Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common form of non-Hodgkin’s lymphoma (1). Prognostic factors include age, general
health, serum lactate dehydrogenase levels, Ann Arbor disease
stage, and the presence of extranodal lesions (2). The introduction of the anti-CD20
antibody rituximab greatly improved the management and treatment of
B-cell malignancies (3), including
DLBCL (4,5). Retrospective analyses of rituximab in
combination with cyclophosphamide, doxorubicin, vincristine,
prednisone (CHOP) or equivalent regimens demonstrated that the
two-year overall survival (OS) increased to 78% in adult DLBCL
patients of all ages, in comparison to 52% in the pre-rituximab era
(6). For DLBCL patients,
combination therapy consisting of rituximab plus CHOP chemotherapy
is now the standard first-line treatment (5).
Despite improved outcomes, a proportion of DLBCL
patients exhibit relapse, particularly the subgroup of patients
with an International Prognostic Index (IPI) ≥2 (according to
National Comprehensive Cancer Network criteria). Currently, the
standard therapy for chemosensitive relapses is based on salvage
chemotherapy followed by high-dose therapy and autologous stem cell
transplantation (auto-SCT) in selected patients (7). The benefits of auto-SCT in combination
with salvage chemotherapy over salvage chemotherapy alone have been
demonstrated in several studies (8,9). In
this clinical setting, the application of rituximab for in
vivo purging of stem cell grafts resulted in a high purging
efficiency and improved response rates (10,11).
Five-year interim results from an ongoing study have shown the
efficacy of in vivo rituximab graft purging and
post-transplant rituximab maintenance therapy in combination with
auto-SCT in patients with relapsed follicular lymphoma (12). However, these results need to be
confirmed in studies using additional patient cohorts, such as
DLBCL patients. Another unknown element is the effect of rituximab
induction treatment on the outcome of second-line treatment
regimens involving the use of rituximab for graft purging and
maintenance therapy. Further clinical evidence is necessary in
order to guide the choice of salvage chemotherapy regimens and
assess the role of rituximab maintenance following auto-SCT.
This study aimed to determine the efficacy and
safety of rituximab purging and maintenance therapy combined with
auto-SCT in younger patients with high-risk DLBCL after
rituximab-based induction therapy.
Materials and methods
Inclusion criteria
DLBCL patients were evaluated at the Department of
Hematology, Peking Union Medical College Hospital, China, between
December 2004 and December 2006. The main inclusion criteria were:
an IPI score ≥2, age 18–65 years, an Eastern Cooperative Oncology
Group (ECOG) status <2, and partial recovery after corresponding
induction therapy. Patients were excluded if they exhibited central
nervous system involvement, a history of other malignancies,
pregnancy, severe non-malignant cardiac, renal, hepatic or
neurological diseases, and severe active infections, including
evidence of hepatitis B virus (HBV) replication. The study protocol
was approved by the local ethics committee, and this study was
performed in accordance with the standards defined by the 1964
Declaration of Helsinki. Informed consent was obtained from all
patients prior to enrollment in the study.
Induction therapy
All 12 patients received 4–6 courses of induction
therapy consisting of rituximab plus CHOP. The patients underwent
evaluation after every two cycles of chemotherapy. Patients who did
not exhibit partial remission (PR) or who exhibited relapse after
remission received salvage chemotherapy, which consisted of 2–4
courses of etoposide, cisplatin, cytarabine and
methylprednisolone/mitoxantrone, ifosfamide and etoposide until PR
was achieved. Stem cells were mobilized in patients without
lymphoma infiltration as assessed by bone marrow examination.
Mobilization and harvesting of autologous
peripheral hematopoietic stem cells
Peripheral hematopoietic stem cells were mobilized
with CHOP (cyclophosphamide: 2.5 g/m2). Granulocyte
colony-stimulating factor (G-CSF) was administered at a
concentration of 5–10 μg/kg/day. A single infusion of rituximab
(600 mg) was administered intravenously as an in vivo purge
one day prior to stem cell mobilization. Peripheral hematopoietic
stem cells were harvested using a Baxter CS3000 Plus cell separator
when peripheral mononuclear cells (MNCs) were
>1.5×109/l with a target count of
>2×108 MNCs and >2×106 CD34+
cells per kg of body weight. Patients in complete (CR) or
unconfirmed complete remission (CRu) after mobilization underwent
auto-SCT.
