Introduction
Gastric cancer is a leading cause of cancer-related
death, ranking fifth in incidence and third in mortality worldwide.
In 2020, there were ~1.1 million new cases and ~769,000 deaths
globally, with the highest rates observed in East Asia, Eastern
Europe and South America (1). China
accounts for nearly half of the global burden, with particularly
high incidence in rural provinces such as Gansu and Henan. Despite
a decline in the age-standardized incidence rate (annual change of
−0.41%), the absolute number of new cases has risen due to
population aging and growth (2).
Together, these trends reflect the need for improved prevention and
treatment, especially in high-burden regions.
Deficient mismatch repair (dMMR) is a key molecular
feature of gastric cancer, often associated with microsatellite
instability (MSI)-high (MSI-H) status, resulting in genomic
instability and a high tumor mutation burden (TMB) (3). This, in turn, increases tumor
immunogenicity and enhances the likelihood of immune checkpoint
inhibitors (ICIs) treatment response (4). In advanced gastric cancer, dMMR
accounts for 7–10% of cases, most frequently in gastric body
adenocarcinoma or elderly patients (5). Compared with proficient mismatch
repair (pMMR), dMMR is generally linked with a better prognosis but
reduced sensitivity to standard chemotherapy (5).
Clinical studies have confirmed that patients with
dMMR gastric cancer respond better to ICIs compared with
traditional treatments. Indeed, programmed death-1
(PD-1)/programmed death-ligand 1 (PD-L1) inhibitors have been
reported to markedly improve objective response rates (ORR) and
pathological complete response (pCR) rates (6). In one trial, the pCR rate was 57.9% in
patients receiving neoadjuvant immunotherapy, compared with 0.0% in
the chemotherapy group (7). In
addition, combining immunotherapy with chemotherapy has been
reported to prolong progression-free survival (PFS) (8). Consequently, dMMR and MSI-H have been
established as key predictive biomarkers for immunotherapy and have
received U.S. Food and Drug Administration approval for guiding
treatment decisions (9).
Nevertheless, a subset of patients with dMMR gastric
cancer still fail to benefit from immunotherapy. Factors such as
the tumor microenvironment, genetic mutations, loss of neoantigens
and acquired resistance may contribute to treatment failure.
Notably, the tumor immune microenvironment (TIME) has emerged as a
critical determinant of immunotherapy success. Research suggests
that tertiary lymphoid structures (TLS) within the tumor can
enhance response to ICIs by improving antigen presentation and
activating T and B cells (10,11).
Therefore, deciphering the immune microenvironment may help explain
variability in patient responses and guide more effective treatment
strategies.
The present study describes the clinical case of a
patient with dMMR gastric cancer who, despite receiving a
combination of immunotherapy and chemotherapy, had suboptimal
treatment outcomes. Through analysis of the TIME before and after
treatment, coupled with bioinformatics analysis of patients with
dMMR gastric cancer, the present study aimed to identify factors
influencing immunotherapy efficacy and explore how these insights
can optimize future treatment strategies for patients with dMMR
gastric cancer.
Materials and methods
Case source
The present case was sourced from the Department of
Oncology at Southern University of Science and Technology Hospital
(Shenzhen, China). The initial consultation date of the patient was
in April 2022. The present study was approved by the Ethics
Committee of Southern University of Science and Technology Hospital
[approval no. 2023(12)], and the patient signed an informed consent
form, agreeing that case information and test results from pre- and
post-treatment specimens may be used for academic reporting. The
standard treatment protocol strictly adhered to the 2021 Chinese
Clinical Oncology Society Guidelines for the Diagnosis and
Treatment of Gastric Cancer (12).
EBER in situ hybridization
EBER in situ hybridization was performed
using the EBER Probe kit (Roche Diagnostics, cat. no. 05997704001)
according to the manufacturer's instructions. Formalin-fixed
paraffin-embedded sections (4 µm, fixed in 10% neutral buffered
formalin at room temperature for 24 h) were first subjected to
permeabilization with kit-supplied protease K (20 µg/ml in 1×PBS,
room temperature, 5 min), followed by post-fixation with 1×PBS
(room temperature, 1 min, 2 washes). Pre-hybridization was
conducted with kit-provided buffer (without probe) at 37°C for 30
min; then, hybridization was performed using the kit probe (diluted
1:50 in hybridization buffer, final concentration 2 ng/µl, 20 µl
per section) in a humidified chamber at 37°C for 16 h. Excess probe
was removed via sequential washes with 2×SSC, 0.5×SSC, and 0.2×SSC
(all at 48°C, 5 min per wash). Post-hybridization steps included
blocking with 5% BSA (cat. no. A7906; Sigma-Aldrich; Merck KGaA;
room temperature, 10 min), incubation with anti-digoxigenin primary
antibody (Roche Diagnostics, cat. no. 11093274910, 1:100 dilution,
37°C, 30 min) and biotinylated goat anti-mouse IgG secondary
antibody (Roche Diagnostics, cat. no. 11093074910, 1:200 dilution,
room temperature, 20 min). Detection was carried out with DAB
chromogenic substrate (Roche Diagnostics, cat. no. 11718096001,
room temperature, 10 min), and images were captured using an
Olympus BX53 light microscope (×200 magnification).
Immune microenvironment detection
methods pre- and post-treatment
Multiplex immunofluorescence staining was performed
by D Medicines (Shanghai) Co., Ltd. The primary antibodies included
CD8, CD4, FoxP3, M1/M2 macrophage markers, and PD-L1; imaging was
conducted with a Leica DM6 B microscope at ×200 magnification.
Scanned images of each slide were subsequently overlaid to generate
a single composite image. The fluorescence images were then
imported into the AP-TIME software (0.3.6; 3D Biotech Ltd.) for
analysis. Pan-CK staining was used to distinguish between tumor
parenchyma and stroma. The number of different cell populations was
quantified by the number of stained cells/mm2 and the
percentage of positively stained cells among all nucleated cells.
In the TIME Profiling (TIME-PRO) test, ‘marge1’ and ‘marge2’ refer
to two different panoramic views or heat maps, illustrating the
distribution and state of immune cells within the tumor
microenvironment, and providing rich information concerning the
immune landscape of the tumor.
Data sources and analysis methods
Non-immune treatment cohort
The gastric adenocarcinoma dataset was downloaded
from The Cancer Genome Atlas (TCGA) database [TCGA-stomach
adenocarcinoma (STAD)] (13),
comprising 440 patients (portal.gdc.cancer.gov/). After filtering
for cases with available MSI status, 383 cases were retained. RNA
sequencing data in transcripts per million format was extracted.
Among these cases, 75 were classified as MSI-H, 52 as MSI-low
(MSI-L) and 256 as microsatellite stability (MSS).
Immune treatment cohorts
A total of three cohorts were analyzed: i) Kim
cohort: Data was sourced from the Harvard Tumor Immune Dysfunction
and Exclusion database (,including 61 patients with gastric cancer
undergoing immunotherapy. After filtering for MSI status, 45 cases
were retained: 5 MSI-H, 1 MSI-L and 39 MSS (14); ii) Peking University Cancer Hospital
(PUCH) cohort: Data was obtained from the literature, comprising 39
patients with gastric cancer treated with immunotherapy (15); and iii) Esophagogastric
Cancer-Memorial Sloan Kettering (EGC-MSK)-2017 cohort: Data was
sourced from the aforementioned literature, including 30 patients
with gastric cancer receiving immunotherapy (16). After filtering for MSI status, 28
cases were included: 5 MSI-H and 23 MSS.
