Introduction
Erlotinib is an oral, small-molecule targeting
therapy that inhibits the epidermal growth factor receptor (EGFR)
of tyrosine kinase, blocking signal transduction pathways
implicated in the proliferation and survival of cancer cells
(1). EGFR is associated with
cellular processes leading to tumorigenesis (2,3). Data
exist concerning erlotinib administration for malignant tumors,
mainly pancreatic cancer, in combination with another cytotoxic
agent, as well as for non-small cell lung cancer (NSCLC) in a large
number of patients as a second-line treatment (4). Erlotinib has provided a survival
benefit for advanced NSCLC patients (5,6). The
data reported by two Phase III studies led to US Food and Drug
Administration (FDA) approval for the use of erlotinib in NSCLC
patients after first-line chemotherapy failure. A survival benefit
was demonstrated in patients with advanced pancreatic cancer when
erlotinib was combined with gemcitabine vs. gemcitabine alone
(7). A survival benefit was even
shown in several subsets of NSCLC patients such as those with
squamous cell carcinoma, smokers and males, where gefitinib did not
appear to be active (5).
Serious adverse reactions are uncommon. The most
common side effects are skin rash and serious grade 3–4 anorexia
followed by fatigue, vomiting and stomatitis which were reported to
be less than 1%. Grade 3–4 diarrhea was also less than 1% (6).
The present study involves erlotinib monotherapy in
pretreated patients with advanced NSCLC. The primary objective was
to determine the response rate and survival in pretreated patients,
and the secondary objective was to determine toxicity.
Materials and methods
Patient eligibility
Eligibility for the study involved histologically or
cytologically confirmed NSCLC, disease staging and a defined
inoperable stage IIIB or IV. Stage IIIA was only included in case
of chronic respiratory insufficiency which did not permit surgery.
A requirement was that patients had to have undergone one or two
lines of prior chemotherapy. Radiation therapy was not excluded as
a previous treatment. Bidimensionally measurable disease criteria
were: physical examination, X-rays, computed tomography (CT), World
Health Organization (WHO) performance status 0–2, expected survival
≥12 weeks, adequate bone marrow reserves (leukocyte count ≥3,500
μl−1, platelet count ≥100,000 μl−1 and
hemoglobin ≥10 g dl−1), adequate renal function (serum
creatinine ≤1.5 mg/dl−1 and serum transaminases ≤3 times
the upper normal limit or ≤5 times the upper normal limit in cases
of liver metastases) and age ≥18 years. In cases of central nervous
system involvement or any secondary malignancy, patients were
excluded. The study was conducted with the approval of our
institutional review boards, and all patients gave their written
informed consent before enrollment.
Treatment
Erlotinib was administered at a dose of 150 mg (1
tablet) per day. In case of adverse reactions, treatment was either
reduced to 100 mg or interrupted for a maximum of two weeks.
Otherwise, treatment was continued until disease progression,
intolerable toxicity or refusal to continue.
Previous treatment
Before entering the study, patients had received
chemotherapy based on cisplatin (44 patients) or carboplatin (5
patients). The second agent of the combination was paclitaxel (40
patients), vinorelbine (4 patients), gemcitabine (3 patients) or
etoposide (2 patients). Eleven patients underwent second-line
chemotherapy 3–9 months after the end of the first-line treatment.