Transplantation of hematopoietic stem
cells
The patients were pretreated with carmustine (300
mg/m2, day -7), etoposide (200 mg/m2, days -6
to -3), cytarabine (400 mg/m2, days -6 to -3) and
melphalan (140 mg/m2, day -2) (BEAM). One day after stem
cell reinfusion, G-CSF (5 μg/kg/day) was administered
subcutaneously until the WBC count reached
>1.5×109/l. Antimicrobial prophylaxis was
administered simultaneously.
In vivo purging with rituximab
A single infusion of rituximab (375
mg/m2) was administered intravenously as an in
vivo purge one day before stem cell mobilization, one day
before conditioning therapy (day -8) and seven days after
reinfusion (day +7). Dexamethasone and Tylenol® were
administered for allergy prevention prior to infusion.
Rituximab maintenance after stem cell
transplantation
At 100 days after auto-SCT, patients underwent
rituximab (375 mg/m2) maintenance therapy every three
months for a total of two years.
Treatment of hepatitis B
Patients who were positive for hepatitis
B-associated antibodies (with the exception of HBsAb) received
lamivudine (0.1 mg orally once daily).
Patient follow-up
Follow-up was carried out every three months, and
included a physical examination, blood testing, testing for HBV
serological markers and quantification of serum immunoglobulins. A
whole body computed tomography (CT) scan was performed every six
months. Response to therapy was assessed every six months. Patients
exhibiting relapse were withdrawn from the study and received
salvage treatment. Rituximab treatment was withdrawn when
treatment-associated adverse effects were greater than second
degree.
Endpoints and statistical
considerations
The primary outcome in this study was
progression-free survival (PFS). Secondary endpoints were OS and
the presence of treatment-related toxicity. OS was defined as the
interval between stem cell reinfusion and patient death, and PFS
was defined as the time between stem cell reinfusion and the first
relapse or last follow-up. Treatment efficacy was evaluated
according to the standardized response criteria for lymphomas
(13). OS and PFS were determined
using the Kaplan-Meier method.
Results
Patient demographics and baseline
characteristics
Seven male and five female patients with stage
III/IV DLBCL were recruited in the present study. Patient baseline
characteristics are summarized in Table
I. The mean age was 45 years (range 18–63). One patient had an
IPI score of 4, while the remaining patients exhibited IPI scores
of 2–3. The patients received an average of seven cycles of
chemotherapy (range 6–9) prior to undergoing auto-SCT. First-line
auto-SCT was performed in nine patients. Among these patients,
residual disease was found in three individuals without CR. Another
three patients who showed chemosensitive disease received salvage
treatment due to relapse, and CRu was achieved after salvage
treatment followed by auto-SCT.
 | Table IPatient baseline characteristics. |
Table I
Patient baseline characteristics.
| Patient no. | Gender | Age (years) | Ann Arbor stage | IPI | No. of previous
chemotherapy regimens | Remission status
before autologous SCT | Remission status
after autologous SCT | Status post-
rituximab maintenance (months) |
|---|
| 1 | M | 36 | III | 2 | 6 | CR1 | CR | CCR 57+ |
| 2 | M | 30 | III | 2 | 6 | CR1 | CR | CCR 55+ |
| 3 | F | 28 | IV | 3 | 7 | CRu1 | CR | CCR 55+ |
| 4 | M | 36 | IV | 3 | 6 | CR1 | CR | CCR 53+ |
| 5 | M | 18 | III | 2 | 6 | CR1 | CR | CCR 53+ |
| 6 | M | 63 | III | 3 | 6 | CRu1 | CR | CCR45+ |
| 7 | F | 45 | IV | 3 | 9 | Relapse 1 sensitive
(CRu) | CR | CCR41+ |
| 8 | M | 55 | III | 2 | 6 | CRu1 | CRu | Relapse, CR |
| 9 | M | 47 | IV | 3 | 9 | Relapse sensitive
(CRu)1 | CR | Relapse, CR |
| 10 | F | 35 | IV | 2 | 6 | CR1 | CR | CCR 35+ |
| 11 | F | 60 | III | 2 | 8 | Relapse 1 sensitive
(CRu) | CR | CCR 34+ |
| 12 | F | 57 | IV | 4 | 7 | CR1 | CR | CCR 33+ |
Response to therapy and relapse
Prior to undergoing auto- SCT, six patients (50%)
had achieved CR and six patients (50%) had achieved CRu. Three
months after transplantation, 11 patients (91.7%) achieved CR and
only one patient achieved CRu. After two cycles of rituximab
maintenance therapy, CR was achieved in all 12 patients. A total of
10 patients achieved long-term CR and the other two experienced
relapse 14 and 20 months after the end of rituximab maintenance
therapy. Of these two patients, one experienced a relapse before
transplantation, and CR was achieved after salvage treatment and
stem cell mobilization. After four cycles of rituximab maintenance
therapy (14 months after auto-SCT), space-occupying lesions were
found, indicating a relapse. The other patient had a residual
lesion in the neck before undergoing transplantation. This lesion
showed partial improvement after auto-SCT. After two cycles of
rituximab maintenance therapy the residual lesion was resolved,
suggesting CR. However, a relapse was noted after six cycles of
rituximab maintenance therapy (20 months after auto-SCT).
