines. Additionally, the longer treatment sessions resulted in lower patient throughput compared with that of conventional modalities, leading to significant opportunity costs.
For broad clinical acceptance akin to established oncotherapies, hyperthermia must be validated by Level I evidence, which is defined by large, prospective, randomized controlled trials that demonstrate a significant improvement in primary endpoints such as overall survival, local control, or disease-free survival, with the results replicated across multiple centers. This requires methodologically rigorous, prospective multicenter trials with reproducible protocols. However, persistent technical heterogeneity and the lack of standardized delivery parameters compromise trial quality. Numerous current randomized studies prioritize composite efficacy endpoints over critical thermometric variables, resulting in gaps in evidence related to treatment optimization. Consequently, outcomes often lack reproducibility across platforms and institutions (160). These collective economic, technical and epistemological challenges have constrained hyperthermia's clinical trajectory, limiting its adoption. However, these are solvable problems that require strategic solutions, which are addressed in the following sections. Thermal therapy, fundamentally a heat-dose modality rather than a temperature-deterministic process, requires evaluation based on thermal energy deposition (similar to the absorbed dose per mass in radiotherapy), rather than merely maintaining a set temperature. Adopting this paradigm would resolve key limitations of isothermal heating, such as heterogeneity-driven dose inhomogeneity and uncontrolled thermal dissipation.
Non-isothermal therapeutic strategies have emerged, harnessing the tumor microenvironment's heterogeneity for selective thermal targeting. Overall, two primary modalities show significant clinical promise, injectable particle-mediated hyperthermia and mEHT.
Injectable particle-mediated hyperthermia: The most extensively studied approach involves the tumor-targeted accumulation of magnetic nanoparticle thermoablation (MNPs) (via passive or active targeting), followed by localized heat generation under alternating magnetic fields (AMF). This enables spatially confined thermoablation, minimizing off-target effects (161). Nanoparticle-based thermal therapy exploits the enhanced permeability and retention (EPR) effect, where particles (~100 nm in diameter) preferentially accumulate in the tumor interstitium due to hyperpermeable neovasculature and impaired lymphatic drainage (162,163). Under AMF, MNPs generate heat via Néel and Brownian relaxation mechanisms (164). This intracellular hyperthermia approach avoids the nonspecific thermal damage inherent in external heating techniques, enabling targeted ablation while preserving healthy tissue (21).
Iron oxide NPs (IONPs), particularly superparamagnetic IONPs, can be considered the most promising thermal sensitizers due to: i) Optimal physicochemical properties (~15-30 nm hydrodynamic diameter); ii) favorable biotoxicological profile with renal and hepatic clearance pathways; iii) dual theranostic functionality (MRI contrast enhancement); iv) high specific absorption rate (SAR) values under clinically feasible AMF conditions; and v) modular surface chemistry for payload conjugation (chemotherapeutics and immunomodulators) (165,166).
Current NP classifications include metallic NPs (Au, Ag), metal oxide NPs (TiO2, ZnO) and magnetic core-shell structures (Fe3O4@SiO2, Fe3O4@Au) (162). Despite their therapeutic potential, several key challenges remain (167,168): i) Inter- and intratumoral heterogeneity in the EPR effect leads to inconsistent nanoparticle accumulation across tumor types, significantly impairing therapeutic predictability and clinical reproducibility (169); ii) long-term biodistribution and biocompatibility concerns stem from sequestration by the reticuloendothelial system, resulting in nanoparticle accumulation in the liver and spleen (>30% of the administered dose), potentially inducing chronic inflammation or iron-overload toxicity; and iii) delivery precision remains inadequate due to stromal barriers (such as abnormal extracellular matrix and elevated interstitial fluid pressure), necessitating advanced active targeting strategies, such as ligand-functionalized NPs (such as folate, RGD peptides) or magnetic guidance systems.
mEHT
mEHT exploits the differential dielectric properties of tumor tissue, particularly selective energy absorption by glycocalyx-coated membrane microdomains (lipid rafts) (170). This results in preferential heating of malignant cells via non-thermal field effects and localized Joule heating (171). By targeting the hypermetabolic activity of tumor microenvironments, mEHT capitalizes on elevated extracellular electrolyte concentrations due to enhanced glucose catabolism (158,172). Mechanistic studies identify glycocalyx-embedded receptor clusters within neoplastic cell membranes as key energy-absorption sites. These nanostructures exhibit efficient energy absorption, minimizing energy loss, with most energy being absorbed by tumor tissue (172,173). Consequently, dose calculation becomes feasible through precise determination of the SAR, measured in watts per kilogram of tissue mass, resolving historical uncertainties in hyperthermia dosimetry.
However, molecular limitations persist; mEHT induction activates heat shock response pathways, resulting in the overexpression of cytoprotective HSP70/90 chaperones and stimulation of pro-survival signaling cascades. These mechanisms collectively promote acquired thermotolerance, potentially undermining therapeutic efficacy and contributing to resistance to multimodal therapies (174).
