Introduction
Unlimited proliferation potential is one of the
hallmarks of cancer cells. Breast cancer is one of the major causes
of morbidity and mortality in women worldwide, with millions
diagnosed annually. Although great strides have been made in
understanding and treating breast cancer, there exists a need for
novel target identification and new therapeutic strategies to
combat the disease (1). Breast
cancer cells express a spectrum of molecular motifs encompassing
various subtypes. Significant advancement has occurred in breast
cancer treatment due to the introduction of hormonal therapy as
well as HER2-directed immunotherapy (1). However, microarray analysis has led to
the discovery of breast cancer types, including ER−
(estrogen receptor-negative), PR− (progesterone
receptor-negative) and HER2− (human epidermal growth
factor receptor-negative) that resemble the basal-like subtype. Due
to the refractory nature of this subtype of breast cancer to the
presently available therapies, efforts have been intensified in the
determination of novel diagnostic markers/therapeutic targets.
The transformation of a normal epithelial cell to a
cancer cell is a multi-step process (2), involving pathways that harbor
mutations in p53, the retinoblastoma gene pRb, BRCA1 (3,4) and
changes in the Ras signaling pathway. Apart from oncogene
activation and alteration in the tumor suppressor genes, the
activation of a unique ribonucleoprotein termed telomerase
(5), plays a pivotal role in the
process of immortalization and is a key regulator of cancer cell
survival. Its function is to restore telomeric sequences which are
unique tandem repeats capping chromosomal termini (6). The in vitro replicative
lifespan of normal cells is limited to a definite number of cell
divisions due to the end replication problem, resulting in the loss
of telomeric sequences (7).
However, the activation of telomerase to restore the telomeric
sequences has been reported in a wide array of tumors and over 80%
of tumors possess telomerase activity (8). Apart from its well-known function of
extending telomeres, telomerase is also reported to have a role in
protecting them (9). Functionally,
different forms of telomerase with processive property, leading to
long telomeric sequences, and non-processive telomerase, generating
short sequences, have been reported in acute myelogenous leukemia
(AML) patients. Moreover, mutations in telomerase reverse
transcriptase (hTERT) leading to reduced enzyme activity are
reported to predispose to AML (10,11).
Xu and Blackburn (12) discovered a
distinct class of extremely short telomeres termed t-stumps.
T-stumps are known to accumulate in telomerase-containing cells
that lack checkpoint pathways involving p53 and/or pRb. Therefore,
telomerase may have another role in protecting t-stumps and
preventing their loss. Notably, the loss of a single telomere is
known to lead to the activation of DNA damage response pathways
(13), causing cell senescence/cell
death. This suggests that a potent inhibitor of telomerase/telomere
maintenance machinery could potentially cause loss of protection of
t-stumps, resulting in cell death.
This study therefore aimed to identify the presence
of unique t-stumps in a panel of breast cancer cell lines. Isogenic
breast cancer cell lines with the immortalized MCF10A and the
highly advanced invasive/metastatic cell line MCF10CA1 were
selected. Furthermore, recent studies showed that the
MCF10A-derived series of cell lines have the ability to acquire a
basal-like phenotype (14).
Utilizing the advantages offered by single telomere length analysis
(STELA) in identifying very short telomeres as described by Xu and
Blackburn (12), the study focused
on the presence of distinct t-stumps in these basal-like breast
cancer cell lines. Moreover, the highly advanced,
invasive/metastatic breast cancer cell line, MCF10CA1, exhibited a
characteristic abundant cluster of telomere t-stumps. These
clusters were not observed in the immortalized MCF10A cell line.
The t-stumps therefore form novel diagnostic markers as well as key
therapeutic targets for chemotherapeutically recalcitrant breast
cancer types.
Materials and methods
Cell lines
MCF10A and MCF10CA1 cell lines were obtained from Dr
Fred R. Miller of the Barbara Ann Karmanos Cancer Institute,
Detroit, MI, USA. The cell lines were grown in Dulbecco’s modified
Eagle’s medium/F12 (1:1) with 10% fetal bovine serum, epidermal
growth factor (2.5 ng/ml), insulin (10 ng/ml), cholera toxin (100
ng/ml) and hydrocortisone (0.5 μg/ml). The MCF-7 cell line was
grown in Dulbecco’s modified Eagle’s medium alone containing 10%
fetal bovine serum and 1% penicillin/streptomycin.
