Introduction
Recently, substantial evidence has shown that cancer
cells exhibit morphological, biological and phenotypical
characteristics similar to normal stem cells. Previous research
provided substantial support for the hypothesis that cancer stem
cells contribute to tumorigenesis (1–3). The
self-renewal of stem and cancer cells may be regulated by similar
signaling pathways (4). Notably,
certain poorly differentiated tumors frequently overexpress genes
preferentially enriched in embryonic stem (ES) cells, such as
NANOG, OCT4 and SOX2 (5–9).
NANOG is a transcription factor that plays a vital
role in maintaining the pluripotency and self-renewal capacity of
ES cells (10,11). It is well known that NANOG is highly
and specifically expressed in ES cells (10,11)
and human germ cell tumors (12–14).
Recent studies indicated that NANOG is also highly expressed in
certain somatic tumors, such as breast (5,8),
prostate (6) and cervical cancer
(15). Of note is that NANOG has 11
highly homologous pseudogenes (16), some of which are normally expressed
in tumors (17–20). Our previous study showed that
NANOGP8, a retrogene of NANOG, is expressed in several tumor
tissues and cell lines together with NANOG (20). Notably, only one amino acid differs
between NANOG and NANOGP8; thus, the two proteins perform similar
activities in promoting cell proliferation (20,21).
Jeter et al (22) recently
reported that in cancer cells, NANOG is derived predominantly from
a retrogene locus termed NANOGP8.
Gastric cancer (GC) is one of the most common types
of cancer and the second highest cause of cancer-related mortality
in the world (23,24). Studies have strongly indicated the
existence of cancer stem cells in GC (25,26).
Investigation of ES cell gene expression in GC tissues may be
helpful in understanding the molecular mechanism for the stem cell
theory of carcinogenesis. To determine its involvement in GC
development, this study analyzed the NANOG/NANOGP8 expression
profile in GC and the correlation between the NANOG/NANOGP8
expression and clinicopathological features.
Materials and methods
Tissue specimens
GCs, as well as their corresponding adjacent
non-neoplastic tissues, intestinal metaplasia and dysplasia tissues
were obtained from the tumor bank of the Beijing Cancer Hospital
and Institute. Each gastric tissue was collected following patient
consent and the approval of the ethics committee of the Beijing
Cancer Hospital and Institute. The cancer tissues were excised from
the central section of the GC. As a control for each GC patient,
normal tissue was excised at least 5 cm from the border of the GC.
The specimens were routinely diagnosed by senior pathologists
according to pathological biopsy and Lauren's classification. The
stage of GC was determined according to the tumor-node-metastasis
(TNM) classification of the American Joint Committee on Cancer.
Follow-up interviews were conducted with patients for at least 5
years or until the patient succumbed to the disease. For tissue
microarray analysis, 40 pairs of GC samples, 40 metaplasia tissues
and 30 dysplasia tissues were fixed in formalin and embedded in
paraffin. For RT-PCR experiments, 12 pairs of samples were frozen
in liquid nitrogen within 30 min after surgery and then stored at
−70˚C until required.
Total RNA extraction and RT-PCR
Total RNA from gastric samples was extracted using
Trizol reagent (Invitrogen, USA) following procedures described by
the manufacturer. To remove any DNA contamination, total RNA was
treated with RNase-free DNase I (Takara Bio Inc., Japan). The
reverse transcription (RT) reaction was carried out with 2 μg total
RNA in a 25-μl reaction containing M-MLV reverse transcriptase
(Promega, USA) using oligo dT primers. An RT reaction was carried
out without reverse transcriptase as a negative control. The PCR
for NANOG/NANOGP8 was carried out using LATaq® DNA
polymerase with GC buffer (Takara Bio Inc.) in a 50-μl volume. The
NANOG/NANOGP8 primers were: forward, 5′-CCTACCCCAGCCTCTACTCT-3′ and
reverse, 5′-CGTCTTCAGGTTGCATGTTC-3′. This pair of primers were able
to identify not only NANOG/NANOGP8, but also other pseudogenes of
NANOG. Amplification of β-actin was used as a normalizing control.
