Introduction
Metastasis is the leading cause of death in bladder
urothelial carcinoma patients. Several events are required for
metastasis to occur, including neovascularization, cell attachment,
invasion and cell proliferation. Interactions of neoplastic cells
with the extracellular matrix are critical steps in the growth and
invasion of cancer. These interactions have been demonstrated in a
wide range of human cancers, including urothelial cancers (1). Matrix metalloproteinases (MMPs), a
family of Zn2+-dependent endogenous proteases, are able
to degrade various components of extracellular matrix. In the
extracellular domain, the activity of these proteases is tightly
regulated by inhibitors known as tissue inhibitors of
metalloproteinases (TIMPs). It has been postulated that TIMPs act
as tumor suppressor genes due to their anti-metalloproteinase
activity and their protective role on the extracellular matrix. The
imbalance between MMPs and TIMPs may be an indicator for cancer
prognosis. To investigate the role of MMPs and TIMPs in urinary
bladder cancers, the expression of MMPs and TIMPs in bladder
transitional cell carcinoma (TCC) cell lines, as well as surgical
specimens was investigated in order to see whether these specimens
were an indicator of cancer prognosis.
Materials and methods
Cell lines and surgical specimens
Six transitional cell carcinoma cell lines (MGH-U1,
MGH-U1R, MGH-U3, MGH-U4, TCC8704 and TSGH8301) were used. The cell
lines were maintained in RPMI-1640 (Gibco) containing 10%
heat-inactivated fetal bovine serum (FBS), 50 U/ml penicillin-G and
50 μg/ml streptomycin (Gibco), 2 mM L-glutamine and 1 mM sodium
pyruvate (Gibco). The four human bladder cancer cell lines, MGH-U1,
-U1R, -U3 and -U4, were generous gifts from Dr C.W. Lin
(Massachusetts General Hospital, Boston, MA, USA). MGH-U1 and -U1R
are sublines of T24, MGH-U3 was established from a grade 1, stage A
bladder tumor, and MGH-U4 was established from a stage O bladder
tumor with atypia. The remaining cell lines, TCC8704 and TSGH8301,
were courtesy of Dr D.S. Yu (Department of Urology, Tri-Service
General Hospital, Taipei, Taiwan, R.O.C.). Cells were incubated at
37°C in a CO2 incubator in humidified atmosphere
containing 95% air and 5% CO2. Table I shows the grade and stage of tumors
from which the cell lines were derived. Following Ethic approval
and patients’ written consent, 30 primary bladder tumor specimens
were resected at Chang Gung Memorial Hospital, Taiwan, and studied.
Tumor staging was performed based on the 1997 tumor, nodes and
metastasis (TNM) classification of bladder cancer, which was agreed
upon by the American Joint Committee on Cancer (AJCC) and the Union
Internationale Contra Cancer (UICC). Of the 30 resected specimens,
15 were superficial involvements (Ta-T1), 9 had muscle invasion
(T2), 4 perivesical invasion (T3) and 2 distant metastases (M1).
The histology was 5 grade 1, 14 grade 2 and 11 grade 3,
respectively. Surgical specimens were immersed in plastic
containers with optimum cutting temperature compound and stored in
a −70°C refrigerator until use.
 | Table IExpression of MMPs and TIMPs in TCC
cell lines determined by immunocytochemistry. |
Table I
Expression of MMPs and TIMPs in TCC
cell lines determined by immunocytochemistry.