Statistical analyses
Categorical variables (such as MSI subtype
distribution in different stages and immunotherapy response between
different MSI types) are summarized as frequencies (n), and the
χ2 test was used to assess intergroup comparisons. For
continuous variables (such as TMB and neoantigen load), the
Shapiro-Wilk test assessed normality. Subsequently, the normally
distributed variables were evaluated using the unpaired t-test
while non-normally distributed variables were analyzed using the
Mann-Whitney U-test for two-group data. For multiple-group data,
ANOVA was used followed by Tukey's post hoc test for normally
distributed data, and the Kruskal-Wallis test was used followed by
Dunn's multiple comparisons test as a non-parametric test. The
abundance of TIME-related indicators was calculated using the
CIBERSORT tool. Survival curves were generated using the
Kaplan-Meier method, and intergroup survival differences were
compared using the log-rank test. All statistical analyses were
performed using R software (The R Foundation; version 4.4.1).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Case report
Patient information and
intervention
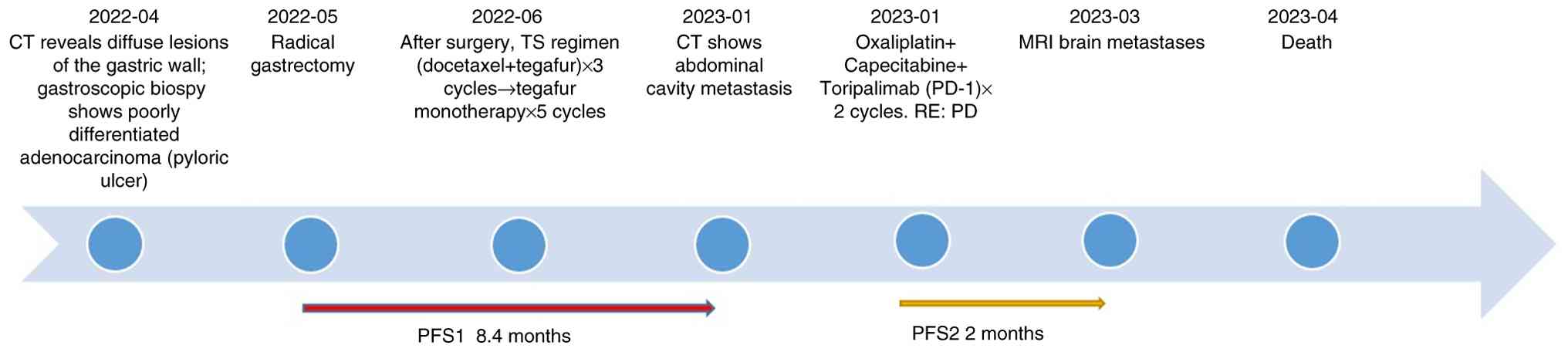
The patient, a 74-year-old man presented to the
Southern University of Science and Technology Hospital (Shenzhen,
China) in April 2022 with complaints of epigastric discomfort and
anorexia for 2 months. CT revealed diffuse gastric wall lesions
accompanied by multiple enlarged lymph nodes around the stomach,
suggesting the possibility of infiltrative gastric cancer (data not
shown). In April 2022, gastroscopy identified a large ulcer near
the pylorus that bled easily upon palpation. Biopsy confirmed a
diagnosis of poorly differentiated adenocarcinoma. In May 2022,
following further examinations including contrast-enhanced
thoracoabdominal computed tomography gastroscopy with biopsy, the
patient underwent a radical gastrectomy. Post-surgical pathology
analysis revealed a moderately-to-poorly differentiated gastric
adenocarcinoma, categorized as diffuse type according to Lauren's
classification (17). Cancer emboli
were observed in blood vessels, and nerve involvement was also
noted. Both surgical margins were negative for cancer; however,
lymph node metastasis was found in the surrounding adipose tissue
(3/16). Metastases were observed in the 8th lymph node
group (1/1), and the 1st and 3rd groups (3/5), while no metastases
were observed in the 9th group (0/3). The immunohistochemistry
results in accordance with standard clinical diagnostic protocols
were as follows: MutL homolog 1 (−, deletion), postmeiotic
segregation increased (S. Cerevisiae) 2 (−, deletion), MutS
homolog (MSH)2 (+, no deletion), MSH6 (+, no deletion), CK7 (+),
CK20 (−), C-erb-2 (0, negative) and EBER in situ
hybridization (−), (Fig. 1; data
for CK and Ki-67 are not shown)
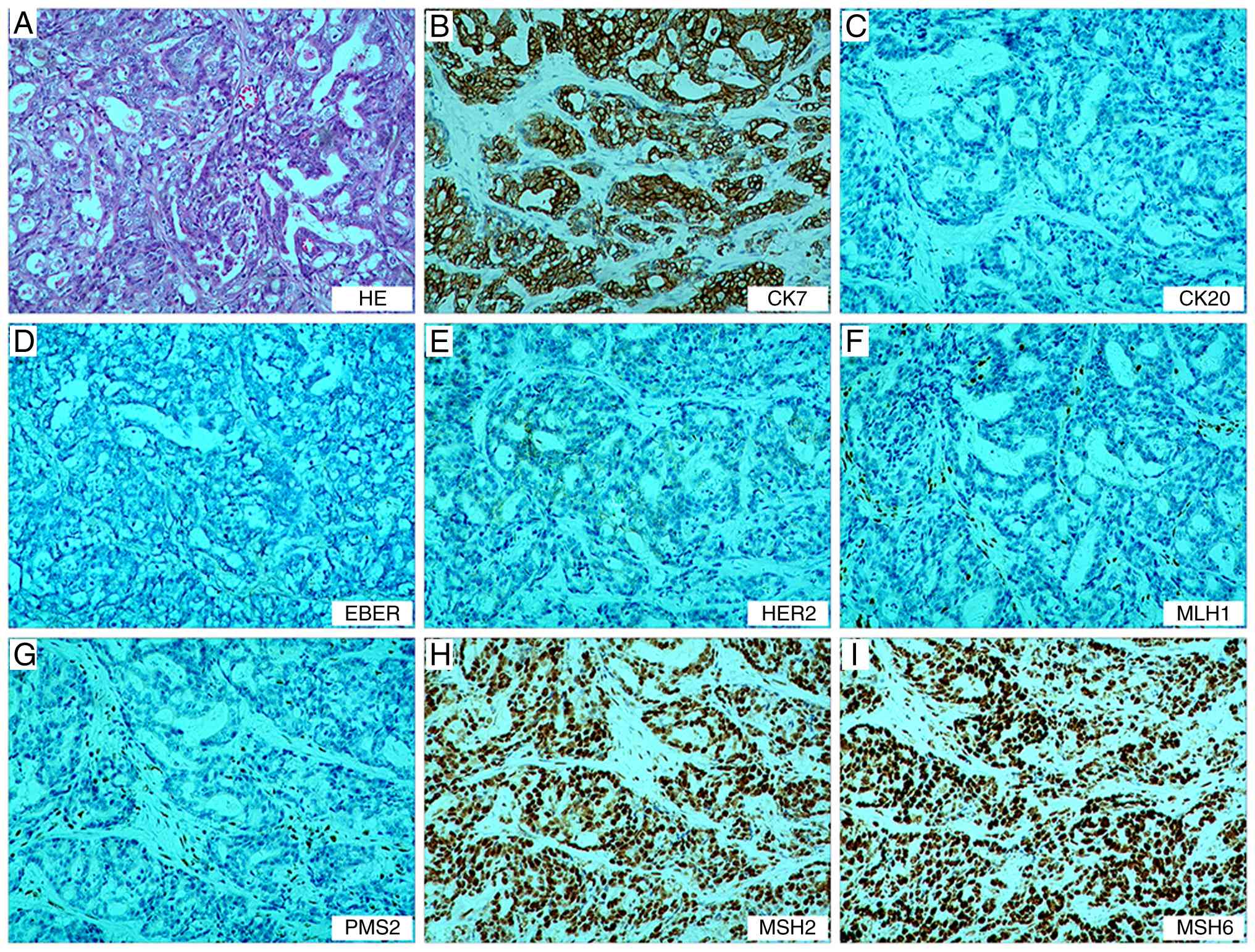 | Figure 1.Postoperative pathological findings
of the gastric tumor. (A) Tumor cells show diffuse/sheet-like
growth with focal glandular differentiation, increased
nuclear-to-cytoplasmic ratio and hyperchromatic nuclei.
Immunohistochemistry demonstrates (B) CK7 (+), (C) CK20 (−), (D)
EBER (−), (E) C-erbB-2 (0), (F) MLH1 (−), (G) PMS2 (−), (H) MSH2
(+) and (I) MSH6 (+), confirming deficient mismatch repair status.