The β-actin primers were: forward, 5′-CGGGACCTGACTGACTACCTC-3′ and
reverse, 5′-TCGTCATACTCCTGCTTGCTG-3′. PCR was performed with the
following cycling profile: 5 min denaturation at 94˚C followed by
38 (NANOG/NANOGP8) or 30 cycles (β-actin) of 30 sec at 94˚C, 30 sec
at 55˚C and 50 sec at 72˚C, with a final extension step at 72˚C for
10 min. Products were analyzed by electrophoresis on a 1.2% (w/v)
agarose gel and the fragments extracted using a gel extraction kit
(Omega, USA). Six clones of each specific PCR product were verified
by sequencing analysis.
Tissue microarray analysis with
immunohistochemistry staining
Tissue microarray blocks of gastric tissues were
constructed for immunohistochemistry staining. For each case, five
tissue cylinders (0.6 mm diameter, 1 mm high) were removed from
individual GC or adjacent normal tissue. The tissue array blocks
were arranged using a puncher (Beecher Instruments, Micro-Array
Technologies, USA). Sections (4 μm) were obtained from the block
and transferred to glass slides. The block contained a
representative control of well-matched cancer and adjacent
non-neoplastic tissue.
For immunohistochemical staining, the slides were
baked overnight at 60˚C, deparaffinized with xylene and rehydrated
in a graded ethanol series. Antigen retrieval was carried out by
microwaving tissue in 1 mM EDTA solution (pH 8.0). The endogenous
peroxidase activity was blocked by incubation in 3%
H2O2 at room temperature for 10 min. After
incubation in 3% milk to prevent non-specific binding, the slides
were immunostained with mouse anti-human NANOG monoclonal antibody
(1:100; Abcam, USA) at 4˚C overnight in a humidified chamber.
Slides were washed in phosphate-buffered saline and incubated with
a secondary antibody conjugated to peroxidase (Zhongshan Golden