Conclusions
The discovery that heat could damage tumor cells led to the development of heat-based therapies. Numerous studies have since explored methods for applying hyperthermia in cancer treatment, capitalizing on the heightened thermal sensitivity of tumor cells within specific temperature ranges. Hyperthermia has proven to be an effective therapeutic strategy when combined with radiation or chemotherapy, enhancing the overall effectiveness of these treatments. Evidence from several phase III trials and pooled data analyses indicates improved patient outcomes when heat therapy is integrated with radiation or chemo-radiation combinations. These findings support the incorporation of thermotherapy into standard cancer treatment protocols, particularly in resource-limited settings (175,176). The combination of heat with radiation and cytotoxic drugs generates diverse antitumor mechanisms. Clinicians have observed that thermal-radiation combinations typically result in fewer adverse effects compared with that of chemotherapy-radiation regimens, making hyperthermia a favorable option for patients who are unable to tolerate aggressive chemotherapy due to compromised health. The data presented in the current review demonstrated hyperthermia's efficacy in treating tumors, including improved tumor response rates, local and distant control rates and extended survival. Additionally, hyperthermia induces fewer low-intensity toxic events (primarily grades 1 and 2), with no reported cardiotoxic effects in any of the existing studies. Overall, hyperthermia combined with other treatments shows promising tumor response and survival benefits with low toxicity. Based on the robust clinical and biological evidence, hyperthermia represents a viable and potent treatment option for numerous patients with malignant tumors. Its successful application, however, is contingent upon careful patient selection and the exclusion of specific contraindications. Key contraindications generally include absolute factors such as coagulopathy, significant uncompensated cardiovascular or cerebrovascular disease, and active severe infections (2). Furthermore, the presence of metallic implants or active medical devices (such as pacemakers) within the treatment field, as well as tumors in immediate proximity to critical, heat-sensitive organs, typically precludes safe treatment (177).
Hyperthermia is a significant adjunct in oncological treatment, forming a core element of multidisciplinary tumor management strategies while demonstrating favorable biocompatibility. Although it can be applied as monotherapy, particularly through thermal ablation techniques, its primary clinical value lies in its synergistic use with established anti-cancer treatments, including radiotherapy, chemotherapy, chemoradiotherapy, immunotherapy and traditional Chinese medicine. Despite ongoing challenges, such as optimizing thermal delivery, improving thermometric precision and standardizing dosimetry, hyperthermia remains promising in combination with other treatments due to its non-toxic nature and minimal contraindications. Hyperthermia is viewed as an interdisciplinary treatment modality and further in-depth research into its mechanisms will advance this field. As the molecular mechanisms of hyperthermia-induced antitumor effects become more defined, thermo-immunotherapy is emerging as an important frontier in oncology. Furthermore, innovations in thermo-responsive nanomaterials and thermal ablation devices are driving technological progress in hyperthermia applications. Future research should prioritize multicenter randomized controlled trials to establish evidence-based therapeutic protocols. Once technical limitations related to thermal targeting precision are resolved, the adoption of image-guided, personalized hyperthermia regimens may notably improve therapeutic outcomes for patients with cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Oncology Department (Radiotherapy), the Zhejiang Provincial Clinical Specialized Subject Construction Project, the Construction Fund of Key Medical Disciplines of Hangzhou (grant no. 2025HZZD07) and the Wu Jieping Medical Foundation (grant no. 320.6750.2021–02-131).
Availability of data and materials
Not applicable.
Authors' contributions
HY was responsible for designing the literature search strategy and performing preliminary screening, while JL was responsible for data extraction and synthesis of findings. HY and KZ developed the methodology. KZ managed project administration and supervision and created the visualizations. YC, QD and CM obtained funding. QD, YC, CM, KZ, HY and JL contributed to writing. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Crezee J, Franken NAP and Oei AL: Hyperthermia-based anti-cancer treatments. Cancers. 13:12402021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Datta NR, Ordóñez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, Marder D, Puric E and Bodis S: Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev. 41:742–753. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maeda H and Khatami M: Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med. 7:112018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beck M, Ghadjar P, Mehrhof F, Zips D, Paulsen F, Wegener D, Burock S, Kaul D, Stromberger C, Nadobny J, et al: Salvage-radiation therapy and regional hyperthermia for biochemically recurrent prostate cancer after radical prostatectomy (Results of the Planned Interim Analysis). Cancers (Basel). 13:11332021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harima Y, Ohguri T, Imada H, Sakurai H, Ohno T, Hiraki Y, Tuji K, Tanaka M and Terashima H: A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int J Hyperthermia. 32:801–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM, et al: Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 11:561–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R and Riess H: The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 43:33–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi GY, Kim MJ, Kim HI, Park J and Baek SH: Hyperthermia treatment as a promising anti-cancer strategy: Therapeutic targets, perspective mechanisms and synergistic combinations in experimental approaches. Antioxid Basel Switz. 11:6252022. View Article : Google Scholar
|
|
9
|