Determination of telomerase activity
Amounts of cellular proteins extracted from cells
were estimated by Quick Start Bradford dye reagent protocol
(Bio-Rad). Telomerase activity was monitored according to the
TRAPeze telomerase detection protocol (Roche).
Extraction of genomic DNA and terminal
restriction fragment (TRF) analysis
Total genomic DNA was extracted using the
REDExtract-N-Amp Tissue PCR kit (Sigma). DNA amounts were
determined using Smartspec (Bio-Rad). Equal amounts of DNA (20 μg)
were then digested with restriction enzymes Rsa1 and
Hinf1, according to the manufacturer's protocol (Roche). The
digested DNA was separated on a 0.8% agarose gel, blotted onto
nylon membrane and hybridized to telomeric sequences. The probe was
generated by random labeling in the presence of α-P32
dCTP. The hybridized sequences were visualized by phosphorimaging
(Molecular Dynamics).
Western blotting
Cells were harvested and washed with 1X PBS and
suspended in RIPA lysis buffer containing protease inhibitors. The
cells were then incubated at 4°C for 30 min and then sonicated
prior to centrifugation at 13,500 rpm for 30 min. The samples were
subjected to electrophoresis using a 10% SDS-PAGE and transferred
to a PVDF membrane. The membrane was probed with a polyclonal
telomerase protein (hTERT) antibody (Abcam) and then to an
anti-β-actin polyclonal antibody to normalize for the levels of
protein loading. The binding of the proteins to the specific
antibodies was detected using a chemiluminescence protocol (Pierce)
and bands were visualized by exposure to X-ray film.
Identification of t-stumps and
primers