| Cell lines | Stagea | Grade | HLA-ABC | MMP1 | MMP2 | MMP3 | MMP9a | MMP9b | TIMP1 |
|---|
| MGH-U1 | B | 3 | 2+ | 3+ | − | − | 1+ | 1+ | − |
| MGH-U1R | B | 3 | 2+ | 3+ | − | − | − | 1+ | ± |
| MGH-U3 | A | 1 | 3+ | 2+ | 2+ | 2+ | ± | 1+ | − |
| MGH-U4 | O | atypia | 3+ | 3+ | 3+ | 2+ | − | 1+ | − |
| TSGH8301 | A | 2 | 1+ | 2+ | 2+ | 1+ | ± | 2+ | ± |
| TCC8704 | D2 | 3 | 2+ | 3+ | 2+ | 2+ | − | 1+ | ± |
Chamber slide cultures and
immunocytochemistry
Cells (5×104) were grown on each well of
Lab-Tek® chamber slides (Nunc, Naperville, IL, USA)
until confluence was achieved. Immunostaining was carried out at
room temperature using the avidin-biotin-peroxidase complex (ABC)
method (Vectastain ABC kit, Vector Laboratories, Burlingame, CA,
USA), according to the manufacturer’s instructions. Briefly, 100 μl
monoclonal antibodies at a concentration of 5 μg/ml, or at the
dilution recommended by the manufacturer were added to each well.
The slides were incubated for 1 h following 1 h of normal horse
serum blocking. After washing twice with PBS solution, secondary
antibodies (anti-mouse IgG) were added and incubated for 1 h. The
slides were washed twice with PBS and then ABC reagent was added
and incubated for another 40 min. After washing, the chromogen,
3-amino-9-ethylcarbazol (Aldrich Chemical Co., Inc., Milwaukee, WI,
USA), containing 0.02% hydrogen peroxide, was used to detect the
peroxidase conjugation. Gill’s hematoxylin solution (Fisher
Scientific, Norcross, CA, USA) was used for counterstaining.
Monoclonal antibodies (mAbs) used in this study were those against
MMP-1 (41-1ES), MMP-2 (42-5D11; Calbiochem, San Diego, CA, USA),
MMP-3 (55-2A4), MMP-9, TIMP-1 and TIMP-2 (Oncogene, Cambridge, MA,
USA). A negative control (PBS instead of primary antibody,
isotype-matched irrelevant mMAbs and normal mouse IgG) and positive
controls (anti-HLA-A-B, C, W6/32) were included in each test.
Western blot analysis
Cell line extracts were obtained as previously
described (2). Blots were incubated
overnight with mouse-anti-MMP-2 (Lab Vision, USA) (1 μg/ml in 3%
BSA) followed by incubation with goat anti-mouse IgG horseradish
peroxidase, developed using the Santa Cruz enhanced
chemiluminescence detection system (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) and exposed by the Chemi-Smart 3000 (Vilber Lourmat)
image system.
Gelatin zymography
Each cell line was grown in culture medium and
protein extract was obtained at days 1, 3 and 5 for zymographic
analysis. Gelatinolytic zymography was performed as described by
Brown et al (3). Briefly, 30
μg of protein extract was mixed with non-reducing electrophoresis
buffer on a 10% polyacrylamide gel containing gelatin (1 mg/ ml).
After electrophoresis, the gels were incubated in a buffer
containing Triton X-100 2.5%, 0.15 M NaCl, 10 mM CaCl2,
50 mM Tris-HCl buffer (pH 7.5) and 0.05% Coomassie brilliant blue
and destained in 7% acetic acid and 5% methanol overnight with
gentle rocking.
Immunohistochemical staining of bladder
tumor tissues
Frozen tissue was cut (5 μm) and placed on
gelatin-coated slides. The tissue was air-dried and fixed in
chilled (4–5°C) acetone for 10 min. Immunostaining was carried out
at room temperature using the ABC method, similar to that described
above.
Statistical analysis
The significance of differences was calculated using
Chi-square and Fisher’s exact probability tests. P<0.05 was
considered to be statistically significant.
Results
Expression of MMPs and TIMPs by TCC cell
lines
Immunohistochemical results of MMPs and TIMPs in TCC
cell lines are shown in Table I.
The cell lines were strongly stained with MMP-1 and weakly stained
with MMP-9b. MMP-2 and -3 were moderately stained on MGH-U3,
MGH-U4, TSGH8301 and TCC8704, but negatively stained on high-grade
TCC MGH-U1 and -U1R. No cell lines expressed TIMP-1 and MMP-9a.
