Introduction
Dynamic computed tomography (Dy-CT) and conventional
B-mode ultrasonography (US) are used for the screening of
hepatocellular carcinomas (HCCs). Although Dy-CT is useful for
assessing the vascularity of hepatic tumors, negative aspects
include exposure to X-rays and the high cost. On the other hand,
B-mode US is economical and easy to perform repeatedly. However,
some nodules are difficult to identify or diagnose, particularly
small-size tumors, due to the sensitivity as well as the
irregularity in the case of chronic injured liver.
Recently, the development of US and
contrast-enhanced ultrasonographic agents for hepatic tumors have
enabled the diagnosis of HCC in the early stage. Perfluorobutane
(Sonazoid®) (1) was
approved as a new contrast-enhanced ultrasonographic agent in Japan
in January 2007. The tumors are phagocyted by Kupffer cells after
injection. The primary characteristic of this agent is the ability
to maintain observations continuously as the tumors are being
phagocyted by Kupffer cells. The present study evaluated the
diagnostic efficacy of contrast-enhanced (CE) US with Sonazoid for
HCCs, particularly small-size HCCs.
Materials and methods
Seventy-nine nodules detected by US and/or Dy-CT
between January and August 2007 in 70 patients examined at Ehime
Prefectural Central Hospital, Japan, were studied. Nodules showing
the typical findings of liver hemangioma were excluded from this