Bridge Biotechnology, China). Immunohistochemical staining was
performed using a commercial DAB kit (Zhongshan Golden Bridge
Biotechnology) which yielded a positive brown signal. Slides were
then counterstained with hematoxylin, dehydrated in a graded
ethanol series and mounted.
Statistical analysis
The data were analyzed using the Chi-square and
Fisher's exact test. The association of NANOG/NANOGP8 expression
with clinicopathological features was analyzed using SPSS software
(version 14). The cumulative survival curve was compared by the
log-rank test. For the analyses, P<0.05 was considered to be
statistically significant.
Results
NANOG/NANOGP8 mRNA expression in GC and
normal tissues
To compare the expression of NANOG/NANOGP8 between
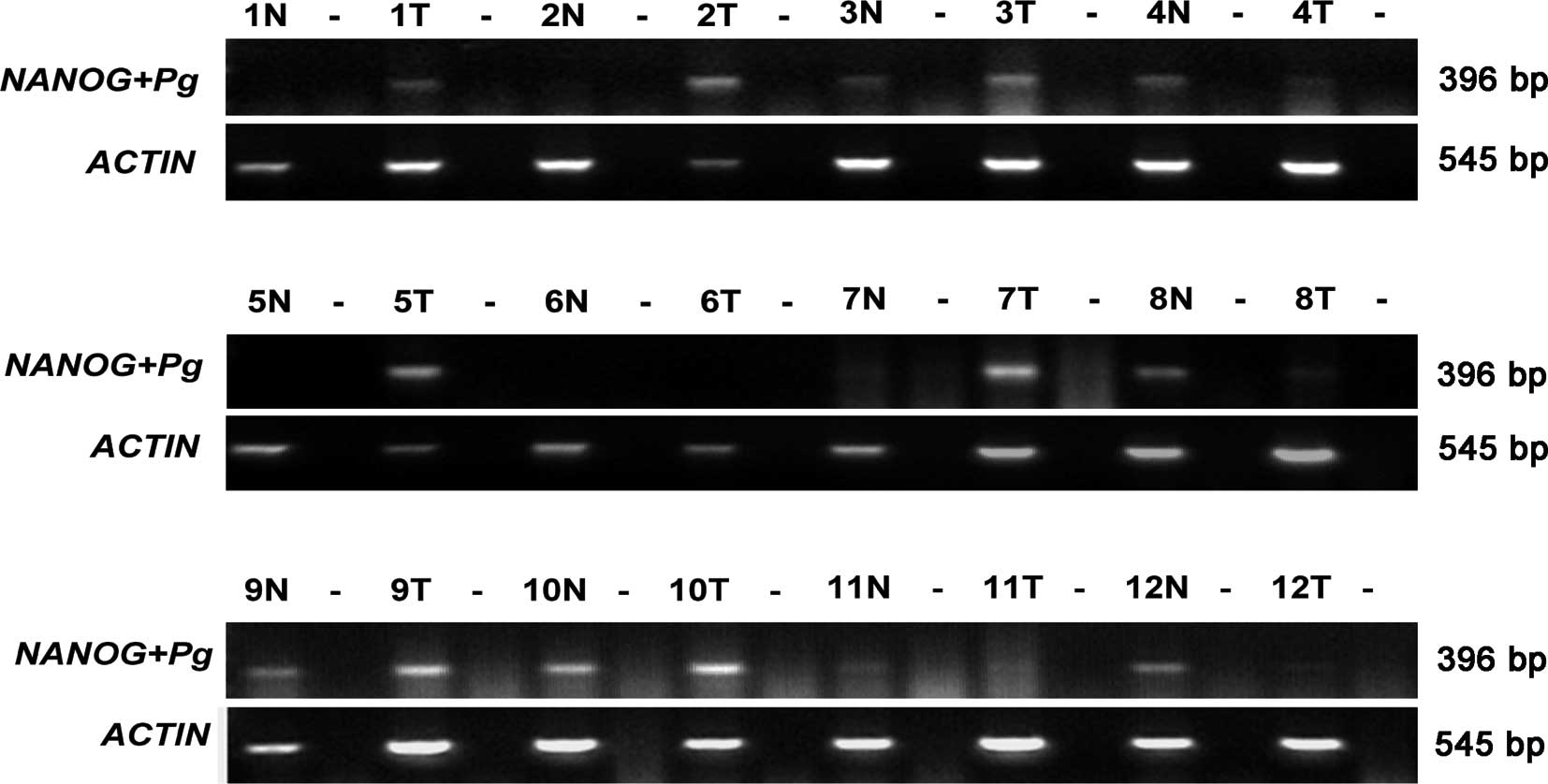
tumor and normal tissues of GC patients, we initally detected mRNA
expression using RT-PCR in 12 pairs of specimens. Due to the
existence of many NANOG pseudogenes, the primers used in this study
were likely able to identify these pseudogenes. Therefore, we
examined the PCR products by sequence analysis. As shown in
Fig. 1, the presence of NANOG
and/or its pseudogene RNA was noted in 10 of the 12 GC cases as
compared to normal tissues (6/12). The sequencing results obtained
from the PCR products indicated that 4 of the 12 GC cases were
specific for NANOGP8 and 2 cases contained NANOG, while only 1 of
the 12 cases in normal tissues contained the NANOGP8 sequence in
the PCR product (Table I). The
NANOG pseudogenes, NANOGP2, NANOGP5, NANOGP7 and NANOGP4, were also
detected in both GC (8/12) and normal tissues (6/12) (Table I).
 | Table ISequencing analysis of RT-PCR products
from 12 pairs of specimens taken from GC patients. |
Table I
Sequencing analysis of RT-PCR products
from 12 pairs of specimens taken from GC patients.