STELA was performed essentially as described by Xu
and Blackburn (12). Genomic DNA
was digested with EcoR1. Twenty ng of this digested DNA was
incubated in a 25-μl ligation reaction along with the telorette3
linker and 1–2 units of T4 DNA ligase (Promega), and then incubated
at 35°C overnight. The ligated DNA was diluted to 2 ng/μl for
further PCR. Each PCR was performed using Master Mix (Qiagen) and
Teltail, with XpYpE2 as primers. The conditions used for PCR and
further visualization of the bands were as described by Xu and
Blackburn (12). Primers used in
STELA were: Telorette3: 5′-TGCTCCGTGCATCTGGCATCCCTAACC-3′; Teltail:
5′-TGCTCCGTGCATCTGGCATC-3′; XpYpE2: 5′-GGTTGT CTCAGGGTCCTAGTG-3′
and XpYpB2: 5′-TCTGAAAGT GGACCAATCAG-3′.
Results
Determination of telomerase activity in
MCF10A, MCF10CA1 and MCF-7 cell lines
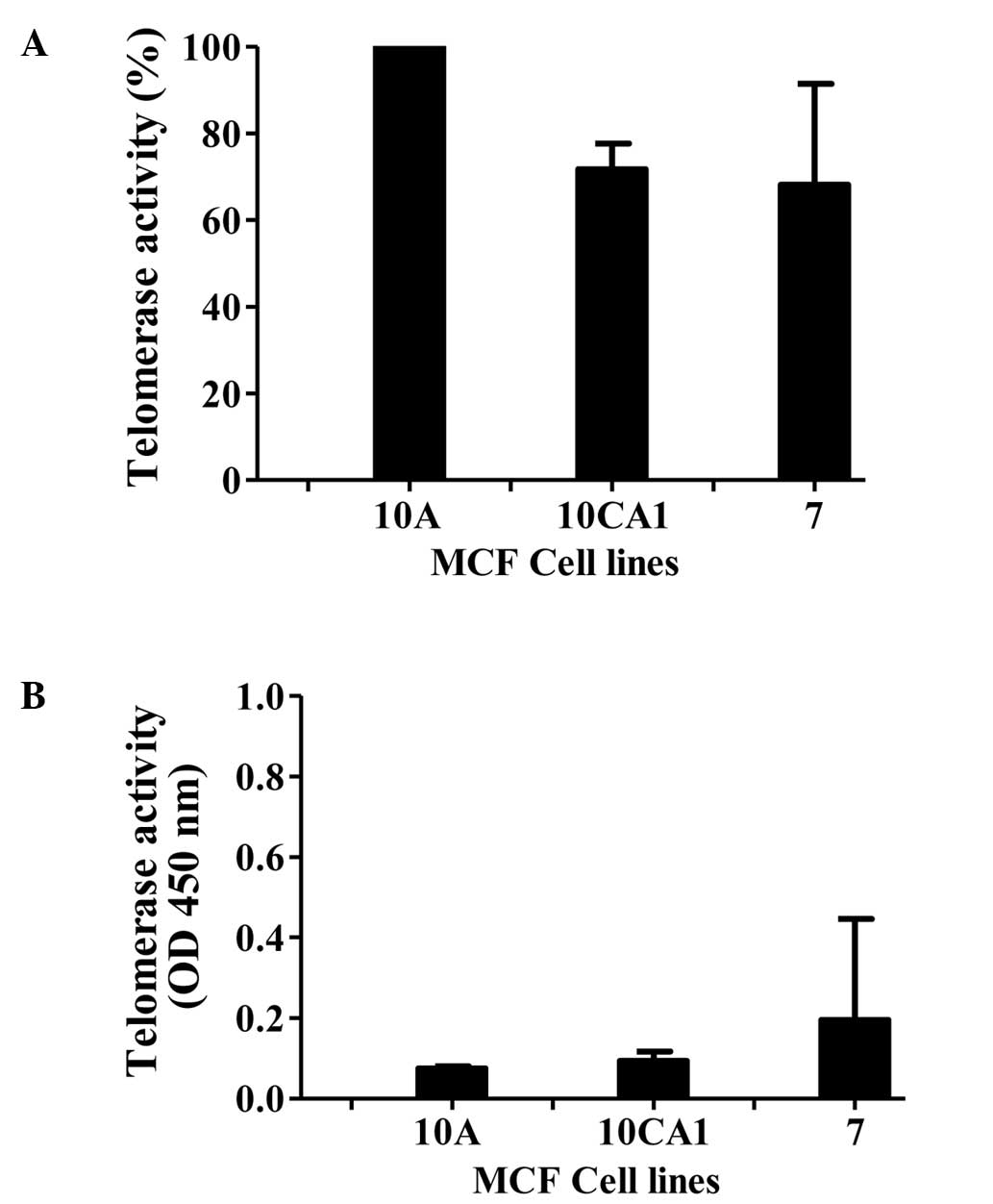
We first monitored the levels of telomerase activity
in the cell-free extracts of the breast cancer cell lines.
Telomerase activity was determined using the TRAPeze protocol, and
the activity is represented as relative telomerase units (RTU,
Fig. 1A). In this determination,
the highest activity observed in the immortalized cell line,
MCF10A, was considered as 100%. Activity in the other lines is
presented as a percentage relative to that of MCF10A (the values
were obtained from three independent experiments carried out in
triplicate). Robust telomerase activity was observed in the breast
cancer cell lines studied. As expected, due to the requirement of
RNA for the telomerase activity, the activity was abolished by
pretreatment of the extract with RNase A (Fig. 1B).