Magnification, ×100. MLH1, MutL homolog; PMS2, postmeiotic
segregation increased (S. Cerevisiae) 2; MSH, MutS
homolog. |
The tumor was staged as pT4bN3aM0 according to the
8th edition of the American Joint Committee on Cancer staging
system (18). Postoperatively, the
patient received three cycles administered every three weeks
(length of each cycle: 21 days) of chemotherapy of the TS regimen
[docetaxel 75 mg/m2 + tegafur 60 mg twice a day (bid)].
During treatment, the patient developed grade IV bone marrow
suppression with infection, which improved after active management.
Due to the advanced age of the patient and refusal of further
aggressive chemotherapy, the patient was switched to single-agent
tegafur (60 mg bid) for five cycles (length of each cycle: 21
days). administered every 3 weeks. The treatment was
well-tolerated, and follow-up CT revealed no disease
progression.
In January 2023, CT revealed abdominal metastases
involving the peritoneum and lymph nodes (Fig. 2A and C), indicating disease
progression. Therefore, 8 days later, the patient began treatment
with oxaliplatin (130 mg/m2) and capecitabine (1.5 g
bid) in combination with the PD-1 inhibitor toripalimab. During the
completion of two cycles administered every three weeks (length of
each cycle: 21 days) of treatment, the patient experienced reduced
appetite. Follow-up CT demonstrated continued lesion enlargement
compared with January 2023 and disease progression was confirmed as
per the Response Evaluation Criteria in Solid Tumors 1.1 criteria
(Fig. 2A and B) (19). Although patients with dMMR gastric
cancer typically respond favorably to immunotherapy (20) the treatment outcome of the patient
in the present case was suboptimal, raising questions about whether
tumor heterogeneity or other factors contributed to the poor
response.
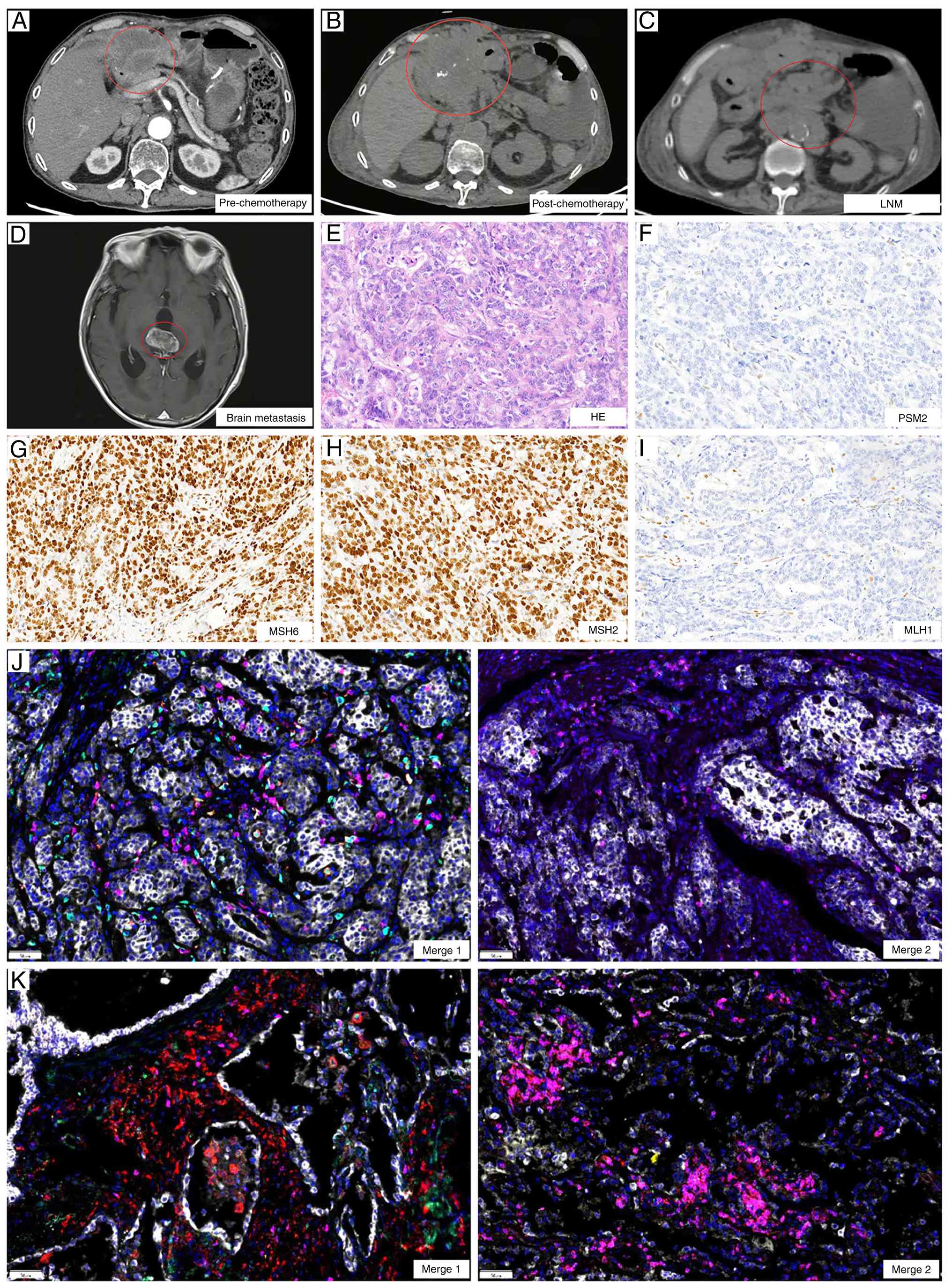 | Figure 2.Imaging and immune microenvironment
changes before and after treatment. CT images (A) before and (B)
after treatment. (C) Lymph node metastases after treatment. (D)
Brain metastases. Pathological examination of peritoneal puncture
lesions, showing (E) HE staining and (F) PMS2, (G) MSH6, (H) MSH2
and (I) MLH1 protein detection, consistent with postoperative
specimens (magnification, ×100). Tumor immune microenvironment
fusion images of (J) pre-treatment and (K) post-treatment
(magnification, ×200). Merge 1 shows PD-1 in green, programmed
death-ligand 1 in yellow, CD8 in pink, CD68 in cyan and CD163 in
red. Merge 2 shows CD3 in pink, CD4 in red, CD20 in green, CD56 in
cyan and forkhead box P3 in yellow. PMS2, postmeiotic segregation
increased (S. Cerevisiae) 2; MSH, MutS homolog; MLH1, MutL
homolog. |
The abdominal metastases were located near the
abdominal wall, and following patient consent, a biopsy of the
lesions was performed to further characterize them. The biopsy
results confirmed metastatic adenocarcinoma and dMMR status,
consistent with baseline findings (Fig.
2E-I). To assess the potential underlying factors, both the
postoperative pathological specimen and the post-treatment biopsy
specimen underwent TIME-PRO assay.
TIME detection results
Before treatment, the TIME of the patient was deemed
‘immune cold’, characterized by low densities of CD3+ T
cells (19.4 cells/mm2) and CD8+ T cells
(19.32 cells/mm2), indicating a scarcity of total and
effector T cells, well below the threshold for a strong immune
response (CD8+ T cell density, ≥330.1
cells/mm2) (21). M1
macrophage density was also low (57.78 cells/mm2) and
forkhead box P3 (FoxP3)+ regulatory T cells (Tregs), TLS
and CD20+ B cell counts were minimal, further confirming
the low immune reactivity (Table I,
Table II, Table III; Fig. 2J).
 | Table I.Immune microenvironment assessment
results summary: Immune checkpoint expression levels. |
Table I.