| Case no. | NANOG | NANOGP8 | NANOGP2 | NANOGP4 | NANOGP5 | NANOGP7 |
|---|
| 1 |
| T | | | | | | 5 |
| N | | | | | | |
| 2 |
| T | | 3 | 3 | | | |
| N | | | | | | |
| 3 |
| T | | | | | 1 | 4 |
| N | | | 1 | | | 5 |
| 4 |
| T | | | | | 3 | 3 |
| N | | 1 | 2 | | | 3 |
| 5 |
| T | 2 | 4 | | | | |
| N | | | | | | |
| 6 |
| T | | | | | | |
| N | | | | | | |
| 7 |
| T | | 2 | | | | 4 |
| N | | | | | | |
| 8 |
| T | 1 | | 5 | | | |
| N | | | 1 | | 3 | 2 |
| 9 |
| T | | 1 | | | 5 | |
| N | | | 4 | | 1 | 1 |
| 10 |
| T | | | 2 | | | 2 |
| N | | | 3 | 1 | | 2 |
| 11 |
| T | | | | | | |
| N | | | | | | |
| 12 |
| T | | | | | | |
| N | | | 2 | | 2 | 1 |
Detection of the NANOG/NANOGP8 protein
expression in GC and normal tissues
Of note is that pseudogenes, including NANOG, cannot
be translated into functional protein products (27). We attempted to detect NANOG/NANOGP8
protein in GC and normal tissues using immunohistochemical
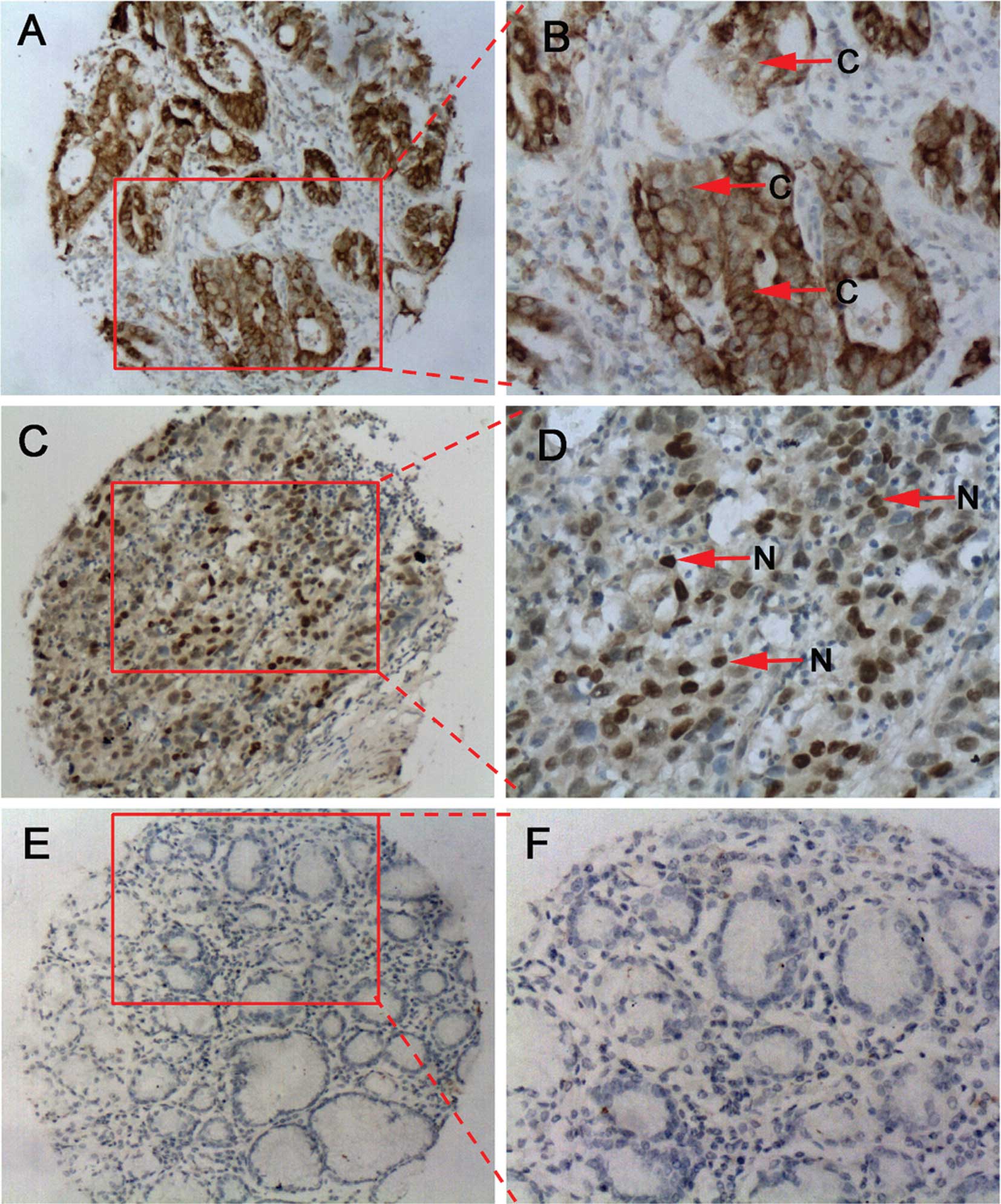
staining. Results indicated that there was an increased
NANOG/NANOGP8 protein expression in GC tissues compared to normal
matched tissues (Fig. 2, Table II). Positive NANOG/NANOGP8 staining
was detected in 75% (30/40) of the GC tissues and only in 12.5%
(5/40) of the corresponding adjacent normal tissues (P<0.001).