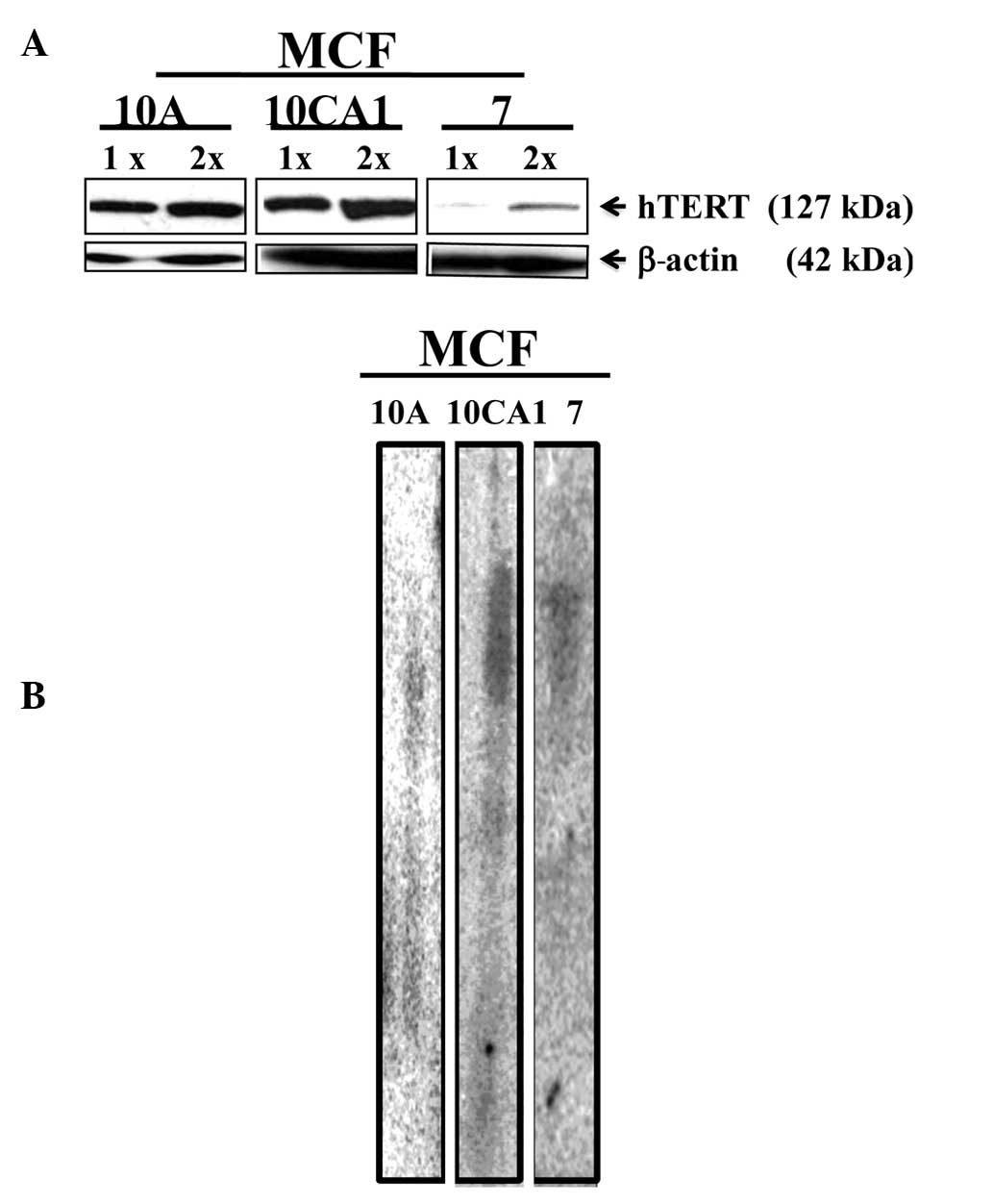
Assessment of the telomerase protein and
terminal telomere lengths
The amount of hTERT in the cell lines was visualized
by semi-quantitative Western blotting using antibodies against
hTERT. Telomerase migrated as an ~127 kDa band. When normalized to
β-actin (~42 kDa band), the MCF-7 cell line exhibited low levels of
hTERT when compared to hTERT levels in the advanced
(invasive/metastatic) breast cancer cell line, MCF10CA1 (Fig. 2A). This assessment was made based on
data from three independent experiments.
The lengths of the terminal bulk telomeres were
measured by terminal restriction fragment (TRF) analysis after
restriction enzyme digestion using the enzymes Rsa1 and
Hinf1. When measurements were conducted using the TRF
analysis, bulk telomere lengths were identified in the cell lines
studied (Fig. 2B).