Immune microenvironment assessment
results summary: Immune checkpoint expression levels.
| A,
Pre-treatment |
|---|
|
|---|
|
|
Count/mm2, % |
|
|
|---|
|
|
|
|
|
|---|
| Test index | Tumor
parenchyma | Stroma | TPS, % | CPS |
|---|
| PD-1 | 0.00 (0.00) | 0.00 (0.00) | - | - |
| PD-L1 | - | - | <1 | <1 |
|
| B,
Post-treatment |
|
|
|
Count/mm2, % |
|
|
|
|
|
|
|
| Test
index | Tumor
parenchyma | Stroma | TPS, % | CPS |
|
| PD-1 | 0.53 (0.01) | 1.53 (0.02) | - | - |
| PD-L1 | - | - | <1 | <1 |
 | Table II.Immune microenvironment assessment
results summary: Tumor immune microenvironment cell
composition. |
Table II.
Immune microenvironment assessment
results summary: Tumor immune microenvironment cell
composition.
| A,
Pre-treatment |
|---|
|
|---|
|
|
Count/mm2, % |
|---|
|
|
|
|---|
| Test index | Tumor
parenchyma | Stroma |
|---|
| T cell-related |
|
|
|
CD3 | 19.40 (0.19) | 83.40 (0.85) |
|
CD3+CD4+ | 0.00 (0.00) | 0.00 (0.00) |
|
CD8 | 19.32 (0.19) | 5.39 (0.08) |
|
FoxP3 | 0.00 (0.00) | 0.00 (0.00) |
|
PD-1+CD8+ | 0.00 (0.00) | 0.00 (0.00) |
|
CD3+CD4+FoxP3+ | 0.00 (0.00) | 0.00 (0.00) |
|
Macrophage-related |
|
|
|
CD68+CD163+ | 4.14 (0.04) | 1.71 (0.02) |
|
CD68+CD163− | 57.78 (0.55) | 57.59 (0.83) |
|
PD-L1+
CD68+ | 2.24 (0.02) | 3.11 (0.04) |
| NK
cell-related |
|
|
|
CD56bright | 0.00 (0.00) | 0.00 (0.00) |
|
CD56dim | 0.00 (0.00) | 0.00 (0.00) |
| B Cell-related |
|
|
|
CD20 | 0.02 (0.00) | 2.38 (0.02) |
|
| B,
Post-treatment |
|
|
|
Count/mm2, % |
|
|
|
| Test
index | Tumor
parenchyma | Stroma |
|
| T cell-related |
|
|
|
CD3 | 439.29 (5.45) | 292.43 (3.59) |
|
CD3+CD4+ | 95.06 (1.18) | 54.84 (0.67) |
|
CD8 | 6.68 (0.10) | 3.00 (0.04) |
|
FoxP3 | 0.00 (0.00) | 0.18 (0.00) |
|
PD-1+CD8+ | 0.00 (0.00) | 0.00 (0.00) |
|
CD3+CD4+FoxP3+ | 0.00 (0.00) | 0.00 (0.00) |
|
Macrophage-related |
|
|
|
CD68+CD163+ | 0.00 (0.00) | 0.00 (0.00) |
|
CD68+CD163− | 0.00 (0.00) | 0.00 (0.00) |
|
PD-L1+
CD68+ | 0.00 (0.00) | 0.00 (0.00) |
| NK
cell-related |
|
|
|
CD56bright | 0.00 (0.00) | 0.00 (0.00) |
|
CD56dim | 0.00 (0.00) | 0.00 (0.00) |
| B cell-related |
|
|
|
CD20 | 17.07 (0.21) | 0.86 (0.01) |
 | Table III.Immune microenvironment assessment
results summary: Tertiary lymphoid structure. |
Table III.
Immune microenvironment assessment
results summary: Tertiary lymphoid structure.
| Group |
Count/mm2 |
µm2/mm2 |
|---|
| Pre-treatment | 0.06 | 6,854.22 |
| Post-treatment | 0.00 | 0.00 |
After treatment, marked changes were observed.
Although PD-L1 remained negative [tumor proportion score, <1%;
combined positive score (CPS), <1], CD3+ T cell
density increased to 439.29 cells/mm2, primarily due to
CD3+CD4+ T cells (95.06
cells/mm2), while CD8+ T cells remained low
(6.68 cells/mm2), indicating a predominantly regulatory
immune response. FoxP3+ T cell density was 0.18
cells/mm2, suggesting potential immune suppression.
Meanwhile, B cell infiltration did not notably increase
(CD20+ B cells, 17.07 cells/mm2) and TLS
remained absent. Overall, despite increased T cell infiltration,
immune escape mechanisms and a suppressive microenvironment
continued to limit effective antitumor immune responses,
potentially compromising treatment efficacy (Table I, Table
II, Table III; Fig. 2K).
Follow-up
In March 2023, the patient developed changes in
mental status, such as bradyphrenia and irrelevant answers to
questions. Brain MRI revealed metastatic lesions (Fig. 2D), indicating poor disease control.
The patient was provided with optimal nutritional support but died
in April 2023 with an overall survival (OS) of 11.6 months