Righini MF, Durham A and Tsoutsou PG: Hyperthermia and radiotherapy: Physiological basis for a synergistic effect. Front Oncol. 14:14280652024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O and Bentzen SM: Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet Lond Engl. 345:540–543. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wessalowski R, Schneider DT, Mils O, Friemann V, Kyrillopoulou O, Schaper J, Matuschek C, Rothe K, Leuschner I, Willers R, et al: Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: An open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol. 14:843–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Zee J, González González D, van Rhoon GC, van Dijk JD, van Putten WL and Hart AA: Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet Lond Engl. 355:1119–1125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Issels RD, Lindner LH, Verweij J, Wessalowski R, Reichardt P, Wust P, Ghadjar P, Hohenberger P, Angele M, Salat C, et al: Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: The EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 4:483–492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Datta NR, Puric E, Klingbiel D, Gomez S and Bodis S: Hyperthermia and radiation therapy in locoregional recurrent breast cancers: A systematic review and Meta-analysis. Int J Radiat Oncol Biol Phys. 94:1073–1087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Datta NR, Rogers S, Klingbiel D, Gómez S, Puric E and Bodis S: Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: A systematic review with conventional and network meta-analyses. Int J Hyperthermia. 32:809–821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dikomey E and Franzke J: Effect of heat on induction and repair of DNA strand breaks in X-irradiated CHO cells. Int J Radiat Biol. 61:221–233. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
IJff M, Crezee J, Oei AL, Stalpers LJA and Westerveld H: The role of hyperthermia in the treatment of locally advanced cervical cancer: A comprehensive review. Int J Gynecol Cancer. 32:288–296. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu W, Pan S, Zhang J, Xu J, Zhang R, Zhang Y, Fu Z, Wang Y, Hu C and Xu Z: The role of hyperthermia in the treatment of tumor. Crit Rev Oncol Hematol. 204:1045412024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JH, Hahn EW and Tokita N: Combination hyperthermia and radiation therapy for cutaneous malignant melanoma. Cancer. 41:2143–2148. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu G, Ma R, Liu P, Wang K and Cai K: An injectable nanocomposite hydrogel prevents postoperative tumor recurrence and wound infection via synergistic photothermal-chemo-therapy. J Colloid Interface Sci. 655:809–821. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farzin L, Saber R, Sadjadi S, Mohagheghpour E and Sheini A: Nanomaterials-based hyperthermia: A literature review from concept to applications in chemistry and biomedicine. J Therm Biol. 104:1032012022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dewey WC: Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia. 10:457–483. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dewhirst MW, Prosnitz L, Thrall D, Prescott D, Clegg S, Charles C, MacFall J, Rosner G, Samulski T, Gillette E and LaRue S: Hyperthermic treatment of malignant diseases: Current status and a view toward the future. Semin Oncol. 24:616–625. 1997.PubMed/NCBI
|
|
24
|
Urano M, Kuroda M and Nishimura Y: For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia. 15:79–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elming PB, Sørensen BS, Oei AL, Franken NAP, Crezee J, Overgaard J and Horsman MR: Hyperthermia: The optimal treatment to overcome radiation resistant hypoxia. Cancers (Basel). 11:602019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dewhirst MW, Vujaskovic Z, Jones E and Thrall D: Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia. 21:779–790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vaupel P, Kallinowski F and Okunieff P: Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 49:6449–6465. 1989.PubMed/NCBI
|
|
28
|
Vaupel P, Mayer A and Höckel M: Tumor hypoxia and malignant progression. Methods Enzymol. 381:335–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tannock IF and Rotin D: Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 49:4373–4384. 1989.PubMed/NCBI
|
|
30
|
van der Zee J, Vujaskovic Z, Kondo M and Sugahara T: The Kadota fund international Forum 2004-clinical group consensus. Int J Hyperthermia. 24:111–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brown JM and Wilson WR: Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dunne M, Regenold M and Allen C: Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv Drug Deliv Rev. 163–164. 98–124. 2020.PubMed/NCBI
|
|
33
|
Westra A and Dewey WC: Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 19:467–477. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Overgaard J and Suit HD: Time-temperature relationship th hyperthermic treatment of malignant and normal tissue in vivo. Cancer Res. 39:3248–3253. 1979.PubMed/NCBI
|
|
35
|
Sapareto SA, Raaphorst GP and Dewey WC: Cell killing and the sequencing of hyperthermia and radiation. Int J Radiat Oncol Biol Phys. 5:343–347. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song CW, Park HJ, Lee CK and Griffin R: Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia. 21:761–767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, MacFall JR, Brizel DM, Meyer RE, Prescott DM, et al: Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys. 46:179–185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thrall DE, Larue SM, Pruitt AF, Case B and Dewhirst MW: Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperthermia. 22:365–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun X, Xing L, Ling CC and Li GC: The effect of mild temperature hyperthermia on tumour hypoxia and blood perfusion: Relevance for radiotherapy, vascular targeting and imaging. Int J Hyperthermia. 26:224–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Winslow TB, Eranki A, Ullas S, Singh AK, Repasky EA and Sen A: A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: Analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int J Hyperthermia. 31:693–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, Brizel DM and Vujaskovic Z: Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res. 10:4287–4293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vaupel PW and Kelleher DK: Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: Heterogeneity is the key issue. Int J Hyperthermia. 26:211–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Issels RD: Hyperthermia adds to chemotherapy. Eur J Cancer. 