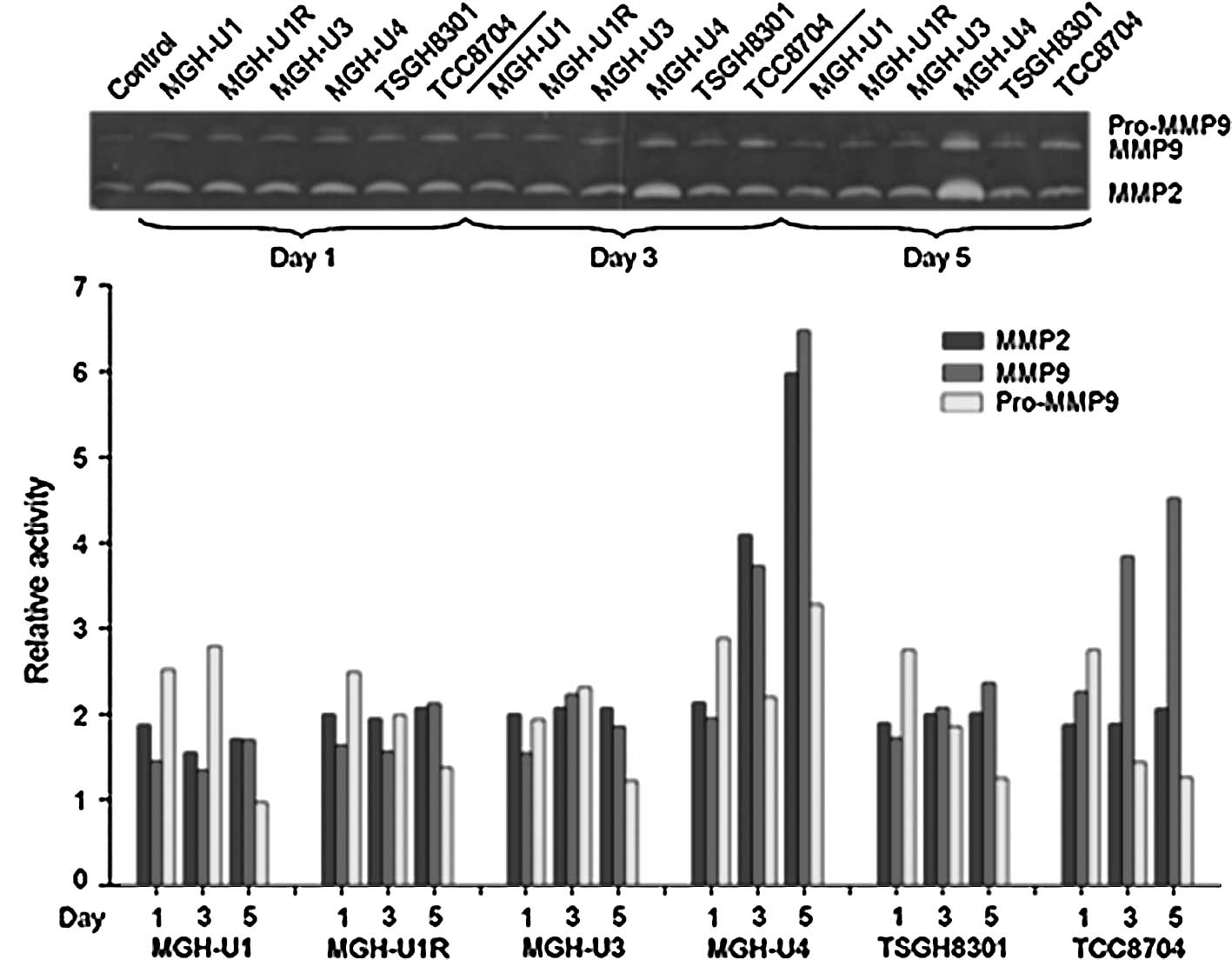
Zymographic analysis of the cell lines showed that the level of
MMP-2 was found to be higher in MGH-U4 as compared to the other
cell lines, and MMP-9 was higher in MGH-U4 and TCC8704 (Fig. 1). Western blotting was used to
verify the presence of MMP-2 and -9 in cultured cell lines used in
zymography. The expression of MMP and TIMP families was not
correlated with the disease status of the original tumors.