The majority of NANOG/NANOGP8 cells were diffusely localized in
both the nucleus and cytoplasm (Fig. 2A
and B), whereas in other GC tissues, NANOG/NANOGP8 cells were
clearly localized in the nucleus (Fig.
2C and D).
 | Table IIComparison of NANOG/NANOGP8 protein
expression between GC and normal matched tissues. |
Table II
Comparison of NANOG/NANOGP8 protein
expression between GC and normal matched tissues.
| Histology | Total cases | NANOG/NANOGP8 | P-value |
|---|
| |
| |
|---|
| | Positive (%) | Negative (%) | |
|---|
| Tumor | 40 | 30 (75.0) | 10 (25.0) | <0.001 |
| Normal | 40 | 5 (12.5) | 35 (87.5) | |
Correlation analysis of NANOG/NANOGP8
expression and clinicopathological features
A correlation analysis of NANOG/NANOGP8 expression
and the clinicopathological features of GC was conducted.
NANOG/NANOGP8 protein expression did not correlate to the following
clinicopathological features: GC histological grade (well vs. poor
differentiation, P=0.589), lymph node metastasis (N0 vs.
N1, P=0.473), distant metastasis (M0 vs.
M1, P=1) or stage (TNM classification, stages I and II
vs. stages III and IV, P=0.915) (Table III). Additionally, the cumulative
survival curve indicated no significant difference in the survival
time of patients with higher levels vs. those with low levels of
NANOG/NANOGP8 expression (P=0.998; data not shown).
 | Table IIICorrelation analysis between