study. Informed consent was obtained from all of the studied
patients and the study protocol was approved by the Ethics
Committee of Ehime Prefectural Central Hospital.
CEUS was performed within 1 month from the Dy-CT
examination in all of the patients. We divided the nodules into two
groups. Forty-nine nodules (41 patients) ≤2 cm in diameter were
defined as the S-group, and 30 nodules (28 patients) >2 cm in
diameter were defined as the L-group. Sonazoid was used as the
contrast-enhanced ultrasonographic agent (4 μl/kg of body weight)
in all examinations, and the target lesions were scanned after
injection in the arterial and Kupffer phases using a ProSound
Alpha-10 (Aloka Co. Ltd., Tokyo, Japan). The arterial phase of CEUS
imaging was identified 10–60 sec after Sonazoid injection, and the
Kupffer phase 10 min after the injection (2). ProSound Alpha-10 was set up in the
extended pure harmonic detection mode and was used with a convex
type probe (Table I). The results
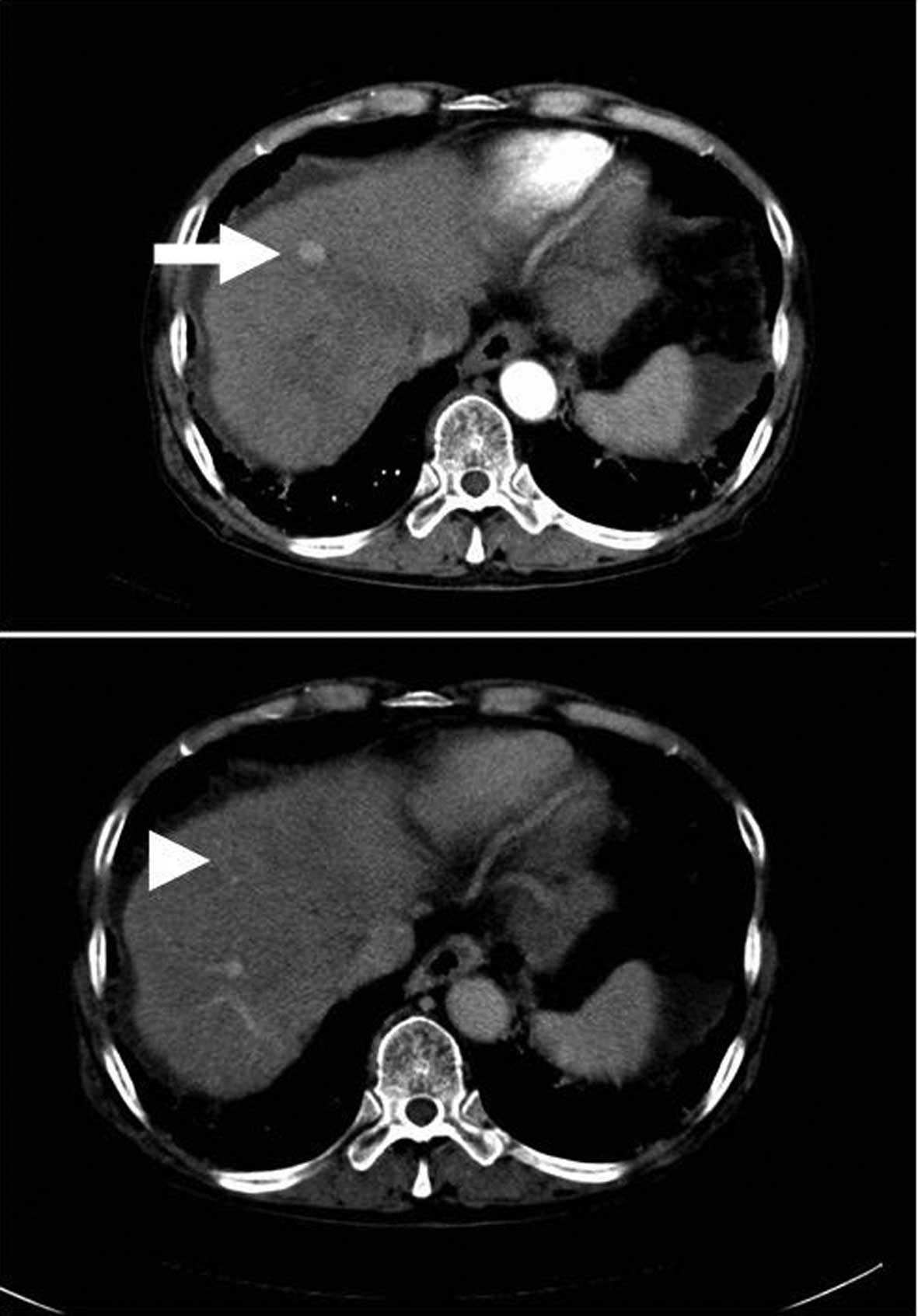
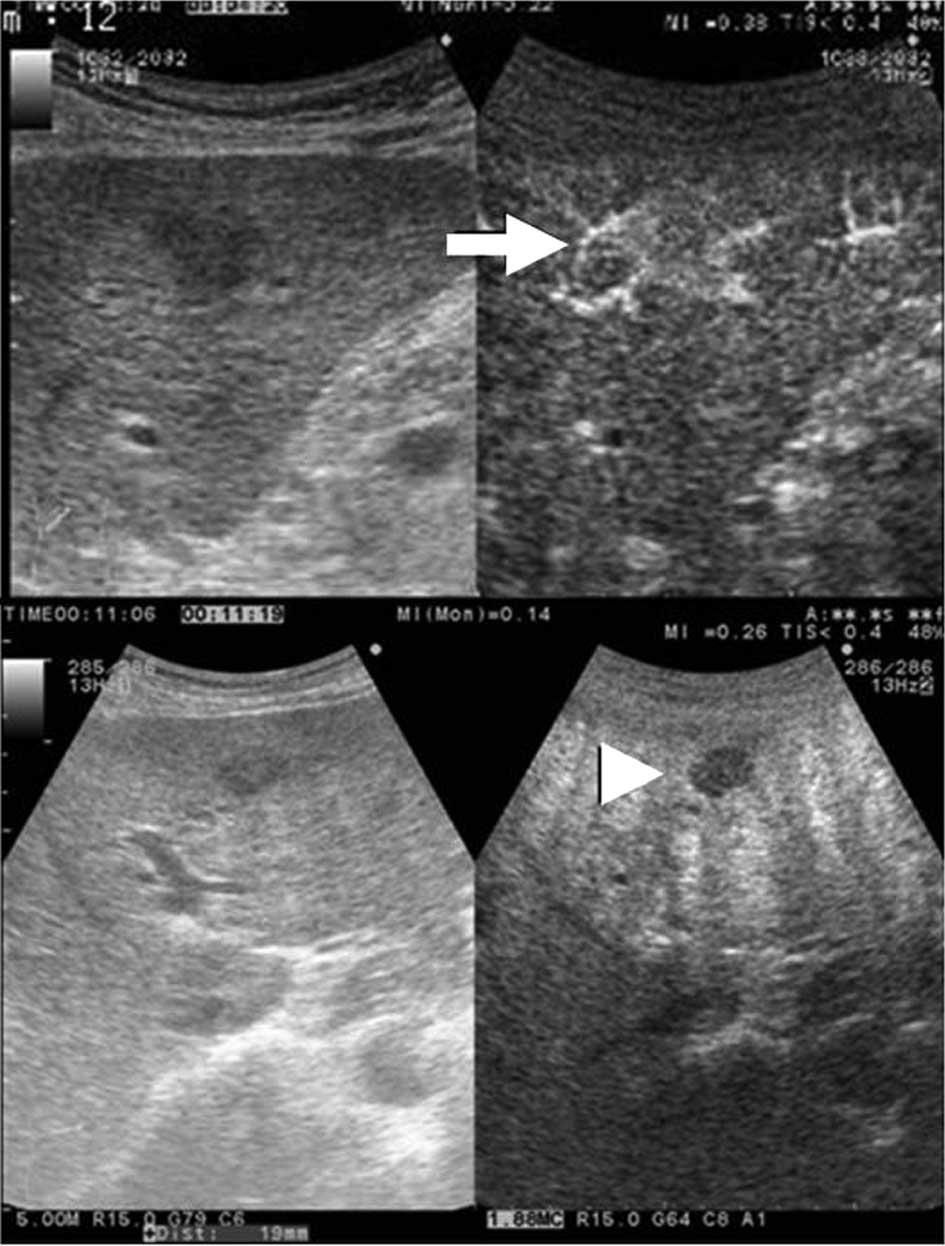
were compared retrospectively between CEUS and Dy-CT. Nodules were
diagnosed as typical HCCs by Dy-CT when they were enhanced in the
arterial phase and were revealed as a defect in the portal phase of
Dy-CT (Fig. 1) (3). Moreover, a nodule was diagnosed as
typical HCC, when it was shown as hypervascular in the arterial
phase and revealed as a defect lesion in the Kupffer phase
(Fig. 2) by CEUS (4,5).