44:2546–2554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vaupel P, Piazena H, Notter M, Thomsen AR, Grosu AL, Scholkmann F, Pockley AG and Multhoff G: From localized mild hyperthermia to improved tumor oxygenation: Physiological mechanisms critically involved in oncologic thermo-radio-immunotherapy. Cancers (Basel). 15:13942023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen T, Guo J, Han C, Yang M and Cao X: Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 182:1449–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mace TA, Zhong L, Kokolus KM and Repasky EA: Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia. 28:9–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang HG, Mehta K, Cohen P and Guha C: Hyperthermia on immune regulation: A temperature's story. Cancer Lett. 271:191–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu X, Kang X, Zhao F, Cui Y, Fu Y, Yang X, Yin J, Li W, Fan J, Yang B, et al: Heterogeneous cellular responses to hyperthermia support combined intraperitoneal hyperthermic immunotherapy for ovarian cancer mouse models. Sci Transl Med. 17:eadp21242025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Demuytere J, Carlier C, Van Helden T, Belza J, Vanhaecke F, Xie F, Vermeulen A, Weerts J, Thomale J, Denys H, et al: Effects of hyperthermia on cisplatin tissue penetration and gene expression in peritoneal metastases: Results from a randomized trial in ovarian cancer. Br J Surg. 111:znae0782024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Bailey J, Burstein HJ, et al: Breast cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 22:331–357. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Abu-Rustum NR, Yashar CM, Arend R, Barber E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Crispens MA, et al: NCCN Guidelines® insights: Cervical cancer, version 1.2024. J Natl Compr Cancer Netw. 21:1224–1233. 2023. View Article : Google Scholar
|
|
52
|
Lomax ME, Folkes LK and O'Neill P: Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin Oncol (R Coll Radiol). 25:578–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sprung CN, Ivashkevich A, Forrester HB, Redon CE, Georgakilas A and Martin OA: Oxidative DNA damage caused by inflammation may link to stress-induced non-targeted effects. Cancer Lett. 356:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
van den Tempel N, Horsman MR and Kanaar R: Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int J Hyperthermia. 32:446–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oei AL, Vriend LEM, Crezee J, Franken NAP and Krawczyk PM: Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat Oncol Lond Engl. 10:1652015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Warters RL and Henle KJ: DNA degradation in Chinese hamster ovary cells after exposure to hyperthermia. Cancer Res. 42:4427–4432. 1982.PubMed/NCBI
|
|
57
|
Kampinga HH and Konings AW: Inhibition of repair of X-ray-induced DNA damage by heat: The role of hyperthermic inhibition of DNA polymerase alpha activity. Radiat Res. 112:86–98. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu Y, Liu P, Chen W, Bai S, Chen S, Chen J, Xu X, Xia J, Wu Y, Lai J, et al: Microwave hyperthermia enhances radiosensitization by decreasing DNA repair efficiency and inducing oxidative stress in PC3 prostatic adenocarcinoma cells. Int J Hyperthermia. 41:23352012024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
VanderWaal RP, Griffith CL, Wright WD, Borrelli MJ and Roti JL: Delaying S-phase progression rescues cells from heat-induced S-phase hypertoxicity. J Cell Physiol. 187:236–243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Coss RA, Dewey WC and Bamburg JR: Effects of hyperthermia on dividing Chinese hamster ovary cells and on microtubules in vitro. Cancer Res. 42:1059–1071. 1982.PubMed/NCBI
|
|
61
|
Dewey WC: Failla memorial lecture. The search for critical cellular targets damaged by heat. Radiat Res. 120:191–204. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vidair CA and Dewey WC: Two distinct modes of hyperthermic cell death. Radiat Res. 116:157–171. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Santivasi WL and Xia F: Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal. 21:251–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ihara M, Takeshita S, Okaichi K, Okumura Y and Ohnishi T: Heat exposure enhances radiosensitivity by depressing DNA-PK kinase activity during double strand break repair. Int J Hyperthermia. 30:102–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Luo Z, Zheng K, Fan Q, Jiang X and Xiong D: Hyperthermia exposure induces apoptosis and inhibits proliferation in HCT116 cells by upregulating miR-34a and causing transcriptional activation of p53. Exp Ther Med. 14:5379–5386. 2017.PubMed/NCBI
|
|
66
|
Roti Roti JL: Heat-induced alterations of nuclear protein associations and their effects on DNA repair and replication. Int J Hyperthermia. 23:3–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lepock JR: Role of nuclear protein denaturation and aggregation in thermal radiosensitization. Int J Hyperthermia. 20:115–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Iliakis G, Wu W and Wang M: DNA double strand break repair inhibition as a cause of heat radiosensitization: Re-evaluation considering backup pathways of NHEJ. Int J Hyperthermia. 24:17–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Datta NR, Puric E, Schneider R, Weber DC, Rogers S and Bodis S: Could hyperthermia with proton therapy mimic carbon ion therapy? Exploring a thermo-radiobiological rationale. Int J Hyperthermia. 30:524–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brenner DJ: The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol. 18:234–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Barendsen GW: Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys. 8:1981–1997. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Overgaard J: The heat is (still) on-the past and future of hyperthermic radiation oncology. Radiother Oncol. 109:185–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Datta NR and Bodis S: Hyperthermia with radiotherapy reduces tumour alpha/beta: Insights from trials of thermoradiotherapy vs radiotherapy alone. Radiother Oncol. 138:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, González González D, et al: Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 35:731–744. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Valdagni R and Amichetti M: Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 28:163–169. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Franckena M, Stalpers LJA, Koper PCM, Wiggenraad RGJ, Hoogenraad WJ, van Dijk JDP, Wárlám-Rodenhuis CC, Jobsen JJ, van Rhoon GC and van der Zee J: Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: An update of the Dutch Deep Hyperthermia trial. Int J Radiat Oncol Biol Phys. 70:1176–1182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y and Sawada S: A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia. 17:97–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Horsman MR and Overgaard J: Hyperthermia: A potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol). 19:418–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tsai TF, Hwang TIS, Chen PC, Chen YC, Chou KY, Ho CY, Chen HE and Chang AC: Hyperthermia reduces cancer cell invasion and combats chemoresistance and immune evasion in human bladder cancer. Int J Oncol. 65:1162024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Overgaard J: Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys. 6:1507–1517. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
van Leeuwen CM, Oei AL, Chin KWTK, Crezee J, Bel A, Westermann AM, Buist MR, Franken NAP, Stalpers LJA and Kok HP: A short time interval between radiotherapy and hyperthermia reduces in-field recurrence and mortality in women with advanced cervical cancer. Radiat Oncol. 12:752017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Notter M, Piazena H and Vaupel P: Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: A retrospective study of 73 patients. Int J Hyperthermia. 33:227–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
van Leeuwen CM, Crezee J, Oei AL, Franken NAP, Stalpers LJA, Bel A and Kok HP: The effect of time interval between radiotherapy and hyperthermia on planned equivalent radiation dose. Int J Hyperthermia. 34:901–909. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Overgaard J: The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys. 16:535–549. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Schildkopf P, Frey B, Mantel F, Ott OJ, Weiss EM, Sieber R, Janko C, Sauer R, Fietkau R and Gaipl US: Application of hyperthermia in addition to ionizing irradiation fosters necrotic cell death and HMGB1 release of colorectal tumor cells. Biochem Biophys Res Commun. 391:1014–1020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee S, Son B, Park G, Kim H, Kang H, Jeon J, Youn H and Youn B: Immunogenic Effect of Hyperthermia on Enhancing Radiotherapeutic Efficacy. Int J Mol Sci. 19:27952018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen R, Zou J, Liu J, Kang R and Tang D: DAMPs in the immunogenicity of cell death. Mol Cell. 85:3874–3889. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Nytko KJ, Weyland MS, Dressel-Böhm S, Scheidegger S, Salvermoser L, Werner C, Stangl S, Carpinteiro AC, Alkotub B, Multhoff G, et al: Extracellular heat shock protein 70 levels in tumour-bearing dogs and cats treated with radiation therapy and hyperthermia. Vet Comp Oncol. 21:605–615. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Van Dieren L, Quisenaerts T, Licata M, Beddok A, Lellouch AG, Ysebaert D, Saldien V, Peeters M and Gorbaslieva I: Combined radiotherapy and hyperthermia: A systematic review of immunological synergies for amplifying radiation-induced abscopal effects. Cancers (Basel). 16:36562024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Overgaard J, Ccm Hulshof M, Dahl O and Arcangeli G; ESHO clinical committee, : ESHO 1–85. Hyperthermia as an adjuvant to radiation therapy in the treatment of locally advanced breast carcinoma. A randomized multicenter study by the European Society for Hyperthermic Oncology. Radiother Oncol. 196:1103132024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang Y, Hong W, Che S, Zhang Y, Meng D, Shi F, Su J, Yang Y, Ma H, Liu R, et al: Outcomes for hyperthermia combined with concurrent radiochemotherapy for patients with cervical cancer. Int J Radiat Oncol Biol Phys. 107:499–511. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chi MS, Yang KL, Chang YC, Ko HL, Lin YH, Huang SC, Huang YY, Liao KW, Kondo M and Chi KH: Comparing the effectiveness of combined external beam radiation and hyperthermia versus external beam radiation alone in treating patients with painful bony metastases: A phase 3 prospective, randomized, controlled trial. Int J Radiat Oncol Biol Phys. 100:78–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu P, Wu J, Chen L, Wu Z, Wu Y, Zhang G, Yu B, Zhang B, Wei N, Shi J, et al: Water-filtered infrared A radiation hyperthermia combined with immunotherapy for advanced gastrointestinal tumours. Cancer Med. 13:e700242024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
van der Zee J and González GD: The Dutch deep hyperthermia trial: Results in cervical cancer. Int J Hyperthermia. 18:1–12. 2002.PubMed/NCBI
|
|
95
|
Servayge J, Olthof EP, Mom CH, van der Aa MA, Wenzel HHB, van der Velden J, Nout RA, Boere IA, van Doorn HC and van Beekhuizen HJ: Survival of women with advanced stage cervical cancer: Neo-adjuvant chemotherapy followed by radiotherapy and hyperthermia versus chemoradiotherapy. Cancers (Basel). 16:6352024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yea JW, Park JW, Oh SA and Park J: Chemoradiotherapy with hyperthermia versus chemoradiotherapy alone in locally advanced cervical cancer: a systematic review and meta-analysis. Int J Hyperth. 38:1333–1340. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mei X, Kok HP, Rodermond HM, van Bochove GGW, Snoek BC, van Leeuwen CM, Franken NAP, Ten Hagen TLM, Crezee J, Vermeulen L, et al: Radiosensitization by hyperthermia critically depends on the time interval. Int J Radiat Oncol Biol Phys. 118:817–828. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
de Martel C, Plummer M, Vignat J and Franceschi S: Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 141:664–670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bol V and Grégoire V: Biological basis for increased sensitivity to radiation therapy in HPV-positive head and neck cancers. BioMed Res Int. 2014:6960282014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Khan I, Harshithkumar R, More A and Mukherjee A: Human papilloma virus: An unraveled enigma of universal burden of malignancies. Pathog Basel Switz. 12:5642023. View Article : Google Scholar
|
|
101
|