NANOG/NANOGP8 protein expression and clinicopathological
features. |
Table III
Correlation analysis between
NANOG/NANOGP8 protein expression and clinicopathological
features.
| Clinicopathological
features | Total cases | NANOG/NANOGP8 | P-value |
|---|
| |
| |
|---|
| | Positive | Negative | |
|---|
|
Differentiation | | | | 0.589 |
| Well | 13 | 9 | 4 | |
| Poor | 27 | 21 | 6 | |
| LN metastasis | | | | 0.473 |
| N0 | 12 | 8 | 4 | |
| N1 | 28 | 22 | 6 | |
| Distant
metastasis | | | | 1.000 |
| M0 | 36 | 27 | 9 | |
| M1 | 4 | 3 | 1 | |
| Stage | | | | 0.915 |
| I, II | 14 | 10 | 4 | |
| III, IV | 26 | 19 | 7 | |
NANOG/NANOGP8 in the early developmental
stages of GC
As NANOG/NANOGP8 protein expression in GC did not
correlate to the afore-mentioned clinicopathological features, we
hypothesized that NANOG/NANOGP8 plays a role in the early
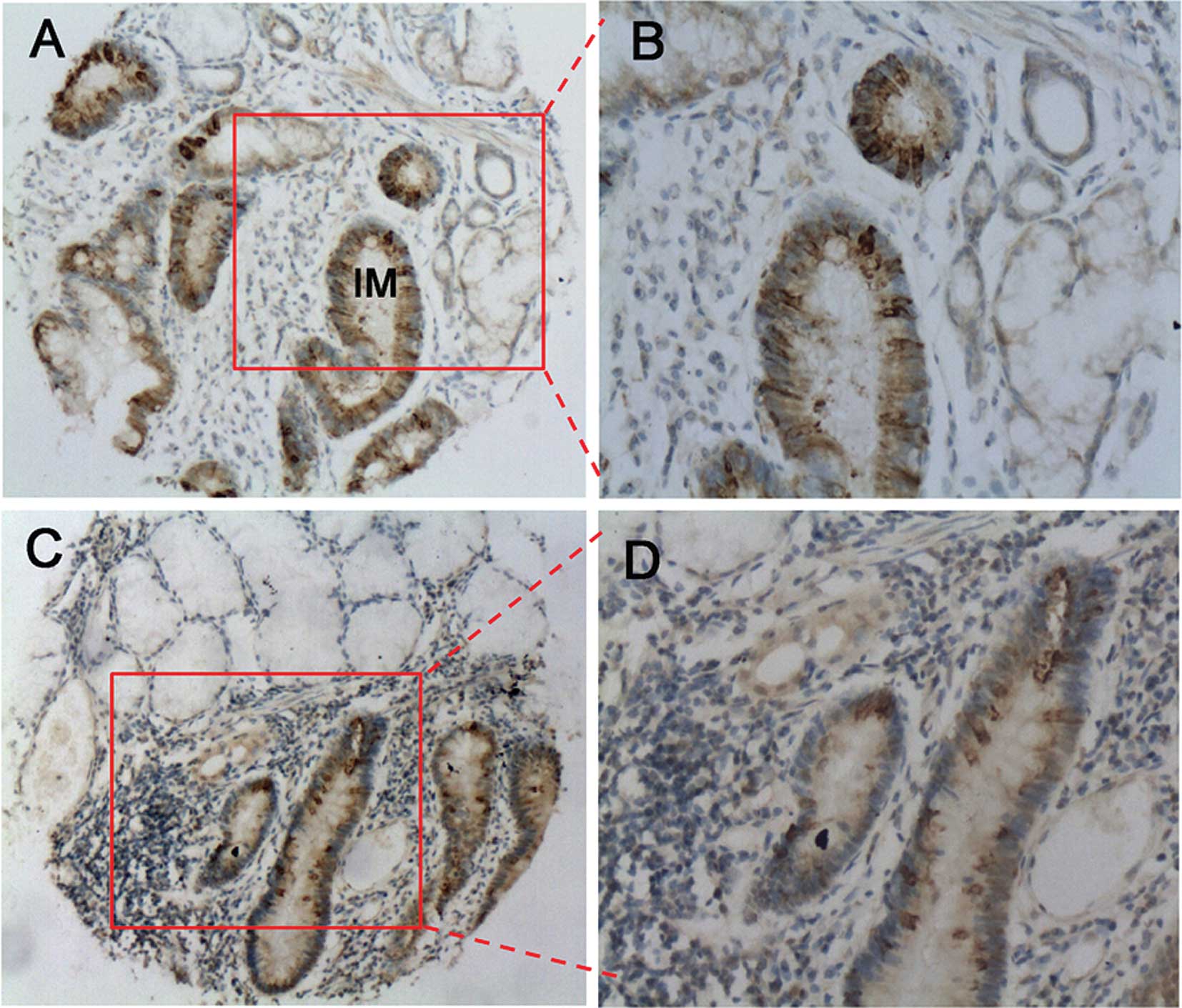
developmental stages of gastric carcinogenesis. We were able to
detect the NANOG/NANOGP8 protein in the intestinal metaplasia and
dysplasia tissues, representative of the early developmental stages
of GC (28,29). As shown in Fig. 3, NANOG/NANOGP8 was highly expressed
in the intestinal mucosa of the metaplasia tissues (60%, 24/40;
Fig. 3A and B). The dysplasia
tissues also demonstrated high levels of NANOG/NANOGP8 expression
(66.7%, 20/30), whereas adjacent normal gastric tissues exhibited
no expression (Fig. 3C and D).