Subsequently, the two patients received salvage therapy and
radiotherapy, which led to CR.
Survival
The mean follow-up period was 44 months (range
35–61). Disease-free survival was noted in 10 patients, and two
patients survived, despite tumor relapse. The median survival time
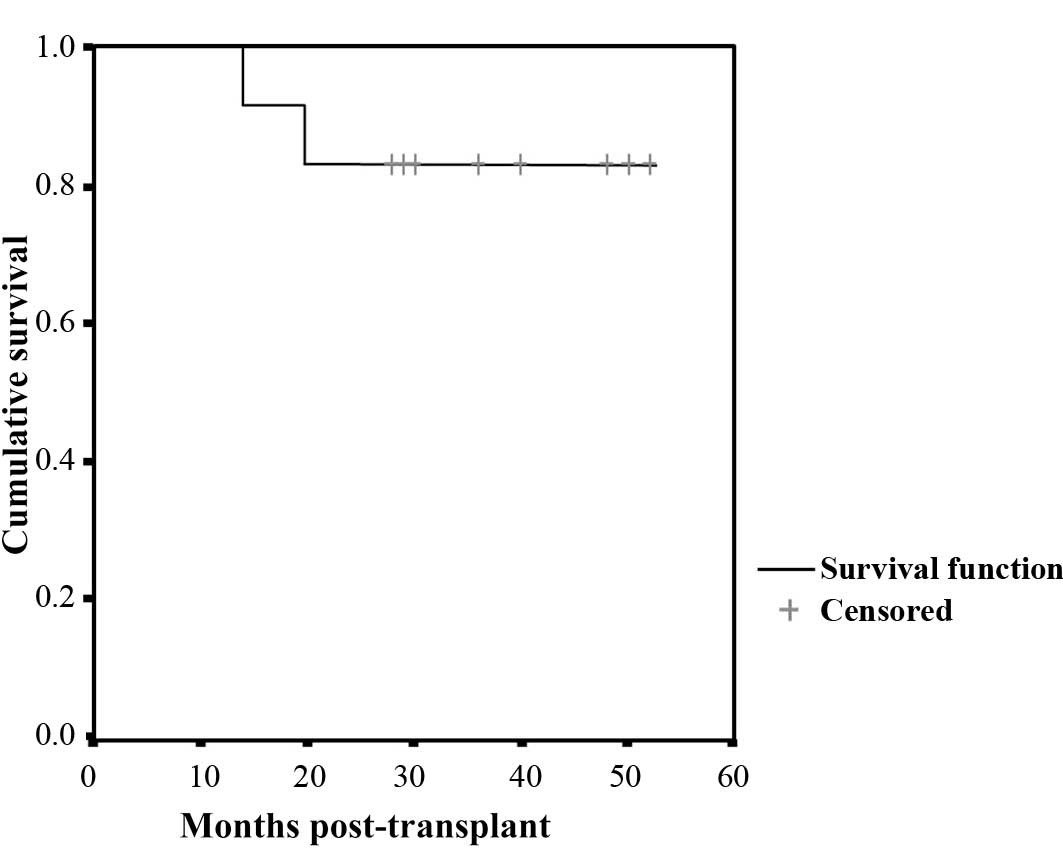
was not achieved in either patient. The three-year OS rate was 100%
and the three-year PFS rate was 83%. The projected five-year OS and
PFS rates are 100 and 83%, respectively (Fig. 1).
