Introduction
Fullerene, the third allotrope of carbon, was
synthesized in 1985 (1). Due to its
unique geometric structure and chemical properties, fullerene
derivatives have attracted interest in physics, chemical,
biological and medical applications (2). The endohedral metallofullerenes
recently attracted attention due to their special biomedical effect
(3,4). Gadolinium fullerene compounds,
Gd@C82, composed of a Gd3+ ion trapped in an
82-carbon fullerene cage, were initially used as a contrast agent
in magnetic resonance imaging (MRI) for intracellular labeling and
T1 enhancement (5). Based on this
structure, Gd@C82(OH)x was studied as a new generation
of high-efficiency MRI contrast agents (6,7).
During an investigation using mouse models to
compare the anti-tumor efficacy of metallofullerenol nanoparticles
with the anti-tumor effects of anti-neoplastic agents CTX
(cyclophosphamide) and CDDP (cisplatin),
Gd@C82(OH)22 (22-nm nanoparticles through
large molecular interactions in aqueous solutions) was identified
and showed a strong anti-tumor activity (4). This high anti-tumor efficacy was not
due to direct toxic effects on tumor cells, and the content of this
nanoparticle was less than 0.05% of the exposed dose (4). It was therefore suggested that
[Gd@C82(OH)22]n plays a role by
inhibiting the migration and/or adhesion ability of glioblastoma
cells.
The present study examined the hypothesis that
[Gd@C82(OH)22]n nanoparticles
directly participate in glioblastoma cell migration and adhesion.
Cells treated with [Gd@C82(OH)22]n
nanoparticles exhibited significant impairment in a series of
migration and adhesion assays in vitro. Furthermore, our
data showed that the key proteins, CD40 and ICAM-1, were involved
in the [Gd@C82(OH)22]n
nanoparticle-mediated adhesion of glioblastoma cells. Results
indicated that [Gd@C82(OH)22]n
inhibited cancer cell adhesion through the down-regulation of CD40
and ICAM-1. Thus, [Gd@C82(OH)22]n
nanoparticle may be a new potential anti-tumor effector and
therapeutic component for malignant glioblastoma infiltration.
Materials and methods
Cell culture and reagents
Cells from the human glioblastoma cell line LN-229
were obtained from the American Type Culture Collection (Manassas,
VA, USA) and cultured in RPMI-1640 with 10% fetal bovine serum
(complete medium). The chemotaxis chambers and membranes were
acquired from Neuroprobe (Gaithersburg, MD, USA). The recombinant
human epithelial growth factor (EGF) was obtained from R&D
Systems (Minneapolis, MN, USA). Fibronectin (0.1%) and MTT were
from Sigma (St. Louis, MO, USA). The antibodies against CD40,
ICAM-1 and β-actin were acquired from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA).
MTT cell proliferation assay
Cells (1×103/well) were plated in 96-well
plates in 100 μl of complete medium. Following overnight
attachment, the cells were exposed to different concentrations of
[Gd@C82(OH)22]n nanoparticles (10,
50 and 100 μg/ml) for 3 days. To perform the assay, 10 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution was added to each well. After 4 h of incubation at 37°C,
followed by the addition of 150 μl dimethyl sulfoxide to each well
and vibration for 10 min, absorbance was measured at 490 nm in a
microplate reader. Triplicate wells with pre-determined cell
numbers were subjected to the above assay concomitantly with the
test samples to normalize the absorbance readings. The experiments
were conducted in triplicate and repeated at least three times
independently.
Western blotting
Western blotting was performed as described by Zhang
et al (8). Briefly, the
LN-229 cells were serum-starved for 2 h and then treated with
[Gd@C82(OH)22]n nanoparticles for
24 h. The treated and control cells were washed twice with ice-cold
phosphate-buffered saline (PBS), and subsequently activated by 10
ng/ml EGF for 0, 5 or 15 min. The reactions were stopped by
ice-cold PBS (pH 6.8). The cells were lysed with 1X SDS lysis
buffer containing Tris-HCl (pH 6.8), 62.5 mM, 2% SDS and 10%
glycerol for 20 min on ice. Samples were boiled for 10 min,
followed by centrifugation at 10,000 rpm for 10 min, and
supernatants were isolated. Equal amounts of protein were separated
by sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and electrotransferred onto nitrocellulose membranes
(Immobilon-P, Millipore, Billerica, MA, USA). The blots were probed
with the appropriate dilutions of primary antibody overnight at 4°C
(CD40, 1:50; ICAM-1, 1:50) and incubated with the proper dilution
of alkaline phosphatase-conjugated secondary antibodies.
Chemotaxis assay
Chemotaxis assay was performed as previously
described (8). The cells were
treated for 2 h in the serum-free medium and then with
[Gd@C82(OH)22]n nanoparticles for
24 h. The cells (8×105 cells/ml) suspended in binding
medium (RPMI-1640, 0.1% BSA and 25 mM HEPES) were added to the
upper chambers. The EGF (10 ng/ml) was loaded into the lower
chemotaxis chamber. The polycarbonate filter (8-μm pore size;
Neuroprobe, Cabin John, MD, USA) was pre-treated with 10 μg/ml
fibronectin overnight, air-dried and inserted between the upper and
lower chambers. The lower chamber was then incubated at 37°C in 5%
CO2 for 3.5 h. The filter membrane was rinsed, fixed and
stained. The number of migrating cells were counted at a
magnification of ×400 in three separate fields by light microscopy.