Expression of MMPs and TIMPs by bladder
carcinoma tissues
The surgical specimens stained positively with
MMP-1, with 19 cases being positive for MMP-2 and only 2 specimens
being positive for MMP-3 (Table
II). The expression of MMP-2 correlated with grade 3 tumors
(p=0.036, Table III). The
expression of MMP-9a and MMP-9b correlated with stage T2/T3/M1
tumors (p=0.012 and 0.023, respectively, Table IV). The expression of MMP-1, MMP-3,
TIMP-1 and TIMP-2 did not correlate with either tumor stage or
tumor grade.
 | Table IIExpression of MMPs and TIMPs in frozen
sections of bladder cancer specimens. |
Table II
Expression of MMPs and TIMPs in frozen
sections of bladder cancer specimens.
| n=30 | Positive (%) |
|---|
| MMP-1 | 30/30 | 100.0 |
| MMP-2 | 19/30 | 63.3 |
| MMP-3 | 2/30 | 6.7 |
| MMP-9a | 21/30 | 70.0 |
| MMP-9b | 15/30 | 50.0 |
| TIMP-1 | 12/30 | 40.0 |
| TIMP-2 | 20/30 | 66.7 |
 | Table IIIExpression of MMPs and TIMPs in frozen
sections according to tumor grade. |
Table III
Expression of MMPs and TIMPs in frozen
sections according to tumor grade.
| MMP or TIMP
expression | Grade I and II (n=19)
(%) | Grade III (n=11)
(%) | p-value |
|---|
| MMP-1 | 19 (100) | 11 (100) | |
| MMP-2 | 9 (47.4) | 10 (90.9) | 0.036 |
| MMP-3 | 0 | 2 (18.2) | 0.068 |
| MMP-9a | 14 (73.7) | 7 (63.6) | 0.463 |
| MMP-9b | 9 (47.4) | 6 (54.5) | 0.699 |
| TIMP-1 | 5 (26.3) | 7 (63.6) | 0.058 |
| TIMP-2 | 12 (63.2) | 8 (72.7) | 0.453 |
 | Table IVExpression of MMPs and TIMPs in
frozen sections according to tumor stage. |
Table IV
Expression of MMPs and TIMPs in
frozen sections according to tumor stage.
| MMP or TIMP
expression | Stage Ta-T1 (n=15)
(%) | Stage T2, T3 and M1
(n=15) (%) | p-value |
|---|
| MMP-1 | 15 (100) | 15 (100) | 1 |
| MMP-2 | 9 (60.0) | 10 (66.7) | 0.686 |
| MMP-3 | 2 (13.3) | 0 | 0.142 |
| MMP-9a | 7 (46.7) | 14 (93.3) | 0.012 |
| MMP-9b | 4 (26.7) | 11 (73.3) | 0.023 |
| TIMP-1 | 6 (40.0) | 6 (40.0) | 1 |
| TIMP-2 | 11 (73.3) | 8 (53.3) | 0.430 |
Discussion
Bladder cancer is the fourth most common malignant
neoplasm in men and the eighth most common in women among
Americans. Bladder cancer can be classified as superficial and
invasive. Additionally, the majority of the bladder tumors are
primarily superficial, but 70% of them will recur and 20–30% result
in progression and metastasis (4).
The aims of management of bladder cancer are two-fold: i) to detect
relapse of the disease prior to the development of overt symptoms,
such as gross hematuria or pain, and ii) to identify tumors that
potentially indicate early recurrence, invasion and dissemination.
Several studies have attempted to define the most predictable
markers for recurrence and metastasis (5). The most common prognostic factors are
staging and grading according to pathological characteristics.
