Introduction
Effort has been devoted to preserving excellent limb
function with a low risk of local tumor recurrence after marginal
or intralesional tumor resection. Thus, over the past 10 years, we
have engaged in the development of photodynamic and radiodynamic
therapy with acridine orange (AO-PDT and AO-RDT) as a minimally
invasive surgery for the treatment of musculoskeletal sarcomas.
Numerous clinical trials for this treatment modality conducted thus
far have shown to be successful (1–7).
However, in these clinical studies on AO-PDT and AO-RDT, AO was
administered by local and not by intravenous injection.
Furthermore, AO excitation was achieved using continuous wave light
(CWL) derived from a xenon lamp, as opposed to a high-power flash
wave light (FWL) from a xenon lamp that has been demonstrated in
previous studies to exert a stronger cytocidal effect, as compared
to CWL, using a mouse osteosarcoma cell line (8,9). These
studies also showed that the intravenous administration of AO may
be useful for the photodynamic diagnosis (PDD) of mouse
osteosarcoma, without entailing any complications (10). Therefore, using a mouse osteosarcoma
model, we undertook to clarify the anti-tumor activity of AO-PDT
with FWL after the intravenous administration of AO.
Materials and methods
Mouse osteosarcoma model
LM8, the mouse osteosarcoma cell line used in the
present study, was derived from Dunn’s osteosarcoma which possesses
strong metastasizing ability (11).
LM8 cells were harvested in Dulbecco’s modified Eagle’s medium
containing 10% fetal bovine serum at 37°C in a 5% CO2
atmosphere. A suspension containing 1×106 cells isolated
from the culture dishes by trypsinization was inoculated into the
soft tissues, including the subcutaneous tissue and muscles of the
back, at the site of implantation in C3H mice after removal of the
hair (5-week-old males; Japan SLC. Inc., Shizuoka, Japan). The
subsequent experiments described below were conducted on tumors
that grew to a macroscopically detectable size (>3 mm in
diameter) within 10 days.
Light sources
A xenon lamp was used as the source of the flash
wave light (FWL) (12). The
illumination machine KFS-30HJ (Ushio Electric Inc., Tokyo, Japan)
was used for the FWL illumination. The FWL light illumination
frequency was 60 Hz and the pulse width was <1 ms. The energy
generated by one-shot illumination with FWL was 15 J and the
illumination level was 1,000,000 lux.
Tumor growth inhibition by AO-PDT using
FWL
The tumor-bearing mice were divided randomly into 4
groups of 5 mice each: group 1, no treatment