The agents administered for the second-line chemotherapy included
docetaxel, pemetrexed or etoposide. Five patients received a
combination of the first two aforementioned agents and 6 received a
single treatment of one of the three agents (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| n (%) |
|---|
| Patients
enrolled | 54 (100) |
| Patients
assessable | 54 (100) |
| Gender |
| Male | 38 (70.37) |
| Female | 16 (29.63) |
| Age |
| Median | 65 |
| Range | 37–81 |
| WHO performance
status |
| 0 | 5 (9.26) |
| 1 | 43 (79.63) |
| 2 | 6 (11.11) |
| Disease stage |
| IIIB | 25 (46.30) |
| IV | 29 (53.70) |
| Histology |
| Adenocarcinoma | 25 (46.29) |
| Squamous cell
carcinoma | 19 (35.19) |
|
Undifferentiated | 10 (18.52) |
| Prior treatment |
| First-line |
|
Cisplatin-paclitaxel | 40 (74.07) |
|
Cisplatin-vinorelbine | 4 (7.41) |
|
Carboplatin-gemzar | 3 (5.56) |
|
Carboplatin-etoposide | 2 (3.70) |
| Second-line |
|
Docetaxel-gemcitabine | 3 (27.27) |
|
Carboplatin-etoposide | 2 (18.18) |
| Docetaxel | 3 (27.27) |
| Vinorelbine | 1 (9.09) |
| Pemetrexed | 2 (18.18) |
Baseline and treatment assessment and
evaluation
Before enrollment, the patients underwent physical
examination, tumor measurement and evaluation, WHO performance
status, electrocardiogram, full blood count, renal and liver
function tests and urinalysis. Staging was determined by chest and
abdominal CT scans, bone scan and occasional magnetic resonance
imaging. Blood counts, blood urea and serum creatinine were
measured before each treatment administration and every 3 weeks
thereafter. During the treatment period, radiologic tests were
conducted: a chest X-ray once every 3 weeks and CT once every 2
months, or whenever there were signs of disease progression.
Imaging-based evaluation was used to assess response. A complete
response (CR) was defined as the disappearance of all measurable
disease, confirmed at 4 weeks at the earliest. A partial response
(PR) was defined as a 30% decrease, confirmed at 4 weeks at the
earliest. In stable disease (SD), neither PR nor progressive
disease (PD) criteria were met; PD involved a 20% increase in tumor
burden but no CR, PR or SD before increased disease. Response data
were based on the response evaluation criteria in solid tumors
(8). A two-step deterioration in
performance status, a >10% loss in pretreatment weight or
increasing symptoms did not by themselves constitute progression of
the disease. However, the appearance of these signs was followed by
a new evaluation of the extent of the disease. Responses had to be
maintained for at least 4 weeks and to be confirmed by two
independent radiologists and two experienced oncologists.
Statistical design
This was an expected two-step Phase II study and an
intent-to-treat analysis. According to the trial design, 30
patients were to be enrolled during the first part of the study and
if an objective response rate of <15% was achieved, the
treatment would have been abandoned; otherwise, 20 additional
patients were to be enrolled. The primary objective of the study
was to determine the efficacy of the regimen with respect to
response and survival, and the secondary objective was to determine
the toxicity. Survival was calculated from the day of enrollment
until death or the end of the study. The median probability of
survival was estimated by the Kaplan-Meier method; confidence
intervals (CIs) for response rates were calculated using methods
for the exact binomial CI.
Results
From April 2007 to December 2008, 54 patients with
advanced or metastatic NSCLC were enrolled in the present trial.
The patients were considered evaluable for response, toxicity and
survival. Patient characteristics at baseline are shown in Table I. The median age was 65 years (range
37–81) and their WHO performance status was 0–2. All patients had
undergone prior chemotherapy mainly based on cisplatin or a
carboplatin combination. Eleven of the 54 patients had undergone
second-line chemotherapy before entering the present trial. The
first- and second-line treatment is shown in Table I. The median duration of treatment
was 4 months (range 1.5–18); 40 patients (70.07%) underwent 4–18
months of treatment.
Response
Of the 54 assessable patients, an objective response
rate was observed in 10 (18.52%) patients, while the median
duration of response was 6 months (range 3–8). Stable disease was
observed in 40 patients (74.07%) and disease progression in 4
patients (7.41%). The response data are documented in Table II.
 | Table IIResponse rate. |
Table II
Response rate.