However, 36% of patients with urothelial cancer lack these
characteristics. Previously, cytological analysis was used for
transitional cell carcinomas. However, new tumor markers and
molecules are currently under investigation (5). Notably, when considering treatment
modalities for patients with transitional cell carcinoma of the
bladder it is crucial to identify tumors that are likely to
progress to invasive disease. Metastasis begins with the growth of
tumor cells and invasion into the stroma surrounding the primary
neoplasm. Degradation of the extracellular matrix and basement
membrane are believed to be associated with tumor invasion and
metastasis (1,6,7). The
principal intrinsic components of the basement membrane are
laminine and type IV collagen. Type IV collagen differs from the
interstitial collagens (type I, II and III) in that it is localized
exclusively in the basement membrane. Various types of matrix
degradative enzymes or proteases are expressed and/or secreted by
tumor cells. These include the metalloproteinases (8), serine proteinases (9) and lysosomal proteases (10,11).
MMPs are a family of peptidae enzymes involved in remodeling
extracellular components (12),
including collagen, gelatin, fibronectin, laminin and proteoglycan.
MMPs are complex regulators of multiple cell functions. Different
cell types express various MMPs in cancer progression and
metastasis (13). Kitagawa et
al (14) noted that MMP-2 or
gelatinase A is able to degrade type IV collagen and plays a role
in tumor angiogenesis and metastasis. Numerous studies focused on
the roles of MMPs in tumor invasion and metastasis (15,16).
The roles of MMPs in angiogenesis are dual and complex and the
increased expression of MMPs has been reported in various human
malignant tumors (17–20). MMP-2 and -9 are critical for the
angiogenic switch when the tumors become vascularized (21). MMPs were secreted in urine or serum
by the tumors, and a zymographic analysis revealed that the urinary
levels of MMP-9 and activated MMP-2 were higher in invasive bladder
cancers than in superficial ones (22,23).
However, in this study, the zymographic analysis of the cell lines
did not yield similar results.
TIMP was originally identified as a mammalian
collagenase inhibitor and is a glycoprotein with a molecular mass
of approximately 28 kDa (24,25).
TIMP-2 is a non-glycosylated protein with a molecular mass of
approximately 21 kDa and a structure homologous to TIMP. TIMPs are
secreted by many types of cells, including tumor cells, and inhibit
the activities of various MMPs. In addition, these inhibitors
suppress tumor cell invasion in vitro (26,27)
and experimental metastasis in vivo (3,27,28).
The hypothesis that TIMPs act as tumor suppressor genes due to
their anti-metalloproteinase activity and their protective role on
the extracellular matrix has been noted (29,30).
On the other hand, it was proposed that the simultaneous cellular
expression of MMPs and TIMPs in patients with breast cancer be
determined as a predictor of clinical outcome (31). No significance of the MMP/TIMP ratio
in predicting invasiveness was found in this study.
Our immunohistochemical results showed that MMP-9
was associated with the invasiveness of TCC and MMP-2 was
associated with the grade of TCC. A discrepancy was found between
the results of frozen sections and chamber slide cell stain, in
which tumors of higher grade were stained negatively with MMP-2.
Possible causes for the discrepancy in the results may be that the
number of cell lines used were insufficent to reflect patient
tumors. Additionally, cell lines able to grow in vitro may
be highly selective subpopulations of the original tumors. The
three-dimensional structure noted in in vivo tumors, as well
as the interaction between tumors and stromal cells may be required
for MMP-2 expression. Another explanation is that these cell lines
were maintained in vitro for more than 2 years and loss of
MMP-2 expression occurred following such a long-term in
vitro culture. Early stage immunohistochemistry is therefore
required to verify this assumption. MMP-9 has been reported to be
overexpressed in invasive bladder cancers (23). However, MGH-U1 and -U1R cells were
stained negatively with MMP-9. The explanation in MMP-2 may be
applied to that of MMP-9. In a given tumor microenvironment, the
interaction among tumor cells in situ and tumor-associated
cells, such as neutrophils, macrophages, lymphocytes and
endothelial cells, as well as environmental factors (hypoxia and
pH), cytokines and growth factors released by these cells may be
required for TCC expression of selective MMPs and TIMPs. The
selective expression of these molecules then regulates tumor
progression and angiogenesis. Therefore, the immunohistochemical
results of the expression of MMPs and TIMPs in TCC tumor specimens
may have greater clinical relevance than those obtained with the
limited number of TCC cell lines in this study.