(AO−/L−); group 2, illumination with FWL
alone for 10 min (AO−/10 min FWL); group 3, intravenous
administration of AO at 1.0 mg/kg alone (1.0 mg/kg
AO/L−) and group 4, intravenous administration of AO at
1.0 mg/kg AO followed by illumination with FWL for 10 min (1.0
mg/kg AO/10 min FWL). The tumor-bearing mice administered with AO
via the tail vein were exposed to FWL illumination for 10 min at 2
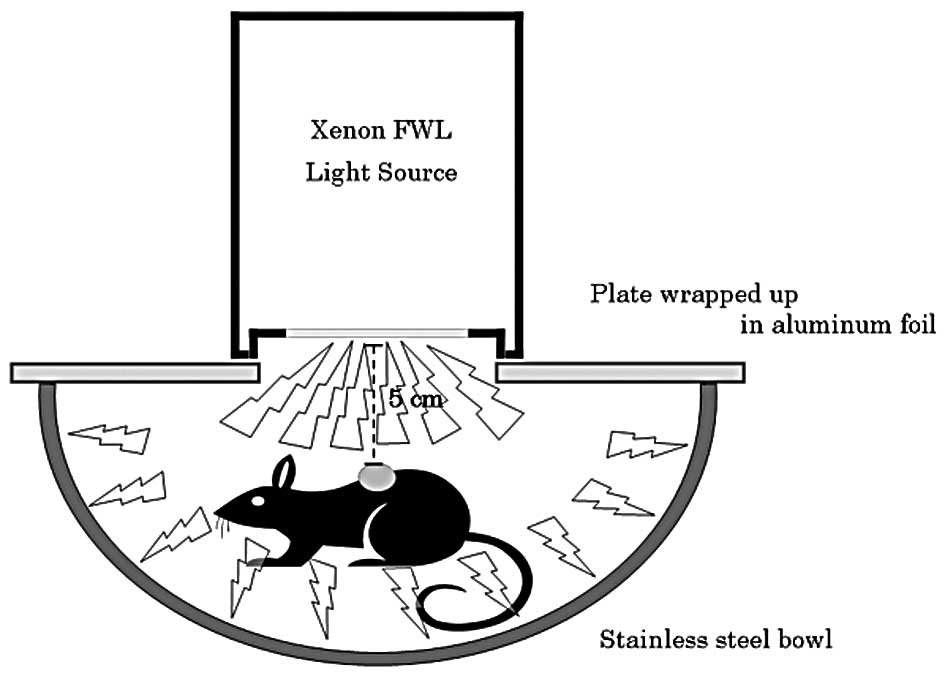
h after the AO injection. Fig. 1
shows the system used for the FWL illumination of the mice. Mice
administered with AO were set in a stainless steel bowl under
anesthesia induced by intraperitoneally administered pentobarbital
sodium, and then exposed to FWL illumination from the illumination
machine KFS-30HJ for 10 min. The tumor volume was sequentially
calculated, until 28 days after this treatment, from the measured
values of the maximum and minimum diameters as maximum diameter ×
minimum diameter 2/2 (13). AO was
used at a concentration of 1.0 mg/kg, since previous studies showed
that this concentration yields the strongest cytocidal effect and
lowest toxicity in mice (10). The
illumination time (10 min) and time-point of illumination (2 h
after AO injection) were also selected based on the results of
previous studies (9,10,14,15).
Histological and immunohistochemical
responses
The histological responses to AO-PDT using FWL were
comparatively evaluated in groups 1 and 4 three days after the
treatment, using sections of the resected tumors stained with
hematoxylin and eosin. Immunohistochemical analysis by TdT-mediated
dUTP-biotin nick end-labeling (TUNEL) was also performed in group
4.
Statistical analysis
Statistical analysis was performed using the
StatView statistical software (version 5.0; SAS Institute Inc.,
Cary, NC, USA). Significant differences among the various groups
were evaluated using Student’s t-test. P<0.05 was considered to
be significant.
Experiments were performed in accordance with the
guidelines in the Declaration of Helsinki and the Interdisciplinary
Principles and Guidelines for the Use of Animals in Research,
Testing and Education.
Results
Tumor growth inhibition by AO-PDT using
FWL
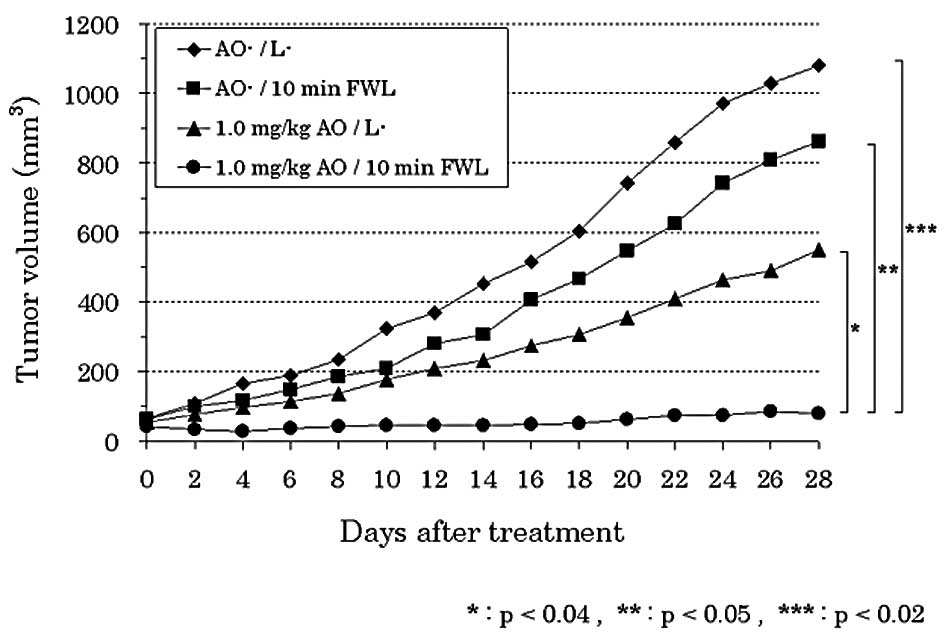
A significant decrease in the tumor volume was
observed in group 4 (1.0 mg/kg AO/10 min FWL) as compared to that
in groups 1 (AO−/L−) (p<0.02), 2
(AO−/10 min FWL) (p<0.05) or 3 (1.0 mg/kg
AO/L−) (p<0.04). The tumor volume tended to decrease
in group 3 as compared to that in groups 1 or 2. However, there
were no significant differences among the three groups (Fig. 2).