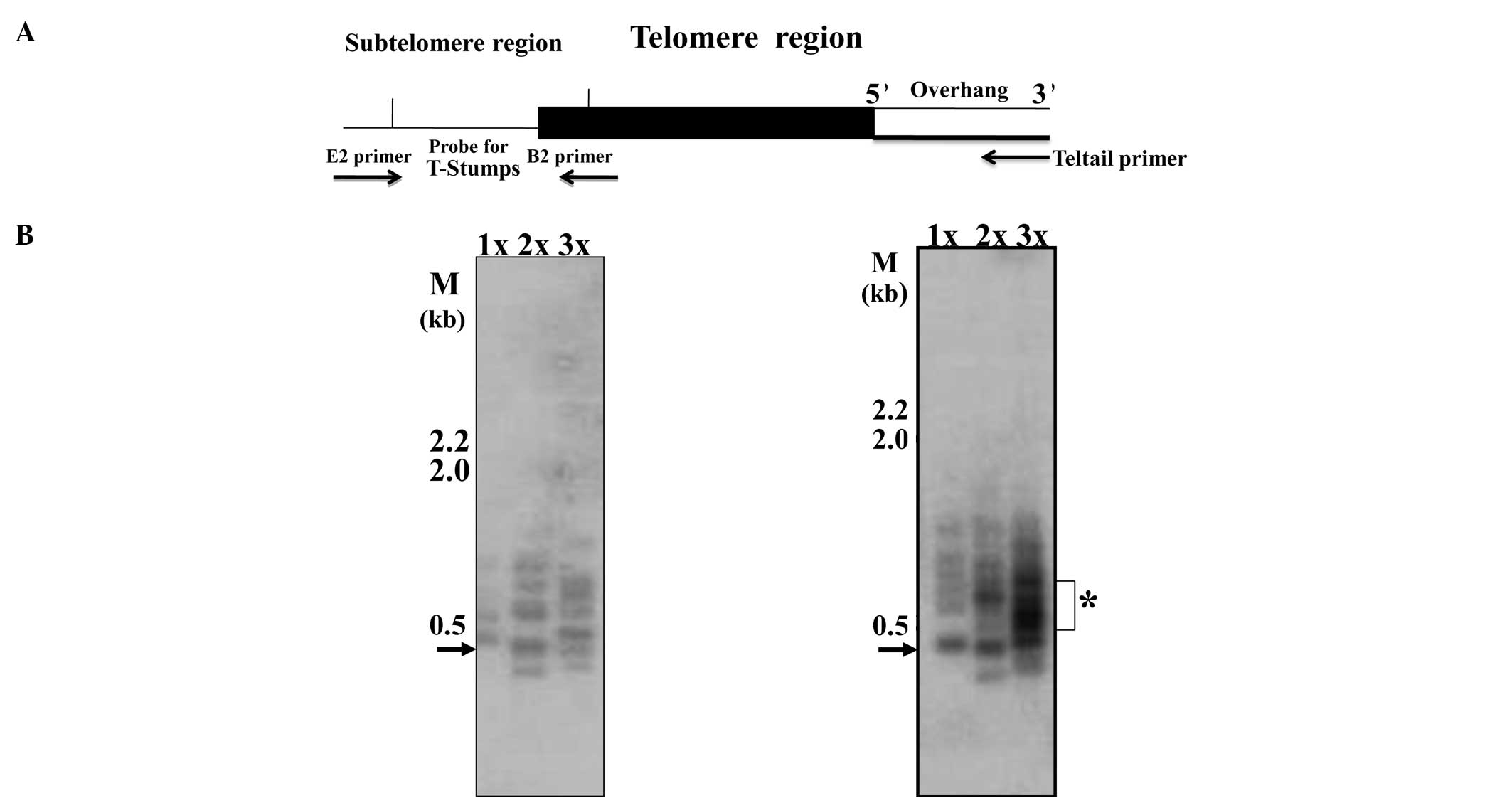
Single telomere length analysis
For a better resolution of telomere lengths and to
determine the distribution of single extremely short telomeres, we
performed STELA as previously described (12,15).