| n (%) |
|---|
| Partial response | 10 (18.52) |
| Stable disease | 40 (74.07) |
| Disease
progression | 4 (7.41) |
Survival data
At the end of the study, 15 of the 54 patients were
still alive (27.77%). The median follow-up was 8 months (range
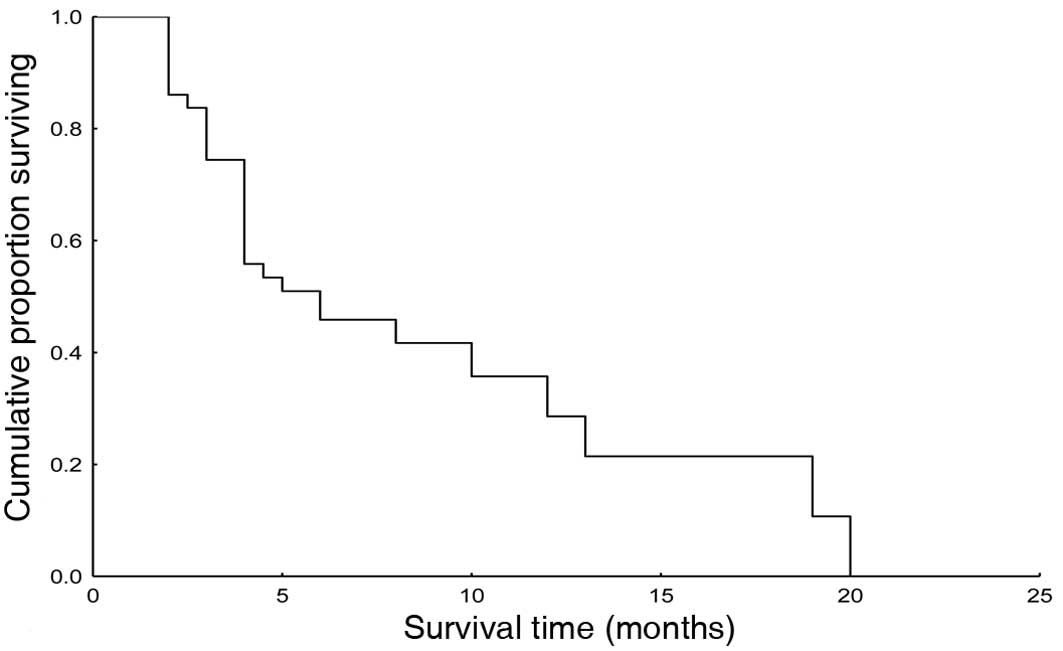
3–20). The median survival time was 6 months (95% CI 1.7–10.3).
Fig. 1 shows the Kaplan-Meier
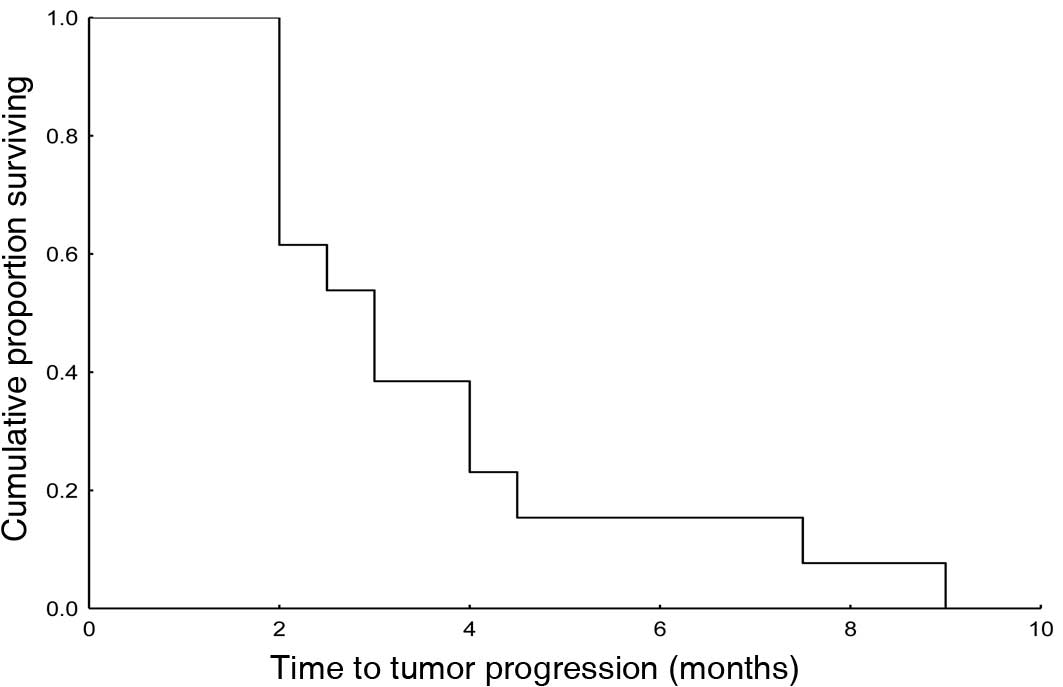
survival curve. The median time to tumor progression (TTP) was 3
months (95% CI 1.9–4.1). TTP is shown in Fig. 2 (Kaplan-Meier).