Discussion
Of note is that malignant cells have a close
relationship to stem cells. It has been postulated that the
self-renewal pathways in stem cells perform a similar role in
cancer cells (4,5). This study attempted to provide
evidence that the ES cell self-renewal gene, NANOG, is also highly
expressed in cancer cells. Since NANOGP8 showed similar activities
to NANOG in promoting cell proliferation (20,21),
we postulated that NANOG and NANOGP8 play similar roles in
maintaining the high proliferative capacity of cancer cells. The
mechanism by which NANOG and NANOGP8 expression is regulated may be
distinctly different. However, the effects of NANOG and NANOGP8 in
carcinogenesis are the same. Furthermore, it is difficult to
distinguish the protein products of NANOG and NANOGP8 by
immunohistochemistry due to only one amino acid difference between
them. Therefore, this study analyzed the expression of NANOG and
NANOGP8 together.
We detected an increase in the level of mRNA
transcripts of NANOG, NANOGP8 and several NANOG pseudogenes in GC
vs. normal tissues. From the 10 RT-PCR-positive cases in GC
tissues, six showed an expression of NANOG/NANOGP8 at the mRNA
level. The protein expression of NANOG/NANOGP8 in GC tissues was
found to be significantly higher (75%) than that of normal tissues
(12.5%), supporting our hypothesis that the gene NANOG is highly
expressed in GC.
It is well known that the transcription factor
NANOG, is localized to the nucleus (21,30).
The same applies to NANOGP8 (20).
Following the exclusion of false-positive staining, we found that
in certain cancer specimens, the localization of NANOG/NANOGP8 is
not restricted to the nucleus but can also be observed in the
cytoplasm. These results are similar to those found in breast
carcinoma samples (8) and malignant
cervical epithelial cells (15). It
provides a hint that NANOG/NANOGP8 plays a more complex role(s) in
cancer than anticipated. The regulation mechanism for localization
is currently unknown and further study is required.
Investigation into the activities of these genes and
their association with cancer may result in a better understanding
of the biological characteristics of carcinoma. For the first time,
we have proven that no correlation exists between NANOG/NANOGP8
protein expression and the differentiation, lymph node metastasis,
distant metastasis, stage or survival of GC patients. Our results
suggest that NANOG/NANOGP8 expression is not related to the
prognosis of GC. With respect to the theory that cancer stem cells
account for carcinogenesis (1–3), we
presume that NANOG/NANOGP8 expression may relate to the initial
stages of GC. Intestinal-type gastric tumors are usually
well-characterized by sequential developmental stages that include
chronic gastritis, atrophy, intestinal metaplasia, dysplasia and
carcinoma (28,29). We identified the expression of
NANOG/NANOGP8 in the early developmental stages of GCs, during
intestinal metaplasia and dysplasia, but not in the adjacent normal
tissues. This suggests a potential role for NANOG/NANOGP8 in the
early developmental stages of gastric carcinogenesis.
Jeter et al (22) recently reported that NANOG and/or
NANOGP8 regulates prostate tumor development. These authors found
that the ectopic expression of patient tumor-derived NANOGP8 in the
K14 cellular compartment of transgenic mice disrupts tissue
homeostasis associated with hyperplasia, dysplasia and abnormal
differentiation. The results obtained by Jeter et al
(22) confirm and significantly
extend our findings in GC.
In conclusion, we detected a significantly increased
expression of NANOG/NANOGP8 in GC compared to normal tissues. Our
results suggest that NANOG/NANOGP8 is not related to the prognosis
of GC, but to the early developmental stages of gastric
carcinogenesis. Further studies and an analysis of the
NANOG/NANOGP8 expression would provide a better understanding of
the biological characteristics of GC.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China (30600304), the Ministry of
Science and Technology of China (2006CB943601; 2004CB518708;
2006AA02A402) and the Beijing Municipal Science and Technology
Commission (D0905001040631). We thank the tissue bank of the
Beijing Cancer Hospital and Institute for the gastric tissues.