(Fig. 3).
Bioinformatics results
Association between MSI and clinical
Features
Table IV summarizes
the age distribution of patients with gastric cancer stratified by
MSI status across different cohorts. In the EGC-MSK-2017 cohort,
patients in the MSI-H group were significantly older than those in
the MSS group, while in the PUCH and TCGA cohorts, the age
difference between the MSI-H and MSS groups was not significant
Furthermore, the proportion of MSI-H patients with in gastric
cancer stages I/II was notably higher compared with those with
stages III/IV; however, statistical analysis revealed no
significant difference between the groups (Fig. 4A).
 | Table IV.Age distribution by MS status in
different cohorts. |
Table IV.
Age distribution by MS status in
different cohorts.
| A, EGC-MSK-2017
cohort |
|---|
|
|---|
| MSI status | n | Minimum | Quartile 1 | Median | Mean | Quartile 3 | Maximum |
|---|
| MSI-H | 5 | 52.34 | 68.66 | 69.43 | 69.45 | 71.10 | 85.72 |
| MSS | 23 | 22.00 | 45.18 | 57.00 | 55.40 | 69.73 | 76.21 |
|
| B, PUCH
cohort |
|
| MSI
status | n | Minimum | Quartile
1 | Median | Mean | Quartile
3 | Maximum |
|
| MSI-H | 6 | 55.00 | 60.75 | 64.00 | 63.33 | 65.00 | 72.00 |
| MSS | 25 | 23.00 | 56.00 | 62.00 | 59.60 | 65.00 | 76.00 |
|
| C, TCGA
cohort |
|
| MSI
status | n | Minimum | Quartile
1 | Median | Mean | Quartile
3 | Maximum |
|
| MSI status | n | Minimum | Quartile 1 | Median | Mean | Quartile 3 | Maximum |
| MSI-H | 75 | 44.89 | 64.57 | 71.32 | 70.40 | 75.97 | 90.00 |
| MSI-L | 52 | 30.02 | 59.56 | 67.99 | 65.69 | 72.13 | 83.42 |
| MSS | 256 | 34.50 | 57.18 | 66.19 | 64.93 | 72.71 | 90.00 |
Association between MSI and immune
checkpoints
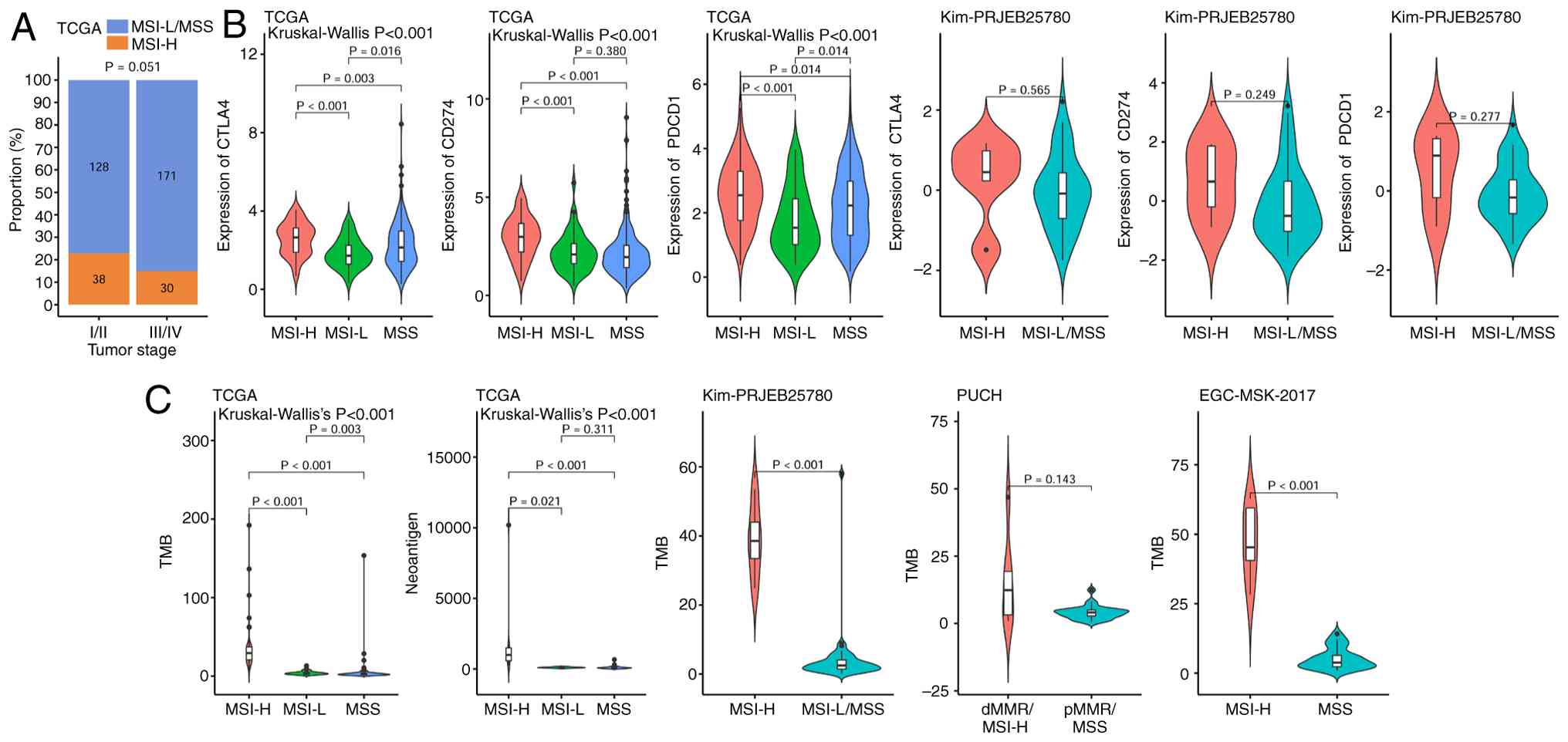
In TCGA gastric cancer cohort, the expression levels
of the immune checkpoint markers cytotoxic T-lymphocyte-associated
protein 4 (CTLA4), CD274 and programmed cell death protein 1
(PDCD1) were significantly higher in the MSI-H population compared
with in the MSI-L and MSS populations. Similarly, in the Kim
immunotherapy cohort, these markers were also notably elevated in
the MSI-H group compared with in the MSS group, although the
differences were not statistically significant, likely due to the
small sample size of only five patients with MSI-H (Fig. 4B).
Association between MSI, TMB and
neoantigen load
In TCGA gastric cancer cohort, the MSI-H group had a
significantly higher TMB and neoantigen load than the MSI-L and MSS
groups. Consistently, in the Kim and EGC-MSK-2017 immunotherapy
cohorts, patients with MSI-H also showed a significantly higher TMB
compared with those with MSI-L/MSS. Only the PUCH cohort
demonstrated no significant difference in TMB between groups,
possibly due to the small sample size (Fig. 4C).
Prognostic differences between MSI-H
and MSS
In TCGA gastric cancer cohort, the MSI-H population
showed a trend toward improved OS, with a hazard ratio (HR) of
0.68; however, the difference between the MSI-H and MSI-L/MSS
populations was not statistically significant. In the PUCH
immunotherapy cohort, OS did not differ significantly between MSI-H
and MSS populations (HR, 1.8; log-rank P=0.379). Similarly, in the
EGC-MSK-2017 cohort, patients with MSI-H exhibited a trend toward
improved OS (HR, 0.42), but the difference was not statistically
significant (log-rank P=0.239) (Fig.
5A-C).
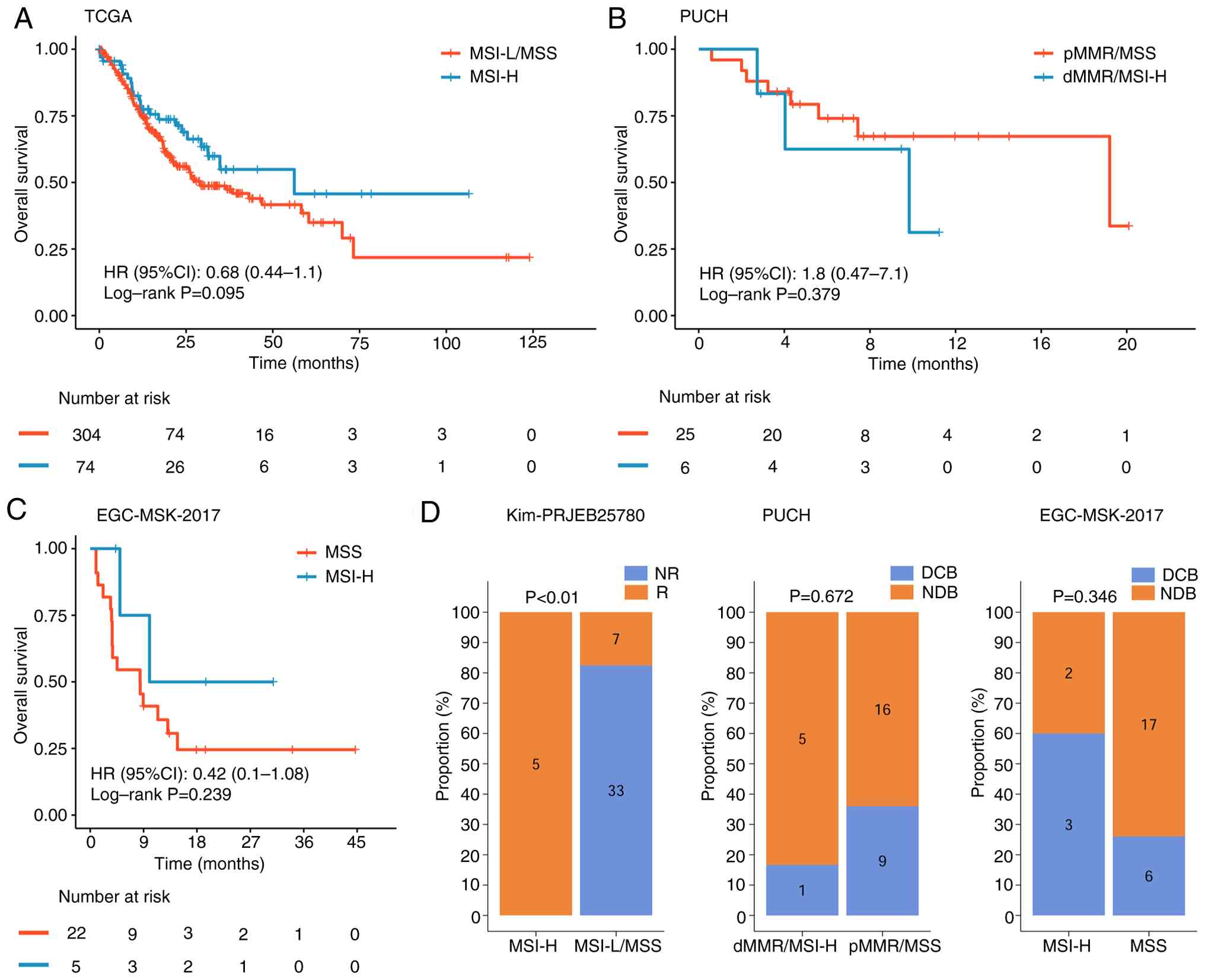 | Figure 5.Prognostic differences between MSI-H
and MSS groups across different databases. Survival analysis
performed in the (A) TCGA, (B) PUCH and (C) EGC-MSK-2017 cohorts.