Adverse effects
No serious adverse events or deaths related to stem
cell mobilization or auto-SCT were noted in this study, nor any
treatment-related mortalities. During stem cell harvesting, the
median MNC count was 3.8×108 per kg of body weight
(range 2.2–7.1×108) and the median CD34+ cell
count was 4.6×106 per kg of body weight (range
2.5–8.4×106). The median time for the recovery of
neutrophils was 10 days (range 8–11). After auto-SCT, eight
patients experienced fever due to agranulocytosis. No symptoms of
infection were observed in seven patients. One patient had
septicemia caused by E. coli but recovered after antibiotic
therapy. No other hematologic or non-hematologic adverse effects
occurred.
A total of eight patients completed the eight cycles
of rituximab maintenance therapy. Two patients withdrew from the
study due to relapse after receiving four and six courses of
rituximab maintenance. The remaining two patients, in whom the
treatment interval was prolonged due to adverse effects, received
four and seven courses of rituximab maintenance therapy. During
maintenance therapy six months after undergoing auto-SCT, transient
severe neutropenia (which lasted nine months) was observed in one
patient. However, no signs of infection were noted, and the regimen
was maintained. Herpes zoster was observed in one patient five
months after undergoing transplantation; the patient recovered
after receiving appropriate treatment. Repeated urinary tract
infections and fungal vaginitis were observed in another patient
six months after stem cell transplantation. However, the patient
recovered two months after administration of antibacterial and
anti-fungal therapy.
HBV monitoring
HBV-related serologic markers (HBsAg, HBsAb, HBcAb,
HBeAg and HBeAb) were not detected in three patients before
auto-SCT. Four patients were HBsAb-positive, three were HBsAb- and
HBcAb-positive, and two were HBcAb-positive. The five patients who
were HBcAb-positive received oral lamivudine. No changes in
HBV-related markers were observed during follow-up. For the two
patients who were only positive for HBcAb, HBV-DNA was also
monitored and showed normal levels during follow-up.
Monitoring of immunoglobulin levels
Serum levels of immunoglobulins were monitored prior
to stem cell transplantation and every three months during
follow-up. The serum levels of immunoglobulins were normal in all
12 patients before auto- SCT. After transplantation, reduced
immunoglobulin levels were noted in two patients, six and 21 months
after transplantation. Serum IgG and IgA levels were reduced by 50
and 30%, respectively, in the first patient, accompanied by
recurrent urinary tract infections and fungal vaginitis. Rituximab
was withdrawn, and antibiotics and immunoglobulins were
administered intravenously. The symptoms resolved two months later,
and the patient’s serum immunoglobulin levels recovered six months
later. Subsequently, rituximab maintenance was reintroduced with no
accompanying decrease in serum immunoglobulin levels. In the second
patient, serum IgM and IgA levels were reduced by >50% 21 months
after auto-SCT, and monoclonal immunoglobulin was not detected by
immunofixation electrophoresis. Rituximab maintenance therapy was
discontinued, and the patient was followed up for six months. Serum
immunoglobulin levels partially recovered, but were lower than
normal; however, no infection was observed in this patient.
Discussion
The introduction of rituximab has greatly improved
treatment response, overall survival and disease-free survival in
patients with DLBCL (4,5). Nevertheless, tumor relapse is a
frequent occurrence in all patient subsets, including younger
patients with high-risk DLBCL. One approach to the treatment of
relapsed DLBCL is the application of high-dose chemotherapy and
auto-SCT (14,15). Although these therapeutic regimens
are effective at improving response to treatment, long-term and
event-free survival have only partially improved. In this setting,
residual disease and tumor contamination of transfused stem cells
are the main causes for relapse of DLBCL. The presence of minimal
residual disease (MRD) is a known poor prognostic indicator in
DLBCL patients who undergo transplantation (16).