Oei AL, van Leeuwen CM, ten Cate R, Rodermond HM, Buist MR, Stalpers LJA, Crezee J, Kok HP, Medema JP and Franken NA: Hyperthermia selectively targets human papillomavirus in cervical tumors via p53-dependent apoptosis. Cancer Res. 75:5120–5129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Datta NR, Rogers S, Ordóñez SG, Puric E and Bodis S: Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int J Hyperthermia. 32:31–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Owadally W, Hurt C, Timmins H, Parsons E, Townsend S, Patterson J, Hutcheson K, Powell N, Beasley M, Palaniappan N, et al: PATHOS: A phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer. 15:6022015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hassanipour-Azgomi S, Mohammadian-Hafshejani A, Ghoncheh M, Towhidi F, Jamehshorani S and Salehiniya H: Incidence and mortality of prostate cancer and their relationship with the human development index worldwide. Prostate Int. 4:118–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Potosky AL, Stanford JL, Stroup AM, et al: Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 368:436–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hurwitz MD, Hansen JL, Prokopios-Davos S, Manola J, Wang Q, Bornstein BA, Hynynen K and Kaplan ID: Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: Long-term results from Dana-Farber cancer institute study 94–153. Cancer. 117:510–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hurwitz MD, Kaplan ID, Hansen JL, Prokopios-Davos S, Topulos GP, Wishnow K, Manola J, Bornstein BA and Hynynen K: Hyperthermia combined with radiation in treatment of locally advanced prostate cancer is associated with a favourable toxicity profile. Int J Hyperthermia. 21:649–656. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Deger S, Taymoorian K, Boehmer D, Schink T, Roigas J, Wille AH, Budach V, Wernecke KD and Loening SA: Thermoradiotherapy using interstitial self-regulating thermoseeds: An intermediate analysis of a phase II trial. Eur Urol. 45:574–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kouloulias V, Nikolakopoulou A, Karanasiou I, Antypas C, Armpilia C and Uzunoglou N: Documentation of a new intracavitary applicator for transrectal hyperthermia designed for prostate cancer cases: A phantom study. J Med Phys. 43:141–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Schouten D, van OS R, Westermann AM, Crezee H, van Tienhoven G, Kolff MW and Bins AD: A randomized phase-II study of reirradiation and hyperthermia versus reirradiation and hyperthermia plus chemotherapy for locally recurrent breast cancer in previously irradiated area. Acta Oncol. 61:441–448. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Linthorst M, Baaijens M, Wiggenraad R, Creutzberg C, Ghidey W, van Rhoon GC and van der Zee J: Local control rate after the combination of re-irradiation and hyperthermia for irresectable recurrent breast cancer: Results in 248 patients. Radiother Oncol. 117:217–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Thomsen AR, Sahlmann J, Bronsert P, Schilling O, Poensgen F, May AM, Timme-Bronsert S, Grosu AL, Vaupel P, Gebbers JO, et al: Protocol of the HISTOTHERM study: Assessing the response to hyperthermia and hypofractionated radiotherapy in recurrent breast cancer. Front Oncol. 13:12752222023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Linthorst M, van Geel AN, Baaijens M, Ameziane A, Ghidey W, van Rhoon GC and van der Zee J: Re-irradiation and hyperthermia after surgery for recurrent breast cancer. Radiother Oncol. 109:188–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Notter M, Stutz E, Thomsen AR and Vaupel P: Radiation-associated angiosarcoma of the breast and chest wall treated with thermography-controlled, contactless wIRA-hyperthermia and hypofractionated re-irradiation. Cancers (Basel). 13:39112021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Matsui K, Takebayashi S, Watai K, Kakehi M, Kubota Y, Yao M and Shuin T: Combination radiotherapy of urinary bladder carcinoma with chemohyperthermia. Int J Hyperthermia. 7:19–26. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Masunaga SI, Hiraoka M, Akuta K, Nishimura Y, Nagata Y, Jo S, Takahashi M, Abe M, Terachi T, Oishi K, et al: Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int J Hyperthermia. 10:31–40. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wittlinger M, Rödel CM, Weiss C, Krause SF, Kühn R, Fietkau R, Sauer R and Ott OJ: Quadrimodal treatment of high-risk T1 and T2 bladder cancer: Transurethral tumor resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiother Oncol. 93:358–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Merten R, Ott O, Haderlein M, Bertz S, Hartmann A, Wullich B, Keck B, Kühn R, Rödel CM, Weiss C, et al: Long-term experience of chemoradiotherapy combined with deep regional hyperthermia for organ preservation in high-risk bladder cancer (Ta, Tis, T1, T2). Oncologist. 24:e1341–e1350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Chan K, Chang S, Friedlander T, et al: NCCN Guidelines® insights: Bladder cancer, version 2.2022. J Natl Compr Cancer Netw. 20:866–878. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Riesterer O, Ademaj A, Puric E, Eberle B, Beck M, Gomez S, Marder D, Oberacker E, Rogers S, Hälg RA, et al: Tetramodal therapy with transurethral resection followed by chemoradiation in combination with hyperthermia for muscle-invasive bladder cancer: Early results of a multicenter phase IIB study. Int J Hyperthermia. 39:1078–1087. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
De Haas-Kock DFM, Buijsen J, Pijls-Johannesma M, Lutgens L, Lammering G, van Mastrigt GAPG, De Ruysscher DK, Lambin P and van der Zee J: Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev. 2009:CD0062692009.PubMed/NCBI
|
|
122
|