CD44 is a cell surface transmembrane glycoprotein
that participates in cell motility and adherence of cells to
extracellular matrix (ECM). CD44 also modulates the secretion and
activation of MMP-2 (32). The
collaboration between MMPs and CD44 at the cell surface may be
essential in mediating degradation of the ECM to facilitate cell
migration (33). In our previous
study, a clear correlation of weak or negative staining of CD44v5
and surgical specimen tumor grades and stages was observed
(2). The combined weight of the
previous (2) and present results
indicate that loss of CD44v5 expression may be induced by MMP-2
expressed by high-grade urothelial carcinomas, exhibiting a more
invasive phenotype.
In conclusion, immunohistochemical testing of MMP
and TIMP expression on 30 TCC surgical specimens revealed that the
overexpression of MMP-2 was correlated with tumor grade, while that
of MMP-9 was correlated with tumor stage. However, the expression
of TIMP-1 or TIMP-2 did not significantly correlate with any of the
disease states analyzed, although there was a tendency for TIMP-2
to correlate with tumor grade.
References
|
1
|
Liotta LA: Tumor invasion and metastasis:
role of the extracellular matrix. Cancer Res. 46:1–7.
1986.PubMed/NCBI
|
|
2
|
Chuang CK and Liao SK: Differential
expression of CD44 variant isoforms by cell lines and tissue
specimens of transitional cell carcinoma. Anticancer Res.
23:4635–4640. 2003.PubMed/NCBI
|
|
3
|
Brown PD, Levy AT, Margulies IMK, Liotta
LA and Stetler-Stevenson WG: Independent expression and cellular
processing of Mr 72,000 type IV collagenase and interstitial
collagenase in human tumorigenic cell lines. Cancer Res.
50:6184–6191. 1990.PubMed/NCBI
|
|
4
|
Fleshner NE, Herr HW, Stewart AK, Murphy
GP, Mettline C and Menck HR: The national cancer data base report
on bladder carcinoma. Cancer. 77:1505–1513. 1996. View Article : Google Scholar
|
|
5
|
Chuang CK and Liao SK: Evaluation of
CA19-9 as a tumor marker in urothelial malignancy. Scand J Urol
Nephrol. 38:359–365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koivunen E, Ristimaki A, Itkonen O, Osman
S, Vuento M and Stenman UH: Tumor-associated trypsin participates
in cancer cell mediated defradation of extracellular matrix. Cancer
Res. 51:2107–2112. 1991.PubMed/NCBI
|
|
8
|
Van Wart HE and Birkedal-Hansen H: The
cysteine switch: a principle of regulation of metalloproteinase
activity with potential applicability to the entire matrix
metalloproteinase gene family. Proc Natl Acad Sci USA.
87:5578–5582. 1990.PubMed/NCBI
|
|
9
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993.PubMed/NCBI
|
|
10
|
Liu BCS and Liotta LA: Biochemistry of
bladder cancer invasion and metastasis: clinical implications. Urol
Clin North Am. 19:621–627. 1992.PubMed/NCBI
|
|
11
|
Redwood SM, Liu BCS, Weiss RE, Hodge DE
and Droller MJ: Abrogation of the invasion of human bladder tumor
cells by using protease inhibitor(s). Cancer. 69:1212–1219. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: an imbalance of positive and negative
regulation. Cancer Res. 51:5054–5059. 1991.PubMed/NCBI
|
|
13
|
Agnes N, Maud J and Erik M: Matrix
metalloproteinases at cancer tumor-host interface. Semin Cell Dev
Biol. 19:52–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitagawa Y, Kunimi K, Ito H, et al:
Expression and tissue localization of membrane-types 1, 2 and 3
matrix metalloproteinases in human urothelial carcinomas. J Urol.
160:1540–1545. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakajima M, Welch DR, Belloni PN and
Nicolson GL: Degradation and basement membrane type IV collagen and
lung subendotherial matrix by rat mammary adenocarcinoma cell
clones of differing metastatic potentials. Cancer Res.