 | Table ITechnical background using the
extended pure harmonic detection method and ProSound Alpha-10. |
Table I
Technical background using the
extended pure harmonic detection method and ProSound Alpha-10.
| Image | Frequency (MHz) | MI index | Range | Gain | Contrast |
|---|
| Fundamental | 5.00 | 0.30 | 17 | 60 | 15 |
| Contrast | 1.88 | 0.24 | 17 | 45 | 18 |
Statistical analysis
Statistical analyses were carried out using the
Chi-square test with StatView version 5.0 (SAS Institute, Inc.,
Berkeley, CA, USA). P<0.05 was considered statistically
significant.
Results
The average diameter of the S-group nodules was
1.42±0.39 cm (range 0.8–2 cm), while that of the L-group was
3.03±1.10 cm (range 2.1–8 cm). The background of each patient is
described in Table II. When
typical HCCs using Dy-CT were defined as the gold standard of
diagnosing HCCs, the sensitivity and specificity of CEUS with
Sonazoid were 97.1% (66/68) and 81.8% (9/11), respectively
(Table III). The sensitivity and
specificity in the S-group were 94.7% (36/38) and 81.8% (9/11),
respectively, while the positive and negative predictive values
were 94.7% (36/38) and 81.8% (9/11), respectively. In the L-group,
the nodules were diagnosed as HCCs by CEUS, which was identical
with the results of Dy-CT.
 | Table IIBackground of the patients. |
Table II
Background of the patients.
| S-groupa | L-groupb |
|---|
| Male | 25 | 20 |
| Female | 16 | 8 |
| Mean age (years) | 71.1±7.4 | 71.0±9.0 |
| Positive for
anti-HCV | 38 | 21 |
| Positive for
HBsAg | 0 | 5 |
| Positive for both
anti-HCVAb and HBsAg | 3 | 0 |
| Negative for both
anti-HCVAb and HBsAg | 0 | 2 |
| Past history of
HCC |
| Positive | 16 | 11 |
| Negative | 25 | 17 |
| No. of tumors | 49 | 30 |
| Tumor size (cm) | 1.42±0.39 | 3.03±1.10 |
| AST (IU/l) | 73.6±100.9 | 66.8±42.5 |
| ALT (IU/l) | 52.3±55.9 | 51.4±31.3 |
| T-bil (mg/dl) | 0.92±0.47 | 1.07±0.80 |
| TP (g/dl) | 7.3±0.8 | 7.1±0.7 |
| ALB (g/dl) | 3.7±0.6 | 3.7±0.6 |
| PLT
(*104/μl) | 11.8±6.10 | 13.3±8.9 |
| PT (%) | 72.1±10.4 | 74.5±14.4 |
| AFP | 89.4±300.0 | 142.1±360.8 |
 | Table IIIComparison between contrast enhanced
ultrasonography and dynamic computed tomography. |
Table III
Comparison between contrast enhanced
ultrasonography and dynamic computed tomography.
