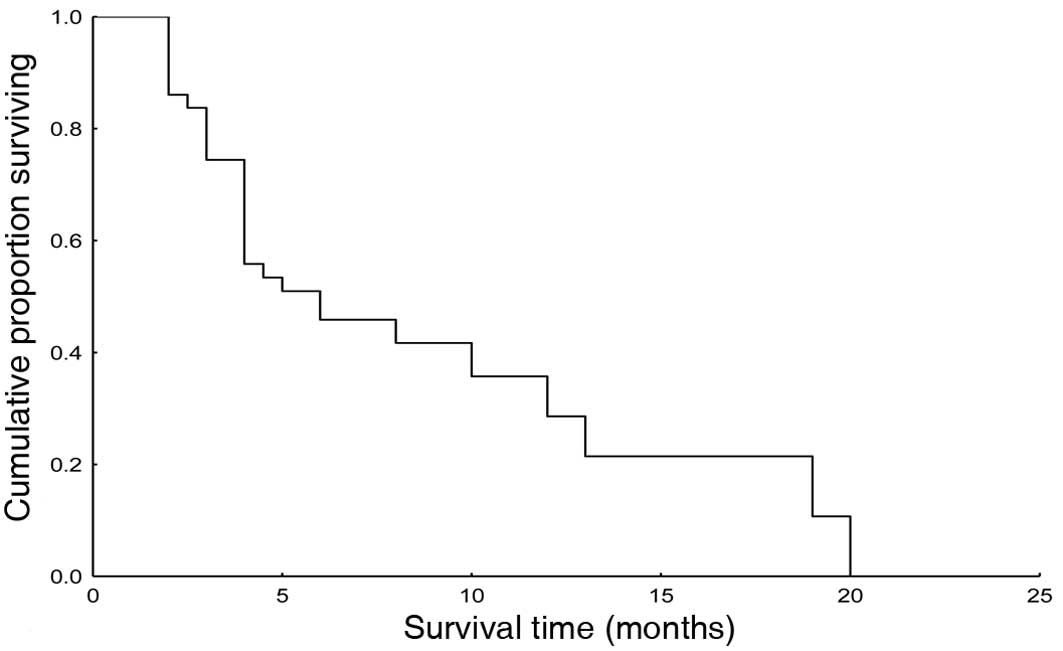
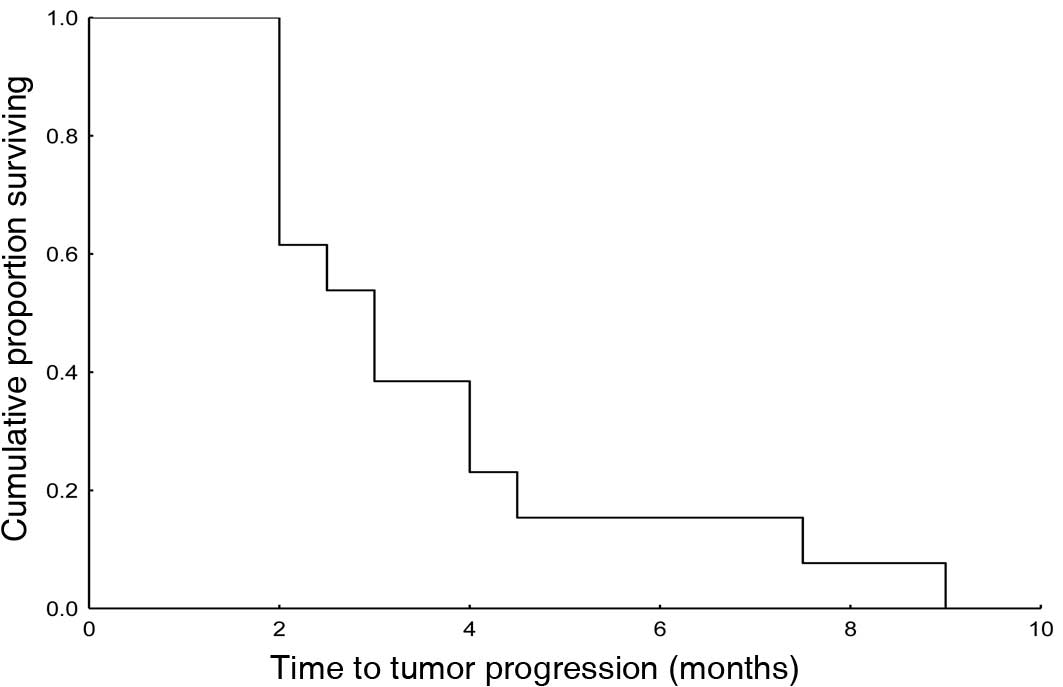
|
1
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jänne PA, Engelman JA and Johnson BE:
Epidermal growth factor receptor mutations in non-small cell lung
cancer: implications for treatment and tumor biology. J Clin Oncol.