(D) Immunotherapy response rates of patients with MSI-H in the Kim,
PUCH and EGC-MSK-2017 cohorts. NR, not reported; R, response; MSI,
microsatellite instability; H, MSI-high; MSS, microsatellite
stability; TCGA, The Cancer Genome Atlas; HR, hazard ratio; CI,
confidence interval; dMMR, deficient mismatch repair; MSI-L,
MSI-low; pMMR proficient mismatch repair; DCB, Durable Clinical
Benefit; NDB, Non-Durable Benefit. |
Differences in immunotherapy response
between MSI-H and MSS
Analysis of the Kim cohort demonstrated a
significant difference in immunotherapy response between the MSI-H
and MSS cohorts. By contrast, no significant difference was
observed in the PUCH cohort between the dMMR/MSI-H and pMMR/MSS
groups. In the EGC-MSK-2017 cohort, the MSI-H group exhibited a
markedly higher durable clinical benefit rate, but this difference
was not statistically significant compared with the MSS group
(Fig. 5D).
Immune microenvironment differences
between MSI-H and MSS
TCGA cohort revealed that, compared with the MSS
population, patients with MSI-H had a significantly higher
proportion of CD4+ T cells, natural killer (NK) cells,
mast cells and M1 macrophages. For CD8+ T cells, a
significant difference was only observed between MSI-H and MSI-L
groups, with no difference detected between MSI-H and MSS groups
(Fig. 6A). In the Kim cohort, no
significant differences between MSI-H and MSS/LMSS groups were
demonstrated for any of the detected immune-related indicators
(Fig. 6B).
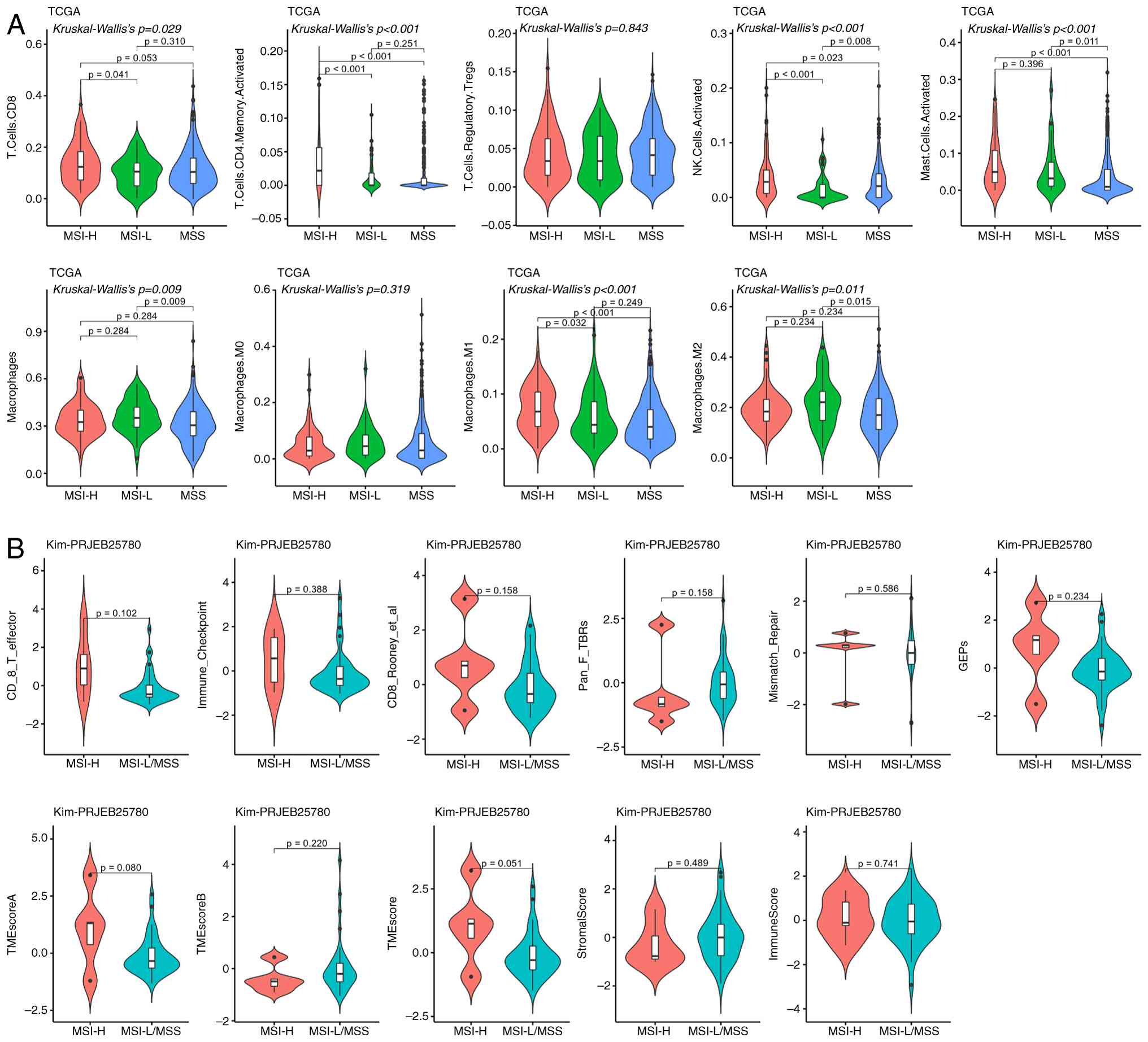 | Figure 6.Differences in the immune
microenvironment between MSI-H and MSS. (A) TCGA analysis
demonstrated that patients with MSI-H exhibited significantly
higher infiltration of memory-activated CD4+ T cells,
activated NK cells, mast cells and M1 macrophages than those with
MSS; CD8+ T cells showed a similar significant
difference between the MSI-H and MSI-L groups (P<0.05). (B) Kim
cohort analysis demonstrated no significant differences in detected
immune-related indicators between MSI-H and MSS/MSI-L (P>0.05).
MSI, microsatellite instability; MSI-H, MSI-high; MSS,
microsatellite stability; TCGA, The Cancer Genome Atlas; NK,
natural killer; MSI-L, MSI-low; NK, natural killer; TME, tumor
microenvironment; M1, type 1 macrophages. |
Discussion
The present study evaluated a patient with gastric
cancer with dMMR features who did not respond to immunotherapy, by
integrating multi-cohort bioinformatics data to elucidate the
complex role of the immune microenvironment in treatment responses.
Despite exhibiting a dMMR/MSI-H molecular profile, the tumor
microenvironment displayed characteristics of a ‘cold tumor’, with
very low PD-L1 expression (<1%), sparse tumor-infiltrating
lymphocytes (TILs) and a modest accumulation of FoxP3+
Treg cells. This suggests that although MSI-H status is typically
considered a marker for sensitivity to immunotherapy, an
immunosuppressive microenvironment can undermine its
effectiveness.
In general, patients with dMMR gastric cancer tend
to have a high TMB and a rich load of tumor neoantigens, which
makes them more responsive to immune therapies (22). In TCGA and EGC-MSK-2017 cohorts,
patients with MSI-H demonstrated a significantly higher TMB;
however, this difference was not observed in the PUCH cohort,
likely due to sample size limitations. In TCGA cohort, the MSI-H
group generally displayed elevated expression of immune checkpoint
markers (such as CTLA4, CD274 and PDCD1) as well as increased
immune cell infiltration (such as CD8+ T and NK cells),
which could potentially serve as biomarkers for predicting
immunotherapy outcomes.
Furthermore, despite the dMMR features of the tumor
in the presented case, the immune microenvironment exhibited clear
signs of immune inactivity, as demonstrated by low TIL levels and
extremely low PD-L1 expression (<1%), indicating the presence of
immune evasion mechanisms. Following treatment, although the number
of CD3+ T cells increased, there was no significant
enhancement in CD8+ T cell activity, and the
accumulation of FoxP3+ Treg cells in the tumor stroma
further supported the notion that the immunosuppressive environment
constrains effective immune responses (23). Furthermore, although there was an
increase in CD20+ B cells, their antitumor immune
effects did not show notable enhancement. The marked accumulation
of Treg cells and myeloid-derived suppressor cells (MDSCs), along
with antigen presentation deficiencies, may have impaired the
function of CD8+ T cells, leading to an overall lack of
immune activation (24). Moreover,
the low expression of PD-L1 could contribute to the poor response
to immunotherapy (25).