Histological and immunohistochemical
responses
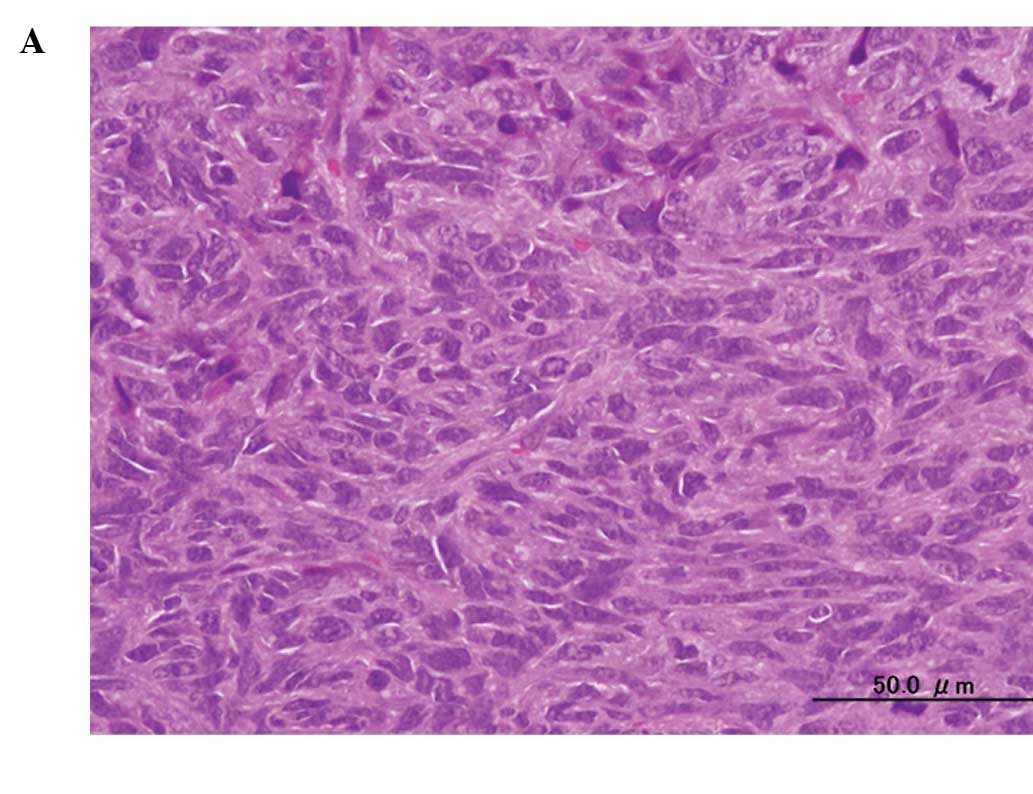
A histological examination of the tumors showed
substantial cell necrosis in group 4, but not in group 1. The
surviving tumor cells in group 4 showed large pyknotic nuclei
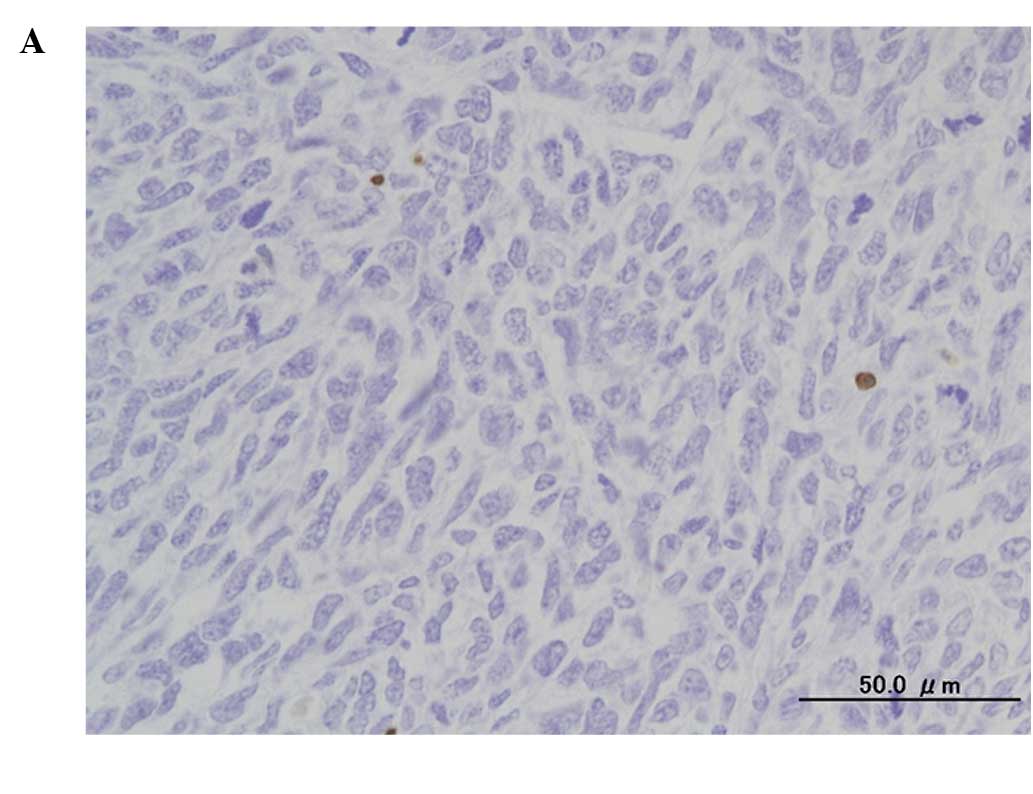
(Fig. 3). Immunohistochemical
analysis by TUNEL assay revealed the presence of numerous apoptotic
cells (Fig. 4).
Discussion
Our previous intensive basic investigation and
clinical trials showed the strong cytocidal effect of photodynamic
and/or radiodynamic therapy with acridine orange (AO-PDT and
AO-RDT). Findings suggest that this type of treatment modality is
useful with minimally invasive surgery for patients with high-grade
malignant musculoskeletal sarcomas, as it allows for the
maintenance of excellent limb function and is correlated with a low
risk of local recurrence (3–10,14–19).
However, further improvements in the techniques of this innovative
modality are required. At present, in clinical AO-PDT, AO is
administrated locally, not systemically, and any residual tumor
after intralesional resection is illuminated by CWL from a xenon
lamp. We contended that for the homogeneous uptake of AO by the
entire tumor burden in the human body, intravenous administration
may be better than local administration and that stronger
excitation with a high-power light may yield a stronger cytocidal
effect. To confirm the latter hypothesis, we performed an in
vitro study using a mouse osteosarcoma cell line in which
AO-PDT was achieved using high-power FWL, which is commonly used
for strobe photos and has a stronger illumination at lower energy
levels than CWL. After a 10-min illumination and following exposure
to AO at the concentration of 1.0 mg/ml, FWL showed a stronger
cytocidal effect than CWL on the mouse osteosarcoma cell line, LM8
(9). Consequently, this in
vivo study was conducted. Previously, we reported that the
intraperitoneal administration of AO at 10 mg/kg followed by blue
light excitation inhibited the tumor growth of osteosarcoma
developing from a different cell line (MOS) than LM8 in vivo
(14,15,18,19).