This methodology involves the ligation of the telorette3 linker to
the 5′ overhang which exists at the end of the ‘X’ chromosome; a
schematic representation of the assay is shown in Fig. 3A. The PCR product was identified by
Southern blotting and hybridizing with a 500-bp telomere-specific
probe (this probe also encompasses ~400 bp of subtelomeric region).
These analyses showed the existence of extremely short telomeres in
the two basal-like breast carcinoma lines, MCF10A and MCF10CA1,
with a molecular size range of 100–1,000 bp (Fig. 3B and C). The 500-bp (0.5 kb shown by
arrow) band actually represents t-stumps only 100 bp in size due to
the obligatory amplification of 400-bp subtelomeric region
(Fig. 3A).
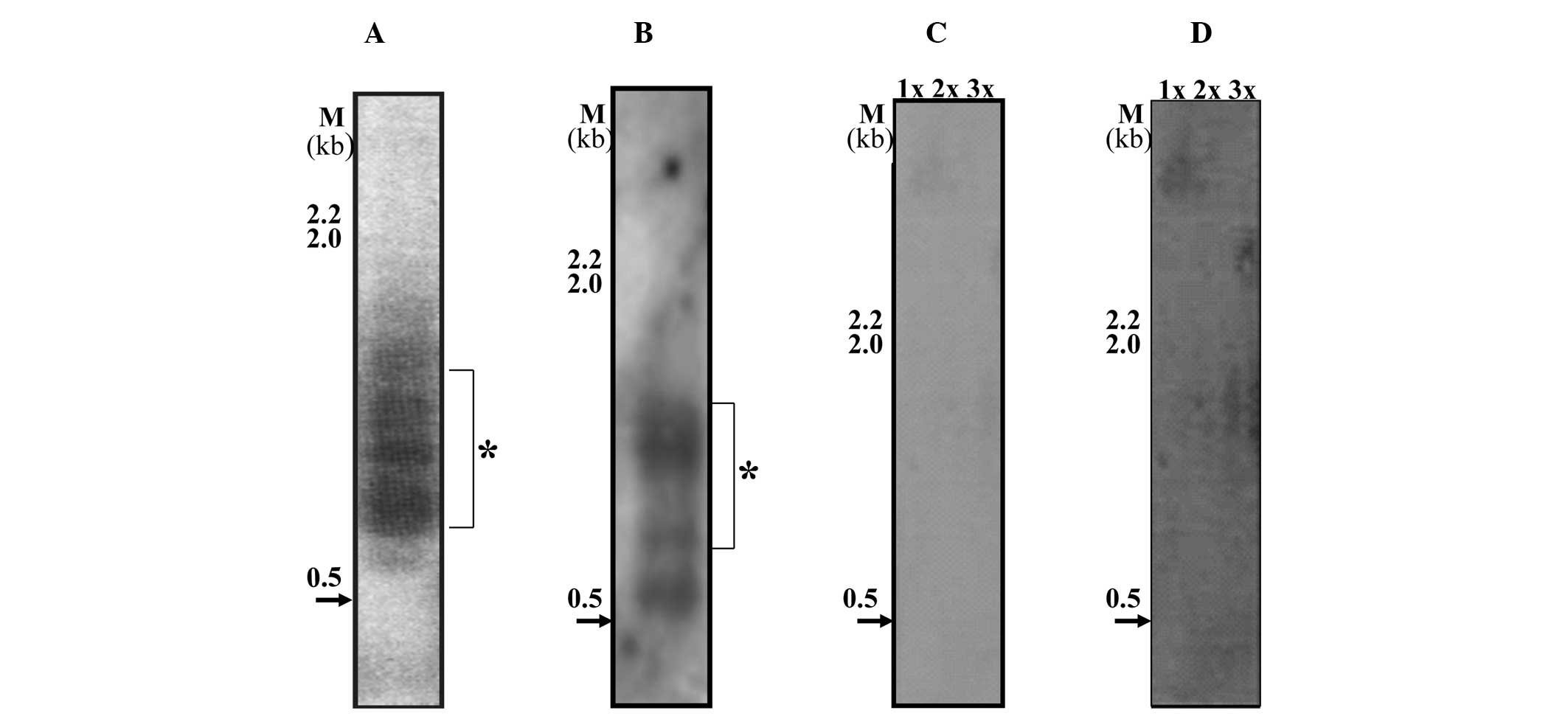
Single telomere length analysis in MCF-7
cell line and in different passage cells of MCF10A and
MCF10CA1
In contrast to the MCF10A and MCF10CA1 cell lines,
MCF-7 showed a low abundance of t-stumps under the same exposure
and conditions (Fig. 4C) with minor
bands being visible after long exposure (Fig. 4D). On the other hand, the highly
invasive/metastatic breast cancer cell line, MCF10CA1 (Fig. 3C), exhibited an abundance of a
cluster of t-stumps with a range of 500–700 bp in three independent
batches of cells. In an earlier passage of cells to the one shown
in Fig. 3C, the amount of 100 bp
t-stumps were minimal, but the 500–700 bp cluster was abundant
(indicated by the star in Fig. 4A).