Kang MK, Kim MS and Kim JH: Clinical outcomes of mild hyperthermia for locally advanced rectal cancer treated with preoperative radiochemotherapy. Int J Hyperthermia. 27:482–490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Schulze T, Wust P, Gellermann J, Hildebrandt B, Riess H, Felix R and Rau B: Influence of neoadjuvant radiochemotherapy combined with hyperthermia on the quality of life in rectum cancer patients. Int J Hyperthermia. 22:301–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gani C, Schroeder C, Heinrich V, Spillner P, Lamprecht U, Berger B and Zips D: Long-term local control and survival after preoperative radiochemotherapy in combination with deep regional hyperthermia in locally advanced rectal cancer. Int J Hyperthermia. 32:187–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zwirner K, Bonomo P, Lamprecht U, Zips D and Gani C: External validation of a rectal cancer outcome prediction model with a cohort of patients treated with preoperative radiochemotherapy and deep regional hyperthermia. Int J Hyperthermia. 34:455–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yu JI, Park HC, Choi DH, Noh JM, Oh D, Park JS, Chang JH, Kim ST, Lee J, Park SH, et al: Prospective phase II trial of regional hyperthermia and whole liver irradiation for numerous chemorefractory liver metastases from colorectal cancer. Radiat Oncol J. 34:34–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Nakajima M, Kato H, Sakai M, Sano A, Miyazaki T, Sohda M, Inose T, Tanaka N, Suzuki S, Masuda N, et al: Planned Esophagectomy after Neoadjuvant Hyperthermo-Chemoradiotherapy using Weekly Low-dose docetaxel and hyperthermia for advanced esophageal carcinomas. Hepatogastroenterology. 62:887–891. 2015.PubMed/NCBI
|
|
128
|
Hulshof MCCM, Van Haaren PMA, Van Lanschot JJB, Richel DJ, Fockens P, Oldenborg S, Geijsen ED, Van Berge Henegouwen MI and Crezee J: Preoperative chemoradiation combined with regional hyperthermia for patients with resectable esophageal cancer. Int J Hyperthermia. 25:79–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Sakamoto T, Katoh H, Shimizu T, Yamashita I, Takemori S, Tazawa K and Fujimaki M: Clinical results of treatment of advanced esophageal carcinoma with hyperthermia in combination with chemoradiotherapy. Chest. 112:1487–1493. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kuwano H, Sumiyoshi K, Watanabe M, Sadanaga N, Nozoe T, Yasuda M and Sugimachi K: Preoperative hyperthermia combined with chemotherapy and irradiation for the treatment of patients with esophageal carcinoma. Tumori. 81:18–22. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nishimura S, Saeki H, Nakanoko T, Kasagi Y, Tsuda Y, Zaitsu Y, Ando K, Nakashima Y, Imamura YU, Ohgaki K, et al: Hyperthermia combined with chemotherapy for patients with residual or recurrent oesophageal cancer after definitive chemoradiotherapy. Anticancer Res. 35:2299–2303. 2015.PubMed/NCBI
|
|
132
|
Sheng L, Ji Y, Wu Q and Du X: Regional hyperthermia combined with radiotherapy for esophageal squamous cell carcinoma with supraclavicular lymph node metastasis. Oncotarget. 8:5339–5348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kang M, Liu WQ, Qin YT, Wei ZX and Wang RS: Long-term efficacy of microwave hyperthermia combined with chemoradiotherapy in treatment of nasopharyngeal carcinoma with cervical lymph node metastases. Asian Pac J Cancer Prev. 14:7395–7400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hua Y, Ma S, Fu Z, Hu Q, Wang L and Piao Y: Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int J Hyperthermia. 27:180–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhao C, Chen J, Yu B and Chen X: Improvement in quality of life in patients with nasopharyngeal carcinoma treated with non-invasive extracorporeal radiofrequency in combination with chemoradiotherapy. Int J Radiat Biol. 90:853–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL and Dewhirst MW: Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 23:3079–3085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kok HP, Van Dijk IWEM, Crama KF, Franken NAP, Rasch CRN, Merks JHM, Crezee J, Balgobind BV and Bel A: Re-irradiation plus hyperthermia for recurrent pediatric sarcoma; a simulation study to investigate feasibility. Int J Oncol. 54:209–218. 2019.PubMed/NCBI
|
|
138
|
Ikuta K, Urakawa H, Kozawa E, Hamada S, Ota T, Kato R, Honda H, Kobayashi T, Ishiguro N and Nishida Y: In vivo heat-stimulus-triggered osteogenesis. Int J Hyperthermia. 31:58–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Dharmaiah S, Zeng J, Rao VS, Zi O, Ma T, Yu K, Bhatt H, Shah C, Godley A, Xia P and Yu JS: Clinical and dosimetric evaluation of recurrent breast cancer patients treated with hyperthermia and radiation. Int J Hyperthermia. 36:986–992. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zagar TM, Higgins KA, Miles EF, Vujaskovic Z, Dewhirst MW, Clough RW, Prosnitz LR and Jones EL: Durable palliation of breast cancer chest wall recurrence with radiation therapy, hyperthermia, and chemotherapy. Radiother Oncol. 97:535–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR and Jones EL: Hyperthermia for locally advanced breast cancer. Int J Hyperthermia. 26:618–624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li G, Mitsumori M, Ogura M, Horii N, Kawamura S, Masunaga S, Nagata Y and Hiraoka M: Local hyperthermia combined with external irradiation for regional recurrent breast carcinoma. Int J Clin Oncol. 9:179–183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Crezee H, van Leeuwen CM, Oei AL, Stalpers LJA, Bel A, Franken NA and Kok HP: Thermoradiotherapy planning: Integration in routine clinical practice. Int J Hyperthermia. 32:41–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in combined treatment of cancer. Lancet Oncol. 3:487–497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M and Hoopes PJ: Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 19:267–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhu Y, Li Q, Wang C, Hao Y, Yang N, Chen M, Ji J, Feng L and Liu Z: Rational design of biomaterials to potentiate cancer thermal therapy. Chem Rev. 123:7326–7378. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Torielli P, McGale J, Liao MJ, Rhaiem R, Bouche O, Botsen D, Gerin O, Lamane A, Lawrence Y, Madelis G, et al: Hepatic metastases management: A comparative review of surgical resection, thermal ablation, and stereotactic body radiation therapy. Eur J Cancer. 228:1156912025. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Gellermann J, Wlodarczyk W, Feussner A, Fähling H, Nadobny J, Hildebrandt B, Felix R and Wust P: Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia. 21:497–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Weihrauch M, Wust P, Weiser M, Nadobny J, Eisenhardt S, Budach V and Gellermann J: Adaptation of antenna profiles for control of MR guided hyperthermia (HT) in a hybrid MR-HT system. Med Phys. 34:4717–4725. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Sapareto SA, Hopwood LE and Dewey WC: Combined effects of X irradiation and hyperthermia on CHO cells for various temperatures and orders of application. Radiat Res. 73:221–233. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kroesen M, Mulder HT, van Holthe JML, Aangeenbrug AA, Mens JWM, van Doorn HC, Paulides MM, Oomen-de Hoop E, Vernhout RM, Lutgens LC, et al: The effect of the time interval between radiation and hyperthermia on clinical outcome in 400 locally advanced cervical carcinoma patients. Front Oncol. 9:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Crezee J, Oei AL, Franken NAP, Stalpers LJA and Kok HP: Response: Commentary: The impact of the time interval between radiation and hyperthermia on clinical outcome in patients with locally advanced cervical cancer. Front Oncol. 10:5282020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Rhee JG, Schuman VL, Song CW and Levitt SH: Difference in the thermotolerance of mouse mammary carcinoma cells in vivo and in vitro. Cancer Res. 47:2571–2575. 1987.PubMed/NCBI