Recently, we also found that the intravenous administration of AO
at 1.0 mg/kg is useful for PDD of mouse osteosarcomas in nude mice.
AO initially binds to both tumor and normal tissues, such as
muscles and adipose tissue. However, after 2 h AO is quickly
excluded from the normal, but not malignant, tissues. This
exclusion produces a significant difference of the AO fluorescence
intensity between tumor and normal tissues, thereby allowing
visualization of the tumor tissue alone which fluoresces under blue
excitation light. In the PDD study, we determined that the LD50 of
the intravenously administered AO was 27.3 mg/kg and that none of
the mice showed fetal complications after administration of the
compound at a dose of <27.3 mg/kg. Therefore, AO at the
concentration of 1.0 mg/kg should be safe for humans (10).
The findings of this study showed that the growth of
mouse osteosarcoma tumors was significantly inhibited in the group
treated with AO-PDT using FWL following the intravenous
administration of AO, as compared with that in the control groups,
including the non-treatment, AO-alone and FWL-alone treatment
groups. Histological and immunohistochemical examinations on day 3,
following AO-PDT, showed that apoptosis and necrosis were induced
in the tumor cells (20,21), although we have yet to clarify the
precise genetic pathway leading to cell death induced by AO-PDT.
None of the mice treated with AO-PDT died. Therefore, we
hypothesize that AO-PDT, with AO intravenously administered at 1.0
mg/kg, followed by excitation using FWL, shows cytocidal effects
against osteosarcoma cells. As demonstrated in our previous in
vitro study, the use of FWL for AO-PDT yields a stronger
cytocidal effect than that of CWL (9). Since the results showed that the
intravenous administration of AO at 1.0 mg/kg is useful for AO-PDT
and is safe for mice, AO may also be applicable in humans, although
intensive clinical studies are needed to confirm the efficacy and
toxicity of AO administered by intravenous injection.
In conclusion, AO-PDT using FWL, following the
intravenous administration of AO, exerted strong anti-tumor
activity against the primary tumor. Consequently, this treatment
modality is applicable for the treatment of sarcomas as well as
carcinomas in humans, and in future, will be employed as an
innovative modality for cancer therapy.
References
|
1
|
Dougherty TJ and Marcus SL: Photodynamic
therapy. Eur J Cancer. 28A:1734–1742. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moan J and Berg K: Photochemotherapy of
cancer: experimental research. Photochem Photobiol. 55:931–948.
1992. View Article : Google Scholar
|
|
3
|
Kusuzaki K, Murata H, Matsubara T, et al:
Clinical trial of photodynamic therapy using acridine orange
with/without low dose radiation as new limb salvage modality in
musculoskeletal sarcomas. Anticancer Res. 25:1225–1236.
2005.PubMed/NCBI
|
|
4
|
Kusuzaki K, Murata H, Matsubara T, et al:
Clinical outcome of a novel photodynamic therapy technique using
acridine orange for synovial sarcomas. Photochem Photobiol.