However, in the batches of cells that were further subcultured,
t-stumps with a size range of 100 bp were observed along with the
distinct and abundant cluster of 500–700 bp (Fig. 4B, the star indicates the abundant
cluster). This consistent presence of the abundant cluster of
distinct t-stumps in the MCF10CA1 (invasive/metastatic) cell line,
leads to the conclusion that it may form a key molecular
target.
Discussion
Breast cancers are heterogeneous and harbor various
subtypes, leading to poor clinical outcomes (16). Moreover, certain subtypes do not
respond to currently available treatment regimens. The breast
cancer cell lines (MCF10A and MCF10CA1) studied have the same
genetic background and therefore are isogenic. These cell lines
exhibit characteristics of basal-like breast cancer types (17). Xu and Blackburn (12), reported on the existence of t-stumps
in human cancer cells. These authors also suggested that telomerase
plays a role in the protection of this distinct class of extremely
short telomeres.
This study, for the first time, reports on the
existence of the unique t-stumps in breast cancer cell lines. These
cell lines exhibited robust telomerase activity and high levels of
hTERT, with the exception of the MCF-7 breast cancer cell line
which had low levels of hTERT, thereby correlating with a low
abundance of t-stumps. This observation is in agreement with
studies carried out by Xu and Blackburn (12) who showed that the overexpression of
hTERT leads to an increase in the amount of t-stumps. However, the
differences we observed between the MCF-7 and the MCF10A and
MCF10CA1 cell lines may also relate to cell type specificity. This
was an observation made by Xu and Blackburn (12) with the HeLa cell line which
exhibited very low levels of t-stumps.
Of note is that STELA reveals dynamic changes at
single telomeres that occur at the chromosomal termini. The
presence of a distinct cluster of telomere t-stumps with a size
range of 500–700 bp, which we identified in the highly advanced
cell line MCF10CA1, may be due to the elongation of the shortest
telomeres by telomerase. This observation was recently made in a
study using human (18) and budding
yeast cells, in which early replication of short telomeres was
noted (19). We cannot disregard
the fact that the cluster of t-stumps observed in the MCF10CA1 line
may also be due to the recombination of the shortest telomeres as
reported recently by Morrish and Greider (20).
The cell lines selected for our study have the same
genetic background. Therefore, it should be noted that during the
progression of tumorigenesis from an immortalized state, i.e.,
MCF10A, to the highly advanced stage, which is the
invasive/metastatic state represented by MCF10CA1, we observed
dynamic changes at the single telomere level. Recently, it was
reported that the elongation of short telomeres can occur in small
size increments in human cells (18). The extension and accumulation of the
short telomeres which occurred in small increments from 100 bp to
500–700 bp in the MCF10CA1 cell line were noted in the present
study. Notably, this increment in short telomere elongation
resulting in a distinct cluster was observed in the
invasive/metastatic cell line and was distinct from the
immortalized cell line (MCF10A) telomere pattern. Therefore, these
unique clusters of t-stumps form signature markers and in
particular molecular targets to treat advanced breast cancer types.