47:4869–4876. 1987.PubMed/NCBI
|
|
16
|
Nakajima M, Welch DR, Wynn DM, Tsuruo T
and Nicolson GL: Serum and plasma Mr 92,000 progelatinase levels
correlate with spontaneous metastasis of rat 13762NF mammary
adenocarcinoma. Cancer Res. 53:5802–5807. 1993.PubMed/NCBI
|
|
17
|
Pyke C, Ralfkiaer E, Tryggvason K and Dano
K: Messenger RNA for two type IV collagenases is located in stromal
cells in human colon cancer. Am J Pathol. 142:359–365.
1993.PubMed/NCBI
|
|
18
|
Stearns ME and Wang M: Type IV collagenase
(Mr 72,000) expression in human prostate: benign and malignant
tissue. Cancer Res. 53:878–883. 1993.PubMed/NCBI
|
|
19
|
Kanayama HO, Yokota KY, Kurokawa Y,
Murakami Y, Nishitani M and Kagawa S: Prognostic values of matrix
metalloproteinase-2 and tissue inhibitor of metalloproteinase-2
expression in bladder cancer. Cancer. 82:1359–1366. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grignon DJ, Sakr W, Toth M, et al: High
levels of tissue inhibitor of metalloproteinase-2 (TIMP-2)
expression are associated with poor outcome in invasive bladder
cancer. Cancer Res. 56:1654–1659. 1996.PubMed/NCBI
|
|
21
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis B, Waxman J, Wasan H, et al: Levels
of matrix metalloproteinase in bladder cancer correlate with tumor
grade and invasion. Cancer Res. 53:5365–5369. 1993.PubMed/NCBI
|
|
23
|
Gerhards S, Jung K, Koenig F, et al:
Excretion of matrix metalloproteinases 2 and 9 in urine is
associated with a high stage and grade of bladder carcinoma.
Urology. 57:675–679. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cawston TE, Galloway WA, Mercer E, Murphy
G and Reynolds JJ: Purification of rabbit bone inhibitor for
collagenase. Biochem J. 195:159–165. 1981.PubMed/NCBI
|
|
25
|
Docherty AJ, Lyons A, Smith BJ, et al:
Sequence of human tissue inhibitor of metalloproteinases and its
identity to erythroid-potentiating activity. Nature. 318:66–69.
1985. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mignatti P, Robbins E and Rifkin DB: Tumor
invasion through the human amniotic membrane: requirement for a
proteinase cascade. Cell. 47:487–498. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schultz RM, Silberman S, Persky B,
Bajkowski AS and Carmichael DF: Inhibition by human recombinant
tissue inhibitor of metalloproteinases of human amnion invasion and
lung colonization by murine B16–F10 melanoma cells. Cancer Res.
48:5539–5545. 1988.PubMed/NCBI
|
|
28
|
Alvarez OA, Carmichael DF and DeClerck YA:
Inhibition of collagenolytic activity and metastasis of tumor cells
by a recombinant human tissue inhititor of metalloproteinases. J
Natl Cancer Inst. 82:589–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stetler-Stevenson WG, Krutzsch HC and
Liotta LA: TIMP2: identification and characterization of a new
member of the metalloproteinases inhibitor family. Matrix
Supplement. 1:299–306. 1992.PubMed/NCBI
|
|
30
|
Denhardt DT, Khokha R, Yagel S, Overall CM
and Parhar RS: Oncogenic consequences of down-regulating TIMP
expression in 3T3 cells with antisense RNA. Matrix Supplement.
1:281–285. 1992.PubMed/NCBI
|
|
31
|
Baker EA, Stephenson TJ, Reed MW and Brown
NJ: Expression of proteinases and inhibitors in human breast cancer
progression and survival. Mol Pathol. 55:300–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi K, Eto H and Tanabe KK:
Involvement of CD44 in matrix metalloproteinase-2 regulation in
human melanoma cells. Int J Cancer. 29:387–395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Isacke CM and Yarwood H: Molecules in
focus. The hyaluronan receptor, CD44. Int J Biochem B. 34:718–721.
2002. View Article : Google Scholar
|