81:705–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida K, Kusuzaki K, Matsubara T, et al:
Periosteal Ewing’s sarcoma treated by photodynamic therapy with
acridine orange. Oncol Rep. 13:279–282. 2005.
|
|
6
|
Kusuzaki K, Murata H, Matsubara T,
Satonaka H, Wakabayashi T, Matsumine A and Uchida A: Acridine
orange could be an innovative anticancer agent under photon energy
(Review). In Vivo. 21:205–214. 2007.PubMed/NCBI
|
|
7
|
Nakamura T, Kusuzaki K, Matsubara T,
Matsumine A, Murata H and Uchida A: A new limb salvage surgery in
cases of high-grade soft tissue sarcoma using photodynamic surgery,
followed by photo- and radiodynamic therapy with acridine orange. J
Surg Oncol. 97:523–528. 2008. View Article : Google Scholar
|
|
8
|
Ueda H, Murata H, Takeshita H, Minami G,
Hashiguchi S and Kubo T: Unfiltered xenon light is useful for
photodynamic therapy with acridine orange. Anticancer Res.
25:3979–3984. 2005.PubMed/NCBI
|
|
9
|
Satonaka H, Kusuzaki K, Matsubara T, et
al: Flash wave light strongly enhanced the cytocidal effect of
photodynamic therapy with acridine orange on a mouse osteosarcoma
cell line. Anticancer Res. 27:3339–3344. 2007.PubMed/NCBI
|
|
10
|
Satonaka H, Kusuzaki K, Matsubara T,
Shintani K, Wakabayashi T, Matsumine A and Uchida A: Extracorporeal
photodynamic image detection of mouse osteosarcoma in soft tissues
utilizing fluorovisualization effect of acridine orange. Oncology.
70:465–473. 2006. View Article : Google Scholar
|
|
11
|
Asai T, Ueda T, Itoh K, Yoshioka K, Aoki
Y, Mori S and Yoshikawa H: Establishment and characterization of a
murine osteosarcoma cell line (LM8) with high metastatic potential
to the lung. Int J Cancer. 76:418–422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimura M, Kashikura K, Yokoi S, Koiwa Y,
Tokuoka Y and Kawashima N: Photodynamic therapy for cancer cells
using a flash wave light xenon lamp. Opt Rev. 12:207–210. 2005.
View Article : Google Scholar
|
|
13
|
Ovejera AA, Houchens DP and Barker AD:
Chemotherapy of human tumor xenografts in genetically athymic mice.
Ann Clin Lab Sci. 8:50–56. 1978.PubMed/NCBI
|
|
14
|
Kusuzaki K, Suginoshita T, Minami G, et
al: Fluorovisualization effect of acridine orange on mouse
osteosarcoma. Anticancer Res. 20:3019–3024. 2000.PubMed/NCBI
|
|
15
|
Kusuzaki K, Minami G, Takeshita H, et al:
Photodynamic inactivation with acridine orange on a
multidrug-resistant mouse osteosarcoma cell line. Jpn J Cancer Res.
91:439–445. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsubara T, Kusuzaki K, Matsumine A,
Shintani K, Satonaka H and Uchida A: Acridine orange used for
photodynamic therapy accumulates in malignant musculoskeletal
tumors depending on pH gradient. Anticancer Res. 26:187–194.
2006.PubMed/NCBI
|
|
17
|
Kusuzaki K, Murata H, Takeshita H, et al:
Intracellular binding sites of acridine orange in living
osteosarcoma cells. Anticancer Res. 20:971–976. 2000.PubMed/NCBI
|
|
18
|
Kusuzaki K, Aomori K, Suginoshita T, et
al: Total tumor cell elimination with minimum damage to normal
tissues in musculoskeletal sarcomas following photodynamic therapy
with acridine orange. Oncology. 59:174–180. 2000. View Article : Google Scholar
|
|
19
|
Hashiguchi S, Kusuzaki K, Murata H, et al:
Acridine orange excited by low-dose radiation has a strong
cytocidal effect on mouse osteosarcoma. Oncology. 65:85–93.
2005.PubMed/NCBI
|
|
20
|
Kessel D and Luo Y: Mitochondrial
photodamage and PDT-induced apoptosis. Photochem Photobiol.
42:89–85. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zdolsek JM, Olson M and Brunk UT:
Photooxidative damage to lysosomes of cultured macrophages by
acridine orange. Photochem Photobiol. 51:67–76. 1990. View Article : Google Scholar : PubMed/NCBI
|