Toxicity
Grade 1–2 skin rash was the main adverse reaction
observed in 53 of the 54 patients (98.15%). Grade 3 skin rash was
observed in 2 patients (3.70%) and this proved to be intolerable.
Thus, the dose of erlotinib was decreased from 150 to 100 mg. Other
non-hematologic toxicities were grade 1–2 diarrhea in 9 patients
(16.66%), nausea and vomiting in 4 (7.41%) and gastritis in 2
(3.70%). Hematologic toxicity (leukopenia and thrombocytopenia) was
not observed in 50 of the 54 patients (92.59%).
Discussion
One of the first growth factors discovered was the
epidermal growth factor (EGF) (9).
It is a protein which binds to a cell surface growth factor
receptor, the EGFR. In binding to the receptor, EGF either induces
cell proliferation or differentiation in mammalian cells (10).
The binding of a ligand to the EGFR induces
conformational changes within the receptor that increases the
catalytic activity of its intrinsic tyrosine kinase, resulting in
autophosphorylation which is necessary for biological activity
(11,12). Protein tyrosine kinase activity
plays a key role in the regulation of cell proliferation and
differentiation (13). A large
number of deletions of the EGFR in RNA have been observed in a
number of neoplasias such as glioblastoma in non-small cell lung
carcinomas, breast cancer, pediatric gliomas, medulloblastomas and
ovarian carcinomas (13).
Overexpression of mRNA and/or the protein encoded by the EGFR gene
has been observed in many types of human malignancies (14), including breast (15), gastric, colorectal (16) and bladder cancer (17). In NSCLC, EGFR expression at
percentages varying from 30 to 70% has been reported (18,19).
Erlotinib is an anti-EGFR targeting agent; studies have already
been performed and reports concerning its value have been
documented (20,21). However, despite the fact that
targeting therapy has been administered in a considerable number of
clinical trials over the recent years, there are many unanswered
questions related to the failure to achieve the expected success
and to explain certain adverse reactions or complications. Tumors
are likely to express variable but excessive numbers of HER1/EGFRs.
Unless all receptors are effectively inhibited from initiating
signaling, there is likely to be sufficient residual tumorigenic
activity to maintain disease (22).
Evidence, although unconfirmed, suggests that cancer types become
dependent on one or more specific elements of the cell signaling
circuit, requiring their continued presence in order to remain
malignant (23).
The results of the present trial showed that the
response rate is higher than that of the 8.5 and 9.5% reported in
previous studies (5,6). Of note is the high disease stability
(74.07%) and the median TTP of 4 months. Three other studies
reported a) a response rate of 13%, a SD rate of 54% and a
progression-free survival (PFS) of 9.7 weeks in 3,338 patients
(24); b) PR 9%, SD 67% and PFS
12.3 weeks in 4,002 patients (25);
and c) PR 12%, SD 56% and PFS 14.3 weeks in 6,809 patients
(26).
Erlotinib may be an eligible second-line treatment
for NSCLC patients. The majority of patients tolerate the treatment
and adverse reactions. Low toxicity increases the tendency to
support the use of erlotinib in pretreated NSCLC patients.
References
|
1
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jänne PA, Engelman JA and Johnson BE:
Epidermal growth factor receptor mutations in non-small cell lung
cancer: implications for treatment and tumor biology. J Clin Oncol.
23:3227–3234. 2005.PubMed/NCBI
|
|
3
|
Swinson DE, Cox G and O’Byrne KJ:
Coexpression of epidermal growth factor receptor with related
factors is associated with poor prognosis in non-small cell lung
cancer. Br J Cancer. 91:1301–1307. 2004. View Article : Google Scholar
|
|
4
|
Comis RL: The current situation: erlotinib
(tarceva) and gefitinib (iressa) in non-small cell lung cancer.