To this end, the eradication of tumor cells has been
a treatment objective in therapeutic regimens involving
transplantation. The use of rituximab for the in vivo
purging of hematopoetic stem cell products has yielded encouraging
results in disease eradication (up to 79% of MRD rendered
undetectable after in vivo purging with rituximab),
resulting in five-year OS and PFS of up to 78% (16–19).
An ongoing prospective study in patients with relapsed follicular
lymphoma showed that the use of rituximab for in vivo graft
purging and maintenance therapy following auto-STC resulted in
undetectable MRD in a high proportion of patients (12). In this study, MRD was present in
56.5% of the patients (13/23) prior to treatment, but became
undetectable in 77% of the patients after auto-SCT and in another
two patients after subsequent rituximab maintenance therapy.
Five-year OS and PFS were 78 and 59%, respectively. Taken together,
these results suggest that the use of rituximab for in vivo
purging in combination with maintenance therapy is a feasible
treatment option for patients with relapsing disease (11).
We hypothesized that rituximab purging and
maintenance therapy in combination with auto-SCT is effective in
DLBCL patients with CR after conventional induction treatment.
Although our study was a single-center, prospective trial with the
limitations of sample size and lack of a comparator group, our
results provide further evidence supporting the use of rituximab in
purging and maintenance therapy regimens.
The three-year and five-year OS rates in this study
were 100%, and the three-year and five-year PFS rates were 83%
after rituximab maintenance therapy. Our results corroborate
previously published findings using similar treatment regimens
incorporating auto-SCT. Two prospective studies (LNH87 and LNH93)
by the Groupe d’Etude des Lymphomes de l’Adulte (GELA) in 137
patients with DLBCL who received first-line transplantation showed
five-year OS and disease-free survival rates of 76 and 68%,
respectively (20). Similar
findings were obtained in an independent study in 112 previously
untreated DLBCL patients who received a combination of rituximab
and high-dose sequential chemotherapy alongside multiple autologous
peripheral blood progenitor cell (PBPC) support (15). A total of 80% achieved CR, and the
projected four-year OS and PFS were 76 and 73%, respectively.
Although rituximab is a well-established component
of induction therapy, concerns have been raised regarding the
potential effects of rituximab-based induction therapy on the
outcomes of re-treatment with rituximab. Further compounding the
issue are conflicting results on the use of rituximab as
maintenance therapy. Some studies demonstrated the benefits of
rituximab in maintenance therapy, but the results are hampered by
the small sample size (21). Other
studies reported no significant differences in OS and PFS between
DLBCL patients who received rituximab purging and maintenance
therapy and those in the control group (22). The results from our study suggest
that rituximab maintenance therapy following auto-STC is beneficial
for DLBCL patients who received rituximab-based induction therapy.
Three months after transplantation, 11 patients (91.7%) achieved CR
and one patient achieved CRu. After two cycles of rituximab
maintenance therapy, CR was achieved in all 12 patients. A total of
10 patients achieved long-term CR and two experienced relapse 14
and 20 months after the end of rituximab maintenance therapy.
The safety findings in the present study did not
differ from those previously reported (12), confirming the safety of rituximab in
maintenance therapy. Treatment was delayed in only two patients due
to rituximab toxicity. One adverse effect of potential concern is
the activation of HBV replication, particularly in countries with a
high incidence of HBV infection (such as China). The long duration
of rituximab maintenance therapy may necessitate an increased
frequency of anti-viral medication. In the present study, the
activation of HBV replication was not observed upon treatment with
rituximab. No patients withdrew from the study, and there were no
delays in the start of therapy. Patients with active HBV
replication were excluded, and lamivudine was administered to those
who were positive for HBV-related antibodies (with the exception of
HBsAb). Nevertheless, the feasibility of rituximab maintenance
therapy in patients with signs of active HBV infection needs to be
confirmed in larger patient cohorts.
Two patients exhibited markedly reduced levels of
serum immunoglobulins. The patients recovered gradually following
discontinuation of therapy, with one of these patients experiencing
recurrent infections. Lim et al (23) and Nishio et al (24) reported that rituximab maintenance
therapy following stem cell transplantation in NHL patients induced
a decrease in serum immunoglobulin levels. Non-fatal infections
were found in approximately 20–30% of patients. In addition, Nishio
et al (24) reported that
the recovery of memory B cells in these patients was delayed,
leading to a reduced expression of CD27, CD80 and CD40, and a
corresponding decrease in the production of plasma cells. Taken
together, these results highlight the importance of monitoring
serum immunoglobulin levels during rituximab maintenance
therapy.