23:3227–3234. 2005.PubMed/NCBI
|
|
3
|
Swinson DE, Cox G and O’Byrne KJ:
Coexpression of epidermal growth factor receptor with related
factors is associated with poor prognosis in non-small cell lung
cancer. Br J Cancer. 91:1301–1307. 2004. View Article : Google Scholar
|
|
4
|
Comis RL: The current situation: erlotinib
(tarceva) and gefitinib (iressa) in non-small cell lung cancer.
Oncologist. 10:467–470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Byoung C-C, Im C-K, Park M-S, et al: Phase
II study of erlotinib in advanced non-small cell lung cancer after
failure of gefitinib. J Clin Oncol. 25:2528–2533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shepherd FA, Pereira JR, Ciuleanu T, et
al: Erlotinib in previously treated non-small cell lung cancer. N
Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a Phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
8
|
Therasse P, Arbuck SG, Eisenhawer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen S: The epidermal growth factor
(EGF). Cancer. 51:1787–1791. 1983. View Article : Google Scholar
|
|
10
|
Yarolen Y and Schlessinger J: Epidermal
growth factor induces rapid reversible aggregation of the purified
epidermal growth factor receptor. Biochemistry. 26:1443–1451. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsuan JJ: Oncogene regulation by growth
factors. Anticancer Res. 13:2521–2522. 1993.PubMed/NCBI
|
|
12
|
Soler C, Beguinot L and Carpenter G:
Individual epidermal growth factor receptor autophosphorylation
sites do not stringently define association motifs for several SH-2
containing proteins. J Biol Chem. 269:12320–12324. 1994.
|
|
13
|
Voldborg RB, Damstrup L, Spang-Thomsen M,
et al: Epidermal growth factor receptor (EGFR) and EGFR mutations,
function and possible role in clinical trials. Ann Oncol.
8:1197–1206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ann J-H, Kim S-W, Hong S-M, et al:
Epidermal growth factor receptor (EGFR) expression in operable
non-small cell lung carcinoma. J Korean Med Sci. 19:529–535. 2004.
View Article : Google Scholar
|
|
15
|
Sainsbury JRC, Nicholson S, Angus B, et
al: Epidermal growth factor receptor status of histological
sub-types of breast cancer. Br J Cancer. 58:458–460. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yasui W, Sumiyoshi H, Hata J, et al:
Expression of epidermal growth factor receptor in human gastric and
colonic carcinomas. Cancer Res. 48:137–141. 1988.PubMed/NCBI
|
|
17
|
Smith K, Fenelly JA, Neal DE, et al:
Characterization and quantitation of the epidermal growth factor
receptor in invasive and superficial bladder tumors. Cancer Res.
49:5810–5815. 1989.PubMed/NCBI
|
|
18
|
Veale D, Kerr N, Gibson GJ, et al:
Characterization of epidermal growth factor receptor in primary
human non-small cell lung cancer. Cancer Res. 49:1313–1317.
1989.PubMed/NCBI
|
|
19
|
Fujino S, Enokibori T, Tezuka N, et al: A
comparison of epidermal growth factor receptor levels and other
prognostic parameters in non-small cell lung cancer. Eur J Cancer.
32A:2070–2074. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hortobagyi GN and Santer G: Challenges and
opportunities for erlotinib (tarceva). What does the future hold?
Semin Oncol. 30(Suppl 7): 47–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arteaga CL: The epidermal growth factor
receptor: from mutant oncogene in nonhuman cancers to therapeutic
target in human neoplasias. J Clin Oncol. 19:S32–S40.
2001.PubMed/NCBI
|
|
22
|
Perez-Soler R: HER1/EGFR targeting:
refining the strategy. Oncologist. 9:58–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weinstein IB: Addiction to oncogenes – the
Achilles heal of cancer. Science. 297:63–64. 2002.
|
|
24
|
Crino L, Eberhardt W, Boyer M, et al:
Erlotinib as second-line therapy in patients (PTS) with advanced
non-small cell lung cancer (NSCLC) and good performance status:
interim analyses from the TRUST Study. In: Abstract Book 33rd ESMO
Congress; (Suppl 8): pp. 2642008
|
|
25
|
Groen H, Barata F, McDermott R, et al:
Efficacy of erlotinib in >4000 patients (PTS) with advanced
non-small cell lung cancer (NSCLC): analysis of the European
subpopulation on the TRUST Study. In: Abstract Book 33rd ESMO
Congress; (Suppl 8): pp. 2662008
|
|
26
|
Reck M, Mali P, Arrieta O, et al: Global
efficacy and safety results from the TRUST study of erlotinib
monotherapy in >7000 patients (PTS) with non-small cell lung
cancer (NSCLC). In: Abstract Book 33rd ESMO Congress; (Suppl 8):
pp. 2622008
|