| A, All cases. |
|---|
|
|---|
| Dy-CT |
|---|
|
|
|---|
| Typical HCC | Not typical HCC | Total |
|---|
| CEUS |
| Typical HCC | 66 | 2a | 68 |
| Not typical HCC | 2b | 9 | 11 |
| Total | 68 | 11 | 79 |
|
| B, S-group. |
|
| Dy-CT |
|
|
| Typical HCC | Not typical HCC | Total |
|
| CEUS |
| Typical HCC | 36 | 2a | 38 |
| Not typical HCC | 2b | 9 | 11 |
| Total | 38 | 11 | 49 |
|
| C, L-group. |
|
| Dy-CT |
|
|
| Typical HCC | Not typical | HCC Total |
|
| CEUS |
| Typical HCC | 30 | 0 | 30 |
| Not typical HCC | 0 | 0 | 0 |
| Total | 30 | 0 | 30 |
In the S-group, 2 nodules were detected by CEUS, but
were not detected by Dy-CT. On the other hand, 2 nodules were
detected by Dy-CT, but not by CEUS. We performed US-guided biopsies
in the 2 nodules which were undetected by Dy-CT. One was detected
as a mixed echoic nodule with a diameter of 0.8 cm in segment 5 of
the liver by B-mode US (male, 63 years of age, positive for
anti-HCVAb). This nodule was revealed as a hypervascular lesion in
the arterial phase and as a defect in the Kupffer phase. Based on
the CEUS findings, the tumor was suspected to be HCC (typical HCC
by CEUS). The result of the biopsy identified it as a
well-differentiated HCC. The other nodule was revealed to be
hypoechoic with a diameter of 1 cm in segment 8 of the liver by
B-mode US (female, 81 years of age, positive for anti-HCVAb). The
tumor was slightly enhanced peripherally in the arterial phase and
was revealed as a partial defect in the Kupffer phase by CEUS. The
CEUS findings were not conclusive, and this tumor was unable to be
diagnosed as a typical HCC. The result of the biopsy identified it
as an atypical adenomatous hyperplasia (AAH). Among the 38 nodules
detected by CEUS, the diameter of 8 nodules including the
above-mentioned 2 nodules were <1 cm. The other 6 nodules larger
by <1 cm were diagnosed as typical HCCs by both Dy-CT and CEUS.
One nodule detected by Dy-CT but not by CEUS was located in a deep
position from the body surface.
Discussion
Sonazoid is a new agent for CEUS which reveals the
arterial flow in tumors. Moreover, malignant hepatic tumors can be
observed continuously and repeatedly throughout the examination as
a defective area in the Kupffer phase due to the lack of Kupffer
cells in tumors (6).
Classic HCC cases have a blood flow from the feeding
artery and a decrease in or lack of a portal vein in the tumor,
whereas those in the early stage have a portal vein and increased
arterial flow. When the diameter of the tumor expands, the portal
flow and Kupffer cells decrease in the tumor (7,8). The
present study validated the usefulness of CEUS using Sonazoid. We
diagnosed all nodules as typical HCCs in cases with tumors >2 cm
in diameter (L-group) using CEUS, identical to the results obtained
using Dy-CT. Therefore, it was confirmed that the diagnostic
ability for detecting HCCs with a diameter >2 cm using CEUS with
Sonazoid was identical to Dy-CT.
A small HCC can be difficult to diagnose. We
performed CEUS with Sonazoid to evaluate small HCC nodules ≤2 cm in
diameter (S-group) and found the sensitivity and specificity to be
94.7 and 81.8%, respectively, while the positive and negative
predictive values were 94.7 and 81.8%, respectively. Although the
study population was small and the study design was retrospective,
the results were encouraging.
The high rates for sensitivity and specificity of
CEUS with Sonazoid are thought to depend on the continuous view
provided in the Kupffer phase. CEUS with other agents (e.g.,
Levovist® and SonoVue®) does not reveal the
Kupffer phase image continuously for >10 min, as the imaging of
the Kupffer phase is performed by bursting microbubbles that
accumulate in Kupffer cells by sound waves (9). Continuous viewing in the Kupffer phase
image with Sonazoid can be obtained since the images are obtained
with vibration, rather than bursting by sound waves, which makes it
easy to observe abnormal findings (10).
Wang et al (9) reported on CEUS imaging with Levovist.
A combination of the characteristics of arterial phase enhancement
and the absence of the Kupffer phase enhancement as determined by
CEUS was highly specific for small HCCs in cirrhosis patients.
However, the use of CEUS with Levovist is difficult for many
operators since the enhancement of the Kupffer phase vanishes
immediately, and the scan cannot be conducted continuously.