There is currently much variability in the frequency
and duration of rituximab use in maintenance therapy regimens.
Treatment in the PRIMA and SAKK trials consisted of treatment
cycles once every two or three months over two years (25). Other protocols define one treatment
course as weekly rituximab treatment cycles over six consecutive
weeks, and recommend one course of treatment every six months to a
total of four courses. No conclusive evidence is currently
available to support the clinical superiority of any treatment
regimen. The results from our study suggest that a treatment cycle
once every three months is an effective and practical alternative.
Due to the long half-life of rituximab, effective plasma
concentrations can be maintained with this regimen (26). Using this strategy, only eight
treatment cycles are needed, thereby reducing treatment costs. The
higher number of Chinese patients with potential HBV infections
precludes the use of a once-weekly treatment regimen, which may
activate HBV replication and increase the risk of liver
deterioration. Nevertheless, this hypothesis needs to be tested in
future clinical trials.
In conclusion, the results from our study suggest
that OS and PFS in younger patients with high-risk DLBCL improved
through rituximab purging and maintenance therapy combined with
auto-SCT. These findings also confirm the safety and tolerability
of rituximab in a clinical setting.
References
|
1
|
The Non-Hodgkin’s Lymphoma Pathologic
Classification Project. National Cancer Institute sponsored study
of classifications of non-Hodgkin’s lymphomas: summary and
description of a working formulation for clinical usage. Cancer.
49:2112–2135. 1982.
|
|
2
|
The International Non-Hodgkin’s Lymphoma
Prognostic Factors Project. A predictive model for aggressive
non-Hodgkin’s lymphoma. N Engl J Med. 329:987–994. 1993.
|
|
3
|
Tilly H and Zelenetz A: Treatment of
follicular lymphoma: current status. Leuk Lymphoma. 49(Suppl 1):
S7–S17. 2008. View Article : Google Scholar
|
|
4
|
Coiffier B, Lepage E, Briere J, et al:
CHOP chemotherapy plus rituximab compared with CHOP alone in
elderly patients with diffuse large-B-cell lymphoma. N Engl J Med.
346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfreundschuh M, Trumper L, Osterborg A, et
al: CHOP-like chemotherapy plus rituximab versus CHOP-like
chemotherapy alone in young patients with good-prognosis diffuse
large-B-cell lymphoma: a randomised controlled trial by the
MabThera International Trial (MInT) Group. Lancet Oncol. 7:379–391.
2006. View Article : Google Scholar
|
|
6
|
Sehn LH, Donaldson J, Chhanabhai M, et al:
Introduction of combined CHOP plus rituximab therapy dramatically
improved outcome of diffuse large B-cell lymphoma in British
Columbia. J Clin Oncol. 23:5027–5033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thieblemont C and Gisselbrecht C:
Second-line treatment paradigms for diffuse large B-cell lymphomas.
Curr Oncol Rep. 11:386–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cerny J, Trneny M, Slavickova A, et al:
Rituximab based therapy followed by autologous stem cell
transplantation leads to superior outcome and high rates of PCR
negativity in patients with indolent B-cell lymphoproliferative
disorders. Hematology. 14:187–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Philip T, Guglielmi C, Hagenbeek A, et al:
Autologous bone marrow transplantation as compared with salvage
chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s
lymphoma. N Engl J Med. 333:1540–1545. 1995.PubMed/NCBI
|
|
10
|
Flohr T, Hess G, Kolbe K, et al: Rituximab
in vivo purging is safe and effective in combination with
CD34-positive selected autologous stem cell transplantation for
salvage therapy in B-NHL. Bone Marrow Transplant. 29:769–775. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magni M, Di NM, Devizzi L, et al:
Successful in vivo purging of CD34-containing peripheral blood
harvests in mantle cell and indolent lymphoma: evidence for a role
of both chemotherapy and rituximab infusion. Blood. 96:864–869.