|
|
154
|
Nah BS, Choi IB, Oh WY, Osborn JL and Song CW: Vascular thermal adaptation in tumors and normal tissue in rats. Int J Radiat Oncol Biol Phys. 35:95–101. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Griffin RJ, Dings RPM, Jamshidi-Parsian A and Song CW: Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int J Hyperthermia. 26:256–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kok HP, Herrera TD and Crezee J: Biological treatment evaluation in thermoradiotherapy: Application in cervical cancer patients. Strahlenther Onkol. 200:512–522. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Notter M, Thomsen AR, Nitsche M, Hermann RM, Wolff HA, Habl G, Münch K, Grosu AL and Vaupel P: Combined wIRA-Hyperthermia and Hypofractionated Re-irradiation in the treatment of locally recurrent breast cancer: Evaluation of therapeutic outcome based on a novel size classification. Cancers (Basel). 12:6062020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Lee SY, Fiorentini G, Szasz AM, Szigeti G, Szasz A and Minnaar CA: Quo vadis oncological hyperthermia (2020)? Front Oncol. 10:16902020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ademaj A, Veltsista PD, Marder D, Hälg RA, Puric E, Brunner TB, Crezee H, Gabrys D, Franckena M, Gani C, et al: A patterns of care analysis of hyperthermia in combination with radio(chemo)therapy or chemotherapy in European clinical centers. Strahlenther Onkol. 199:436–444. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kwon S, Jung S and Baek SH: Combination therapy of radiation and hyperthermia, focusing on the synergistic anti-cancer effects and research trends. Antioxid Basel Switz. 12:9242023. View Article : Google Scholar
|
|
161
|
Bañobre-López M, Teijeiro A and Rivas J: Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep Pract Oncol Radiother. 18:397–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Szwed M and Marczak A: Application of nanoparticles for magnetic hyperthermia for cancer treatment-the current state of knowledge. Cancers (Basel). 16:11562024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhou X, Zhang D, Han M, Ma Y, Li W and Yu N: Carbohydrate polymer-functionalized metal nanoparticles in cancer therapy: A review. Int J Biol Macromol. 306:1412352025. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Mohammad F, Bwatanglang IB, Al-Lohedan HA, Shaik JP, Moosavi M, Dahan WM, Al-Tilasi HH, Aldhayan DM, Chavali M and Soleiman AA: Magnetically controlled drug delivery and hyperthermia effects of core-shell Cu@Mn3O4 nanoparticles towards cancer cells in vitro. Int J Biol Macromol. 249:1260712023. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Beik J, Abed Z, Ghoreishi FS, Hosseini-Nami S, Mehrzadi S, Shakeri-Zadeh A and Kamrava SK: Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J Control Release. 235:205–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY and Aldughaim MS: Nanoparticles as drug delivery systems: A review of the implication of nanoparticles' physicochemical properties on responses in biological systems. Polymers. 15:15962023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wang X, Liu J, Durga L, Beeraka NM, Zhou R, Lu P, Song R, Sinelnikov MY, Chen K, Fan R and Zhao D: Recent updates on the efficacy of Mitocans in Photo/Radio-therapy for targeting metabolism in Chemo/radio-resistant cancers: Nanotherapeutics. Curr Med Chem. 32:2156–2182. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Shirvalilou S, Tavangari Z, Parsaei MH, Sargazi S, Sheervalilou R, Shirvaliloo M, Ghaznavi H and Khoei S: The future opportunities and remaining challenges in the application of nanoparticle-mediated hyperthermia combined with chemo-radiotherapy in cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 15:e19222023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Aloss K and Hamar P: Augmentation of the EPR effect by mild hyperthermia to improve nanoparticle delivery to the tumor. Biochim Biophys Acta Rev Cancer. 1879:1891092024. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Shimizu T and Kondo T: Cellular response to physical stress and therapeutic applications. Cell Biology Research Progress. Nova Biomedical; New York: pp. p2162013
|
|
171
|
Revia RA and Zhang M: Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater Today Kidlington Engl. 19:157–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Krenacs T, Meggyeshazi N, Forika G, Kiss E, Hamar P, Szekely T and Vancsik T: Modulated Electro-hyperthermia-induced tumor damage mechanisms revealed in cancer models. Int J Mol Sci. 21:62702020. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Hegyi G, Szasz O and Szasz A: Oncothermia: A new paradigm and promising method in cancer therapies. Acupunct Electrother Res. 38:161–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Viana P and Hamar P: Targeting the heat shock response induced by modulated electro-hyperthermia (mEHT) in cancer. Biochim Biophys Acta Rev Cancer. 1879:1890692024. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Datta NR, Jain BM, Mathi Z, Datta S, Johari S, Singh AR, Kalbande P, Kale P, Shivkumar V and Bodis S: Hyperthermia: A potential game-changer in the management of cancers in low-middle-income group countries. Cancers (Basel). 14:3152022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Schildkopf P, Ott OJ, Frey B, Wadepohl M, Sauer R, Fietkau R and Gaipl US: Biological rationales and clinical applications of temperature controlled hyperthermia-implications for multimodal cancer treatments. Curr Med Chem. 17:3045–3057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Kok HP, Cressman ENK, Ceelen W, Brace CL, Ivkov R, Grüll H, Ter Haar G, Wust P and Crezee J: Heating technology for malignant tumors: A review. Int J Hyperthermia. 37:711–741. 2020. View Article : Google Scholar : PubMed/NCBI
|