High TMB and dMMR status are typically considered
strong predictors of immunotherapy response (20), yet the present case, together with
the cohort data, revealed considerable heterogeneity between these
molecular features and clinical outcomes. Although the MSI-H cohort
in TCGA study exhibited elevated immune checkpoint expression, the
patient in the presented case had low PD-L1 expression, and
variability observed in the Kim cohort suggests that the level of
immune checkpoint expression may have an individual threshold
effect. Only when this threshold (such as CPS ≥1) was surpassed did
MSI-H features translate into therapeutic benefit. Therefore,
personalized treatment strategies should account for immune
microenvironment features and incorporate targeted therapies and
chemotherapy to enhance immune responses.
The present case stands in contrast to the
‘pro-inflammatory’ phenotype (increased M1 macrophages) observed in
the MSI-H group of TCGA cohort, suggesting functional heterogeneity
within the MSI-H population regarding immune infiltration. While
certain patients exhibit predominant immune infiltration by
effector cells, others, such as the patient in the presented case,
may experience immune suppression driven by regulatory cells,
resulting in treatment resistance.
The Keynote-062 study reported that patients with
dMMR have a markedly higher ORR (55%) compared with patients with
pMMR (25%) (26). Moreover, in the
Keynote-158 study, patients with dMMR reported an ORR of 50%
(including a complete response of 15% and a partial response of
34%), while patients with pMMR had an ORR of 7%. In addition, the
median PFS for patients with dMMR was 13.1 months, compared with
2.1 months for patients with pMMR (27). Nonetheless, 20–30% of patients with
dMMR do not respond to immune therapy (28). The present case represents a
‘non-responder’, in which the immune microenvironment (low PD-L1
and high Treg/MDSCs) deviates from the general trends observed in
the MSI-H cohort of TCGA study, yet aligns with reports of immune
evasion mechanisms documented in other studies (29,30).
This discrepancy may result from the interplay of several factors.
On the one hand, dysregulated WNT/β-catenin pathway activation can
impair neoantigen presentation, rendering high TMB tumors
non-immunogenic. Notably, activated β-catenin stemming from
dysregulated WNT pathways in dMMR tumors also suppresses TLS
formation via downstream effects on the tumor microenvironment,
specifically by modulating target molecules (such as c-Myc and
cyclin D1) or interfering with the Wnt/TCF/lymphoid enhancer factor
signaling axis (which regulates B cell chemokines such as chemokine
(C-X-C motif) ligand 13, critical for TLS assembly) (31). This explains the sparse TILs
observed in such cases. On the other hand, suppressive cells may
directly inhibit CD8+ T cell function through the
secretion of factors such as TGF-β and IL-10 (32,33).
Based on these findings, future predictive models should integrate
genomic features such as MSI status and TMB, immune
microenvironment composition such as CD8+ T cell/Treg
ratios, and dynamic functional indicators such as changes in PD-L1
expression, rather than relying on a single biomarker.
The present study highlights the importance of
individualized TIME evaluation. While traditional biomarkers, such
as dMMR and high TMB, predict the effectiveness of immune
therapies, immunosuppressive mechanisms within the microenvironment
may lead to treatment failure. Therefore, future strategies should
explore combined approaches based on TIME characteristics and
genomic profiles, such as novel antigen vaccines, adoptive T cell
therapy, anti-VEGF drugs (such as bevacizumab) or Claudin
18.2-targeted monoclonal antibodies (such as zolbetuximab) that
could reverse immune suppression and enhance efficacy (34,35).
Claudin 18.2, a tight junction protein aberrantly expressed in
30–50% of gastric cancers, has emerged as a promising target for
combination therapy with immunotherapy (33). This potential is further supported
by data from two phase III clinical trials, SPOTLIGHT and GLOW,
which reported that zolbetuximab in combination with chemotherapy
markedly improved PFS and OS in patients with gastric cancer
(36,37). However, as this agent was
unavailable at the time of treatment, the patient in the presented
case did not receive Claudin 18.2-targeted therapy, which may have
contributed to the suboptimal response.
However, the present study has certain limitations,
specifically the small number of cases included in the analysis may
restrict the generalizability of the observed results regarding
immune markers. Furthermore, the present study focused on
descriptive associations between immune markers and clinical
outcomes, without exploring the underlying biological mechanisms
linking these markers to immunotherapeutic sensitivity in
dMMR/MSI-H gastric cancer. Future studies should expand the study
population to include a larger and more diverse cohort, and
integrate functional experiments to elucidate the mechanistic
basis, thereby identifying more optimal immune markers for clinical
application.
In conclusion, the key conclusions of the present
study are as follows: First, in dMMR/MSI-H gastric cancer
(traditionally a subgroup with high TMB/neoantigen load favoring
immunotherapy), TIME with low CD8+ T cell infiltration,
high FoxP3+ Treg accumulation, absent TLS and low PD-L1
expression (<1%) is a critical indicator of immunotherapy
resistance. This is exemplified by the presented non-responsive
dMMR case. Second, cross-cohort analyses (TCGA-STAD, Kim, PUCH and
EGC-MSK-2017) indicate that despite the generally favorable
immunological features of MSI-H gastric cancers (such as higher
TMB, CD8+ T cells, M1 macrophages and immune
checkpoints), their immunotherapy outcomes remain heterogeneous.
Third, dMMR/MSI-H status alone is insufficient to predict
immunotherapy responses; instead, individualized TIME evaluation
(integrating CD8+ T cell/Treg ratios, TLS presence and
PD-L1 expression) is necessary for precise treatment guidance.
Ultimately, combining genomic profiling (MSI and TMB) with TIME
assessment may address treatment heterogeneity in dMMR/MSI-H
gastric cancer and support exploring combined therapies (such as
Claudin 18.2-targeted agents and anti-VEGF drugs) to reverse
immunosuppression in non-responders.
Acknowledgements
Not applicable.
Funding
The present research was funded by the Major Science and
Technology Project of Nanshan District Health and Wellness System
(grant no. NSZD2024067).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LW contributed to the study conception and design,
bioinformatics analyses, manuscript drafting, and integrated
clinical bioinformatics data interpretation. XZ and MD contributed
to clinical data acquisition and verification, clinical-molecular
feature association analysis, and drafting of the clinical section
of the manuscript. WD contributed to pathological data provision
and pathology-molecular subtype association interpretation. LW and
WD confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethical Review Committee of Southern University of Science and
Technology Hospital [approval no. 2023(12)]. The patient provided
written informed consent for the use of their samples and data in
the present study.
Patient consent for publication
The patient provided written informed consent for
the publication of this manuscript, including the use of any
potentially identifiable data and images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MMR
|
mismatch repair
|
|
dMMR
|
deficient MMR
|
|
pMMR
|
proficient MMR
|
|
TMB
|
tumor mutational burden
|
|
ORR
|
objective response rate
|
|
PFS
|
progression-free survival
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
PD-L1
|
programmed death-ligand 1
|
|
Treg
|
regulatory T cells
|
|
MDSC
|
myeloid-derived suppressor cells
|
|
TCGA
|
The Cancer Genome Atlas
|
|
MSI-H
|
MSI-high
|
|
MSS
|
microsatellite stability
|
|
NK
|
natural killer
|
|
VEGF
|
vascular endothelial growth factor
|
|
CPS
|
combined positive score
|
References
|
1
|
Sung H, Ferlay J, Siegel R, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN Estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Zhang TC, Chen H, Yin X, He Q, Man J, Yang
X and Lu M: Changing trends of disease burden of gastric cancer in
China from 1990 to 2019 and its predictions: Findings from Global
Burden of Disease Study. Chin J Cancer Res. 33:11–26. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Xiu J, Farrell A, Battaglin F,
Arai H, Millstein J, Soni S, Zhang W, Shields A, Grothey A, et al:
Genomic landscapes to characterize mismatch-repair deficiency
(dMMR)/microsatellite instability-high (MSI-H) gastrointestinal
(GI) cancers stratified by tumor mutation burden (TMB). J Clin
Oncol. 41 (16 Suppl):S2618. 2023. View Article : Google Scholar
|
|
4
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrelli F, Antista M, Marra F, Cribiù F,
Rampulla V, Pietrantonio F, Dottorini L, Ghidini M, Luciani A,
Zaniboni A and Tomasello G: Adjuvant and neoadjuvant chemotherapy
for MSI early gastric cancer: A systematic review and
meta-analysis. Ther Adv Med Oncol. 16:1948–1957. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takei S, Kawazoe A and Shitara K: The new
era of immunotherapy in gastric cancer. Cancers (Basel).