2000.PubMed/NCBI
|
|
12
|
Hicks LK, Woods A, Buckstein R, et al:
Rituximab purging and maintenance combined with auto-SCT: long-term
molecular remissions and prolonged hypogammaglobulinemia in
relapsed follicular lymphoma. Bone Marrow Transplant. 43:701–708.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheson BD, Horning SJ, Coiffier B, et al:
Report of an international workshop to standardize response
criteria for non-Hodgkin’s lymphomas. NCI Sponsored International
Working Group. J Clin Oncol. 17:12441999.PubMed/NCBI
|
|
14
|
Haioun C, Lepage E, Gisselbrecht C, et al:
Survival benefit of high-dose therapy in poor-risk aggressive
non-Hodgkin’s lymphoma: final analysis of the prospective LNH87–2
protocol - a Groupe d’Etude des Lymphomes de l’Adulte study. J Clin
Oncol. 18:3025–3030. 2000.
|
|
15
|
Tarella C, Zanni M, Di NM, et al:
Prolonged survival in poor-risk diffuse large B-cell lymphoma
following front-line treatment with rituximab-supplemented,
early-intensified chemotherapy with multiple autologous
hematopoietic stem cell support: a multicenter study by GITIL
(Gruppo Italiano Terapie Innovative nei Linfomi). Leukemia.
21:1802–1811. 2007.
|
|
16
|
Jacquy C, Soree A, Lambert F, et al: A
quantitative study of peripheral blood stem cell contamination in
diffuse large-cell non-Hodgkin’s lymphoma: one-half of patients
significantly mobilize malignant cells. Br J Haematol. 110:631–637.
2000.PubMed/NCBI
|
|
17
|
Goldberg S, Pecora A, Jennis A, et al:
Rituximab permits in vivo purging and collection of tumor-free stem
cells prior to autologous transplantation for B-cell non-Hodgkin’s
lymphoma. Blood. 94:141a1999.
|
|
18
|
Haioun C, Delrau-Larue M, Beaujean F, et
al: Efficiency of in vivo purging with rituximab before autologous
peripheral blood stem cell transplantation (PBSCT) in B-cell
non-Hodgkin’s lymphoma (NHL). Blood. 96:384a2000.PubMed/NCBI
|
|
19
|
Salles G, Moullet I, Charlot C, et al: In
vivo purging with rituximab before autologous peripheral blood
progenitor cell (PBPC) transplantation in lymphoma patients. Blood.
94:141a1999.
|
|
20
|
Mounier N, Gisselbrecht C, Briere J, et
al: All aggressive lymphoma subtypes do not share similar outcome
after front-line autotransplantation: a matched-control analysis by
the Groupe d’Etude des Lymphomes de l’Adulte (GELA). Ann Oncol.
15:1790–1797. 2004.
|
|
21
|
Horwitz SM, Negrin RS, Blume KG, et al:
Rituximab as adjuvant to high-dose therapy and autologous
hematopoietic cell transplantation for aggressive non-Hodgkin
lymphoma. Blood. 103:777–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Habermann TM, Weller EA, Morrison VA, et
al: Rituximab-CHOP versus CHOP alone or with maintenance rituximab
in older patients with diffuse large B-cell lymphoma. J Clin Oncol.
24:3121–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim SH, Zhang Y, Wang Z, et al:
Maintenance rituximab after autologous stem cell transplant for
high-risk B-cell lymphoma induces prolonged and severe
hypogammaglobulinemia. Bone Marrow Transplant. 35:207–208. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishio M, Fujimoto K, Yamamoto S, et al:
Delayed redistribution of CD27, CD40 and CD80 positive B cells and
the impaired in vitro immunoglobulin production in patients with
non-Hodgkin lymphoma after rituximab treatment as an adjuvant to
autologous stem cell transplantation. Br J Haematol. 137:349–354.
2007. View Article : Google Scholar
|
|
25
|
Ghielmini M, Schmitz SF, Cogliatti S, et
al: Effect of single-agent rituximab given at the standard schedule
or as prolonged treatment in patients with mantle cell lymphoma: a
study of the Swiss Group for Clinical Cancer Research (SAKK). J
Clin Oncol. 23:705–711. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cartron G, Blasco H, Paintaud G, Watier H
and Le GC: Pharmacokinetics of rituximab and its clinical use:
thought for the best use? Crit Rev Oncol Hematol. 62:43–52. 2007.
View Article : Google Scholar : PubMed/NCBI
|