References
|
1
|
Burkert J, Wright NA and Alison MR: Stem
cells and cancer: an intimate relationship. J Pathol. 209:287–297.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li L and Neaves WB: Normal stem cells and
cancer stem cells: the niche matters. Cancer Res. 66:4553–4557.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marx J: Cancer research. Mutant stem cells
may seed cancer. Science. 301:1308–1310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu G, Yuan J, Wills M and Kasper S:
Prostate cancer cells with stem cell characteristics reconstitute
the original human tumor in vivo. Cancer Res. 67:4807–4815. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monk M and Holding C: Human embryonic
genes re-expressed in cancer cells. Oncogene. 20:8085–8091. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitsui K, Tokuzawa Y, Itoh H, Segawa K,
Murakami M, Takahashi K, Maruyama M, Maeda M and Yamanaka S: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hart AH, Hartley L, Parker K, Ibrahim M,
Looijenga LH, Pauchnik M, Chow CW and Robb L: The pluripotency
homeobox gene NANOG is expressed in human germ cell tumors. Cancer.
104:2092–2098. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoei-Hansen CE, Almstrup K, Nielsen JE,
Brask Sonne S, Graem N, Skakkebaek NE, Leffers H and Rajpert-De
Meyts E: Stem cell pluripotency factor NANOG is expressed in human
fetal gonocytes, testicular carcinoma in situ and germ cell
tumours. Histopathology. 47:48–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clark AT, Rodriguez RT, Bodnar MS, Abeyta
MJ, Cedars MI, Turek PJ, Firpo MT and Reijo Pera RA: Human STELLAR,
NANOG and GDF3 genes are expressed in pluripotent cells and map to
chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells.
22:169–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye F, Zhou C, Cheng Q, Shen J and Chen H:
Stem-cell-abundant proteins Nanog, Nucleostemin and Musashi1 are
highly expressed in malignant cervical epithelial cells. BMC
Cancer. 8:1082008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Booth HA and Holland PW: Eleven daughters
of NANOG. Genomics. 84:229–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kandouz M, Bier A, Carystinos GD,
Alaoui-Jamali MA and Batist G: Connexin43 pseudogene is expressed
in tumor cells and inhibits growth. Oncogene. 23:4763–4770. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujii GH, Morimoto AM, Berson AE and Bolen
JB: Transcriptional analysis of the PTEN/MMAC1 pseudogene, psiPTEN.
Oncogene. 18:1765–1769. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suo G, Han J, Wang X, Zhang J, Zhao Y,
Zhao Y and Dai J: Oct4 pseudogenes are transcribed in cancers.
BBRC. 337:1047–1051. 2005.PubMed/NCBI
|
|
20
|
Zhang J, Wang X, Li M, Han J, Chen B, Wang
B and Dai J: NANOGP8 is a retrogene expressed in cancers. FEBS J.
273:1723–1730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Wang X, Chen B, Suo G, Zhao Y,
Duan Z and Dai J: Expression of Nanog gene promotes NIH3T3 cell
proliferation. BBRC. 338:1098–1102. 2005.PubMed/NCBI
|
|
22
|
Jeter CR, Badeaux M, Choy G, Chandra D,
Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ and Tang
DG: Functional evidence that the self-renewal gene NANOG regulates
human tumor development. Stem Cells. 27:993–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
24
|
Fox JG and Wang TC: Inflammation, atrophy
and gastric cancer. J Clin Invest. 117:60–69. 2007. View Article : Google Scholar
|
|
25
|
Takaishi S, Okumura T and Wang TC: Gastric
cancer stem cells. J Clin Oncol. 26:2876–2882. 2008. View Article : Google Scholar
|
|
26
|
Schier S and Wright NA: Stem cell
relationships and the origin of gastrointestinal cancer. Oncology.
1:9–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balakirev ES and Ayala FJ: Pseudogenes:
are they ‘junk’ or functional DNA? Ann Rev Genet. 37:123–151.
2003.
|
|
28
|
Correa P: A human model of gastric
carcinogenesis. Cancer Res. 48:3554–3560. 1988.PubMed/NCBI
|
|
29
|
Correa P: Human gastric carcinogenesis: a
multistep and multifactorial process – first American Cancer
Society award lecture on cancer epidemiology and prevention. Cancer
Res. 52:6735–6740. 1992.
|
|
30
|
Do HJ, Lim HY and Kim JH, Song H, Chung HM
and Kim JH: An intact homeobox domain is required for complete
nuclear localization of human Nanog. BBRC. 353:770–775.
2007.PubMed/NCBI
|