14:10542022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang P and Gou H: Immunotherapy-based
therapy versus chemotherapy for the neoadjuvant treatment of
locally advanced dMMR/MSI-H gastric cancer. J Clin Oncol. 42
(16_suppl):e161212024. View Article : Google Scholar
|
|
8
|
Mou P, Ge Q, Sheng R, Zhu T, Liu Y and
Ding K: Research progress on the immune microenvironment and
immunotherapy in gastric cancer. Front Immunol. 14:12911172023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcus L, Lemery S, Keegan P and Pazdur R:
FDA approval summary: Pembrolizumab for the treatment of
microsatellite Instability-High solid tumors. Clin Cancer Res.
25:3753–3758. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu L, Li T, Deng S, Gao H, Jiang Y, Chen
Q, Chen H, Xiao Z, Shuai X and Su Z: Tertiary lymphoid structure
formation induced by LIGHT-engineered and photosensitive
nanoparticles-decorated bacteria enhances immune response against
colorectal cancer. Biomaterials. 314:1228462025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fridman W, Sibéril S, Pupier G, Soussan S
and Sautès-Fridman C: Activation of B cells in Tertiary Lymphoid
Structures in cancer: Anti-tumor or anti-self? Semin Immunol.
65:1017032023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ,
Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, et al: Clinical guidelines
for the diagnosis and treatment of gastric cancer. Cancer Commun.
41:747–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim ST, Cristescu R, Bass AJ, Kim KM,
Odegaard JI, Kim K, Liu XQ, Sher X, Jun H, et al: Comprehensive
molecular characterization of clinical responses to PD-1 inhibition
in metastatic gastric cancer. Nat Med. 24:1449–1458. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiao X, Wei X, Li S, Liu C, Chen H, Gong
J, Li J, Zhang X, Wang X, et al: A genomic mutation signature
predicts the clinical outcomes of immunotherapy and characterizes
immunophenotypes in gastrointestinal cancer. NPJ Precis Oncol.
5:362021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janjigian YY, Sanchez-Vega F, Jonsson P,
Chatila WK, Hechtman JF, Ku GY, Riches JC, Tuvy Y, Kundra R,
Bouvier N, et al: Genetic predictors of response to systemic
therapy in esophagogastric cancer. Cancer Discov. 8:49–58. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a Histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
American Joint Committee on Cancer (AJCC),
. AJCC Cancer Staging Manual. 8th edition. Edge SB, Byrd DR,
Compton CC, et al: New York: Springer; 2017
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
André T, Tougeron D, Piessen G, de la
Fouchardière C, Louvet C, Adenis A, Jary M, Tournigand C, Aparicio
T, Desrame J, et al: Neoadjuvant nivolumab plus ipilimumab and
adjuvant nivolumab in localized deficient mismatch
Repair/Microsatellite Instability-high gastric or esophagogastric
junction adenocarcinoma: The GERCOR NEONIPIGA Phase II study. J
Clin Oncol. 41:255–265. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obeid J, Hu Y, Erdag G, Leick K and
Slingluff C: The heterogeneity of tumor-infiltrating CD8+ T cells
in metastatic melanoma distorts their quantification: How to manage
heterogeneity? Melanoma Res. 27:211–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma W, Pham B and Li T: Cancer neoantigens
as potential targets for immunotherapy. Clin Exp Metastasis.
39:51–60. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hugo W, Zaretsky J, Sun L, Song C, Moreno
B, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to Anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Fátima Aquino Moreira-Nunes C, De Souza
Almeida Titan Martins C, Feio D, Lima IK, Lamarão LM, de Souza CRT,
Costa IB, da Silva Maués JH, Soares PC, de Assumpção PP and Burbano
RMR: PD-L1 expression associated with Epstein-Barr virus status and
Patients' survival in a large cohort of gastric cancer patients in
northern brazil. Cancers (Basel). 13:31072021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shitara K, Van Cutsem E, Bang YJ, Fuchs C,
Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al:
Efficacy and safety of pembrolizumab or pembrolizumab plus
chemotherapy vs chemotherapy alone for patients With First-line,
advanced gastric cancer: The KEYNOTE-062 phase 3 randomized
clinical trial. JAMA Oncol. 6:1571–1580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Malley DM, Bariani GM, Cassier PA,
Marabelle A, Hansen AR, Acosta AJ, Miller WH Jr, Safra T, Italiano
A, Mileshkin L, et al: Pembrolizumab in microsatellite
instability-high/mismatch repair deficient (MSI-H/dMMR) and
non-MSI-H/non-dMMR advanced endometrial cancer: Phase 2 KEYNOTE-158
study results. Gynecol Oncol. 193:130–135. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng D, Wu J, Luo H, Li Y, Xiao J, Peng J,
Ye Z, Zhou R, Yu Y, Wang G, et al: Tumor microenvironment
evaluation promotes precise checkpoint immunotherapy of advanced
gastric cancer. J Immunother Cancer. 9:e0024672021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li N and Li Y, Li J, Tang S, Gao H and Li
Y: Correlation of the abundance of MDSCs, Tregs, PD-1, and PD-L1
with the efficacy of chemotherapy and prognosis in gastric cancer.
Lab Med. 56:259–270. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim Y, Bae YJ, Kim JH, Kim H, Shin SJ,
Jung DH and Park H: Wnt/β-catenin pathway is a key signaling
pathway to trastuzumab resistance in gastric cancer cells. BMC
Cancer. 23:9222023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bernal M, Ruiz-Cabello F, Concha A,
Paschen A and Garrido F: Implication of the β2-microglobulin gene
in the generation of tumor escape phenotypes. Cancer Immunol
Immunother. 61:1359–1371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moaaz M, Lotfy H, Elsherbini B, Motawea M
and Fadali G: TGF-β enhances the Anti-inflammatory effect of
Tumor-infiltrating CD33+11b+HLA-DR Myeloid-derived suppressor cells
in gastric cancer: A possible relation to MicroRNA-494. Asian Pac J
Cancer Prev. 21:3393–3403. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niu Q, Liu J, Luo X, Su B and Yuan X:
Future of targeted therapy for gastrointestinal cancer: Claudin
18.2. Oncol Transl Med. 17:P102–P107. 2021. View Article : Google Scholar
|
|
35
|
Zhang D, Huang G, Liu J and Wei W:
Claudin18.2-targeted cancer theranostics. Am J Nucl Med Mol
Imaging. 13:64–69. 2023.PubMed/NCBI
|
|
36
|
Shitara K, Lordick F, Bang YJ, Enzinger P,
Ilson D, Shah MA, Van Cutsem E, Xu RH, Aprile G, Xu J, et al:
Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive,
HER2-negative, untreated, locally advanced unresectable or
metastatic gastric or gastro-oesophageal junction adenocarcinoma
(SPOTLIGHT): A multicentre, randomised, double-blind, phase 3
trial. Lancet. 401:1655–1668. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shah MA, Shitara K, Ajani JA, Bang YJ,
Enzinger P, Ilson D, Lordick F, Van Cutsem E, Gallego Plazas J,
Huang J, et al: Zolbetuximab plus CAPOX in CLDN18.2-positive
gastric or gastroesophageal junction adenocarcinoma: The
randomized, phase 3 GLOW trial. Nat Med. 29:2133–2141. 2023.
View Article : Google Scholar : PubMed/NCBI
|