Oncologist. 10:467–470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Byoung C-C, Im C-K, Park M-S, et al: Phase
II study of erlotinib in advanced non-small cell lung cancer after
failure of gefitinib. J Clin Oncol. 25:2528–2533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shepherd FA, Pereira JR, Ciuleanu T, et
al: Erlotinib in previously treated non-small cell lung cancer. N
Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a Phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
8
|
Therasse P, Arbuck SG, Eisenhawer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen S: The epidermal growth factor
(EGF). Cancer. 51:1787–1791. 1983. View Article : Google Scholar
|
|
10
|
Yarolen Y and Schlessinger J: Epidermal
growth factor induces rapid reversible aggregation of the purified
epidermal growth factor receptor. Biochemistry. 26:1443–1451. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsuan JJ: Oncogene regulation by growth
factors. Anticancer Res. 13:2521–2522. 1993.PubMed/NCBI
|
|
12
|
Soler C, Beguinot L and Carpenter G:
Individual epidermal growth factor receptor autophosphorylation
sites do not stringently define association motifs for several SH-2
containing proteins. J Biol Chem. 269:12320–12324. 1994.
|
|
13
|
Voldborg RB, Damstrup L, Spang-Thomsen M,
et al: Epidermal growth factor receptor (EGFR) and EGFR mutations,
function and possible role in clinical trials. Ann Oncol.
8:1197–1206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ann J-H, Kim S-W, Hong S-M, et al:
Epidermal growth factor receptor (EGFR) expression in operable
non-small cell lung carcinoma. J Korean Med Sci. 19:529–535. 2004.
View Article : Google Scholar
|
|
15
|
Sainsbury JRC, Nicholson S, Angus B, et
al: Epidermal growth factor receptor status of histological
sub-types of breast cancer. Br J Cancer. 58:458–460. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yasui W, Sumiyoshi H, Hata J, et al:
Expression of epidermal growth factor receptor in human gastric and
colonic carcinomas. Cancer Res. 48:137–141. 1988.PubMed/NCBI
|
|
17
|
Smith K, Fenelly JA, Neal DE, et al:
Characterization and quantitation of the epidermal growth factor
receptor in invasive and superficial bladder tumors. Cancer Res.
49:5810–5815. 1989.PubMed/NCBI
|
|
18
|
Veale D, Kerr N, Gibson GJ, et al:
Characterization of epidermal growth factor receptor in primary
human non-small cell lung cancer. Cancer Res. 49:1313–1317.
1989.PubMed/NCBI
|
|
19
|
Fujino S, Enokibori T, Tezuka N, et al: A
comparison of epidermal growth factor receptor levels and other
prognostic parameters in non-small cell lung cancer. Eur J Cancer.
32A:2070–2074. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hortobagyi GN and Santer G: Challenges and
opportunities for erlotinib (tarceva). What does the future hold?
Semin Oncol. 30(Suppl 7): 47–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arteaga CL: The epidermal growth factor
receptor: from mutant oncogene in nonhuman cancers to therapeutic
target in human neoplasias. J Clin Oncol. 19:S32–S40.
2001.PubMed/NCBI
|
|
22
|
Perez-Soler R: HER1/EGFR targeting:
refining the strategy. Oncologist. 9:58–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weinstein IB: Addiction to oncogenes – the
Achilles heal of cancer. Science. 297:63–64. 2002.
|
|
24
|
Crino L, Eberhardt W, Boyer M, et al:
Erlotinib as second-line therapy in patients (PTS) with advanced
non-small cell lung cancer (NSCLC) and good performance status:
interim analyses from the TRUST Study. In: Abstract Book 33rd ESMO
Congress; (Suppl 8): pp. 2642008
|
|
25
|
Groen H, Barata F, McDermott R, et al:
Efficacy of erlotinib in >4000 patients (PTS) with advanced
non-small cell lung cancer (NSCLC): analysis of the European
subpopulation on the TRUST Study. In: Abstract Book 33rd ESMO
Congress; (Suppl 8): pp. 2662008
|
|
26
|
Reck M, Mali P, Arrieta O, et al: Global
efficacy and safety results from the TRUST study of erlotinib
monotherapy in >7000 patients (PTS) with non-small cell lung
cancer (NSCLC). In: Abstract Book 33rd ESMO Congress; (Suppl 8):
pp. 2622008
|