Sonazoid is considered to be superior for the evaluation of hepatic
tumors compared with other agents, since nodules that are invisible
in B-mode but visible in Dy-CT are not detected in the Kupffer
phase with a continuous view due to the character of Sonazoid. the
detection of the target lesion, we observed the arterial flow by
re-injection into the defect area (11). Hohmann et al reported that
SonoVue markedly improved the characterization of focal hepatic
lesions in comparison with unenhanced sonography (12). Giorgio et al reported that
the enhancement pattern related to tumor hypervascularity as well
as sensitivity and specificity with SonoVue were high for nodules
1–3 cm in diameter, whereas these were very low (sensitivity,
27.3%; specificity, 100%) for nodules <1 cm (13).
In the present study, 8 nodules were <1 cm and
detectable by CEUS with Sonazoid. Two nodules, which were invisible
by Dy-CT but visible in the Kupffer phase of CEUS, were diagnosed
as a well-differentiated HCC and an AAH. This indicates that
certain HCC nodules which are not detectable by Dy-CT may be
detectable by CEUS. The characteristics of these nodules should be
investigated. Although these 2 nodules were diagnosed by biopsy,
another 6, which were <1 cm, were diagnosed as typical HCCs by
CEUS with Sonazoid.
There are several negative aspects associated with
CEUS, such as the difficulty in discerning lesions deeply
positioned, liver surface lesions confirmed by US, and lesions
located in the blind spot. In addition, the internal echo is very
irregular in the case of chronic liver injury. While all nodules of
the L-group were detected by CEUS, 2 nodules in the S-group were
not as they were located in a deep position. The diagnostic
efficacy for small HCCs is evident when understanding the
characteristics and disadvantages of CEUS particularly in the
Kupffer phase.
In conclusion, the low cost and non-invasive
characteristics of CEUS with Sonazoid are useful in screening
examinations and for diagnosing small HCCs.
References
|
1
|
Sontum PC: Physiochemical characteristics
of Sonazoid, a new contrast agent for ultrasound imaging.
Ultrasound Med Biol. 34:824–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe R, Matsumura M, Chen CJ, et al:
Characterization of tumor imaging with microbubble-based ultrasound
contrast agent, sonazoid, in rabbit liver. Biol Pharm Bull.
28:972–977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma. Hepatology. 42:1208–1236. 2005.
View Article : Google Scholar
|
|
4
|
Watanabe R, Matsumura M, Munrmasa T, et
al: Mechanism of hepatic parenchyma-specific contrast of
microbubble-based contrast agent for ultrasonography: microscopic
studies in rat liver. Invest Radiol. 42:643–651. 2007. View Article : Google Scholar
|
|
5
|
Kindberg GM, Tolleshaug H, Roos N, et al:
Hepatic clearance of Sonazoid perfluorobutane microbubbles by
Kupffer cells does not reduce the ability of liver to phagocytose
or degrade albumin microspheres. Cell Tissue Res. 312:49–54.
2003.
|
|
6
|
Kudo M: New sonographic techniques for the
diagnosis and treatment of hepatocellular carcinoma. Hepatol Res.
37(Suppl 2): 193–199. 2007. View Article : Google Scholar
|
|
7
|
Nakashima O, Sugihara S, Kage M, et al:
Pathomorphologic characteristics of small hepatocellular carcinoma:
a special reference to small hepatocellular carcinoma with
indistinct margins. Hepatology. 22:101–105. 1995.
|
|
8
|
Matsui O, Kadoya M, Kameyama T, et al:
Benign and malignant nodules in cirrhotic livers: distinction based
on blood supply. Radiology. 178:493–497. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JH, Lu SN, Hung CH, et al: Small
hepatic nodules (< or =2 cm) in cirrhosis patients:
characterization with contrast-enhanced ultrasonography. Liver Int.
26:928–934. 2006.
|
|
10
|
Numata K, Morimoto M, Ogura M, et al:
Ablation therapy guided by contrast-enhanced sonography with
Sonazoid for hepatocellular carcinoma lesions not detected by
conventional sonography. Ultrasound Med. 27:395–406.
2008.PubMed/NCBI
|
|
11
|
Minami Y, Chung H, Kudo M, et al:
Radiofrequency ablation of hepatocellular carcinoma: value of
virtual CT sonography with magnetic navigation. Am J Roentgenol.
190:W335–W341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hohmann J, Skrok J, Puls R, et al:
Characterization of focal liver lesions with contrast-enhanced low
MI real time ultrasound and SonoVue. Rofo. 175:835–843.
2003.PubMed/NCBI
|
|
13
|
Giorgio A, De Stefano G, Coppola C, et al:
Contrast-enhanced sonography in the characterization of small
hepatocellular carcinomas in cirrhotic patients: comparison with
contrast-enhanced ultrafast magnetic resonance imaging. Anticancer
Res. 27:4263–4269. 2007.
|