It has been reported that single telomere loss results in cell
senescence (13). Therefore, these
very short telomeres, or t-stumps, may form key targets for
chemotherapeutic intervention especially for the class of breast
cancer types which do not respond to the currently available
therapeutic regimens.
Acknowledgements
This study was partially supported by grants from
NIH (NCI R01CA095317) and from the University Research Committee of
the Emory University to H.C.J. We thank Rohith B. Pai for help
during the preparation of the manuscript.
References
|
1
|
Chu D and Lu J: Novel therapies in breast
cancer: what is new from ASCO 2008. J Hematol Oncol. 1:162008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pai SB, Steele VE and Nettesheim P:
Neoplastic transformation of primary tracheal epithelial cell
cultures. Carcinogenesis. 4:369–374. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elenbaas B, Spirio L, Koerner F, Fleming
MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC and Weinberg RA:
Human breast cancer cells generated by oncogenic transformation of
primary mammary epithelial cells. Genes Dev. 15:50–65. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosen EM, Fan S and Isaacs C: BRCA1 in
hormonal carcinogenesis: basic and clinical research. Endocr Relat
Cancer. 12:533–548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greider CW and Blackburn EH: A telomeric
sequence in the RNA of Tetrahymena telomerase required for telomere
repeat synthesis. Nature. 337:331–337. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Wang H, Bishop JM and Blackburn EH:
Telomerase extends the lifespan of virus-transformed human cells
without net telomere lengthening. Proc Natl Acad Sci USA.
96:3723–3728. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pai RB, Pai SB, Kukhanova M, Dutschman GE,
Guo X and Cheng YC: Telomerase from human leukemia cells:
properties and its interaction with deoxynucleoside analogues.
Cancer Res. 58:1909–1913. 1998.PubMed/NCBI
|
|
11
|
Calado RT, Regal JA, Hills M, Yewdel WT,
Dalmazoo LF, Zago MF, Zago MA, Lansdorp PM, Hogge D, Chanock SJ,
Estey EJ, Falcao RP and Young NS: Constitutional hypomorphic
telomerase mutations in patients with acute myeloid leukemia. Proc
Natl Acad Sci USA. 106:1187–1192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L and Blackburn EH: Human cancer cells
harbor T-stumps, a distinct class of extremely short telomeres. Mol
Cell. 28:315–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
D'Adda di Fagagna F, Reaper PM,
Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G,
Carter NP and Jackson SP: A DNA damage checkpoint response in
telomere-initiated senescence. Nature. 426:194–198. 2003.PubMed/NCBI
|
|
14
|
Sarrio D, Rodriguez-Pimilla SM, Hardisson
D, Cano A, Moreno-Bueno G and Palacios J: Epithelial-mesenchymal
transition in breast cancer relates to the basal-like phenotype.
Cancer Res. 68:989–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baird DM, Rowson J, Wynford-Thomas D and
Kipling D: Extensive allelic variation and ultrashort telomeres in
senescent human cells. Nat Genet. 33:203–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu ZB, Liu GY, Yang WT, Di GH, Lu JS,
Shen KW, Shen ZZ, Shao ZM and Wu J: Triple-negative breast cancer
types exhibit a distinct poor clinical characteristic in lymph
node-negative Chinese patients. Oncol Rep. 20:987–994.
2008.PubMed/NCBI
|
|
17
|
Hu M, Yao J, Carroll DK, Weremowicz S,
Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T,
Nikolsky Y, Bauerlein EL, Hahn WC, Gelman RS, Allred C, Bissell MJ,
Schnitt S and Polyak K: Regulation of in situ to invasive breast
carcinoma transition. Cancer Cell. 13:394–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Britt-Compton B, Capper R, Rowson J and
Baird DM: Short telomeres are preferentially elongated by
telomerase in human cells. FEBS Lett. 583:3076–3080. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bianchi A and Shore D: Early replication
of short telomeres in budding yeast. Cell. 128:1051–1062. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morrish TA and Greider CW: Short telomeres
initiate telomere recombination in primary and tumor cells. PLoS
Genet. 5:e10003572009. View Article : Google Scholar : PubMed/NCBI
|