The chemotaxis index was determined as the ratio of the number of
migrating cells in the nanoparticle gradient to the number of
migrating cells in the control medium. For the chemokinesis assay
(checkerboard assay), the control and treated cells were suspended
in medium containing different concentrations of EGF before being
loaded to the upper chambers. Different concentrations of EGF were
added to the lower chambers. All of the samples were tested in
triplicate, and data are expressed as the mean ± SD. Statistical
analysis was carried out to determine the significance of the
chemotactic response by using PRISM 3, a two-way analysis of
variance analysis.
Scratch assay
The LN-229 cells were plated in 35-mm dishes
overnight and starved for 2 h, and subsequently treated with
different concentrations (1, 10, 50 and 100 μg/ml) of
[Gd@C82(OH)22]n nanoparticles for
24 h. A scratch was then made with an even trace in the middle of
the cells using a 10-μl pipette tip (8). The cells were incubated at 37°C in 5%
CO2 for an appropriate time period, and the distance of
the wound was measured under a light microscope. The samples were
tested in triplicate, and the data are expressed as the mean ±
SD.
Adhesion assay
The adhesion assay was carried out as previously
described (8). The cells were
treated for 2 h in the serum-free medium and then with
[Gd@C82(OH)22]n nanoparticles for
24 h. The control or treated cells (2.7×105/ml) were
suspended in complete medium. Then, 1.5 ml cells in the presence of
10 ng/ml EGF were immediately added to a 35-mm dish containing
glass coverslips. The coverslips were pre-treated with 10 μg/ml of
fibronectin in serum-free medium for 2 h at 37°C and then air-dried
for 30 min at room temperature. After 5, 15 and 30 min of
incubation, the cells were gently washed twice with ice-cold PBS
and fixed. The cells attached to the coverslips were counted under
a light microscope at a magnification of ×200.
Statistical analysis
Statistical analysis was carried out using PRISM 3
from GraphPad Software (San Diego, CA, USA). Data are presented as
mean ± SD. Statistical significance for comparisons between the
groups was determined using Student’s paired two-tailed t-test. The
results were generated from three independent experiments.
Results
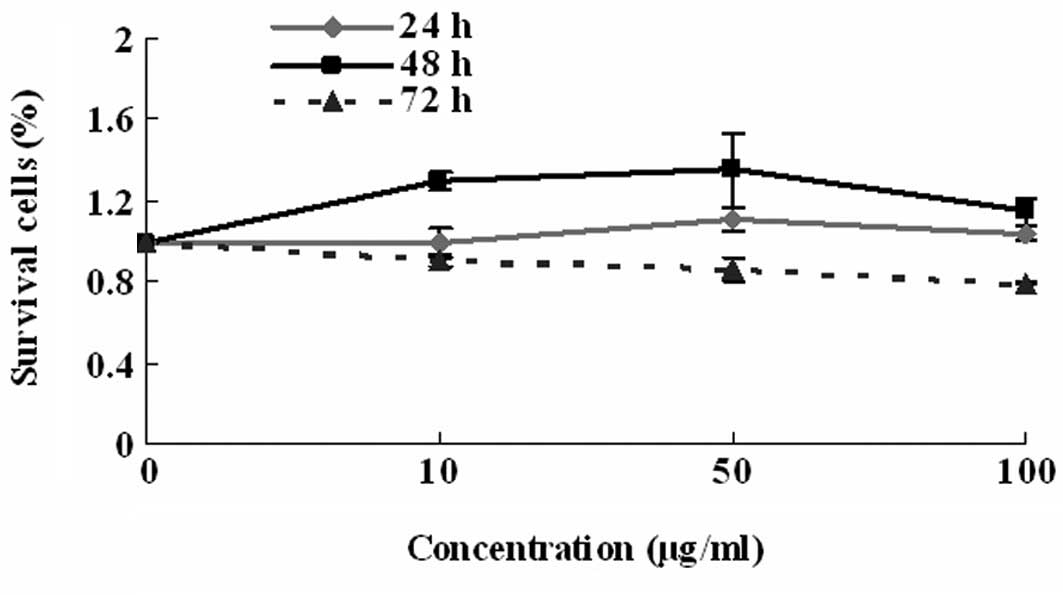
The MTT assay was initially applied to confirm that
the [Gd@C82(OH)22]n nanoparticles
had no toxicity towards the LN-229 glioblastoma cells. A total of 3
doses (10, 50 and 100 μg/ml) of
[Gd@C82(OH)22]n nanoparticles were
used in this assay. The
[Gd@C82(OH)22]n nanoparticles did
not show any cellular toxicity at 24 h (Fig. 1), which was consistent with a
previous report by Chen et al (4).
Cell migration is a highly dynamic phenomenon that
is essential to biological morphogenesis, wound healing, cancer
metastasis and immune response (9–12). In
order to examine the manner in which
[Gd@C82(OH)22]n nanoparticles
affect cell migration, we performed cell chemotaxis and scratch
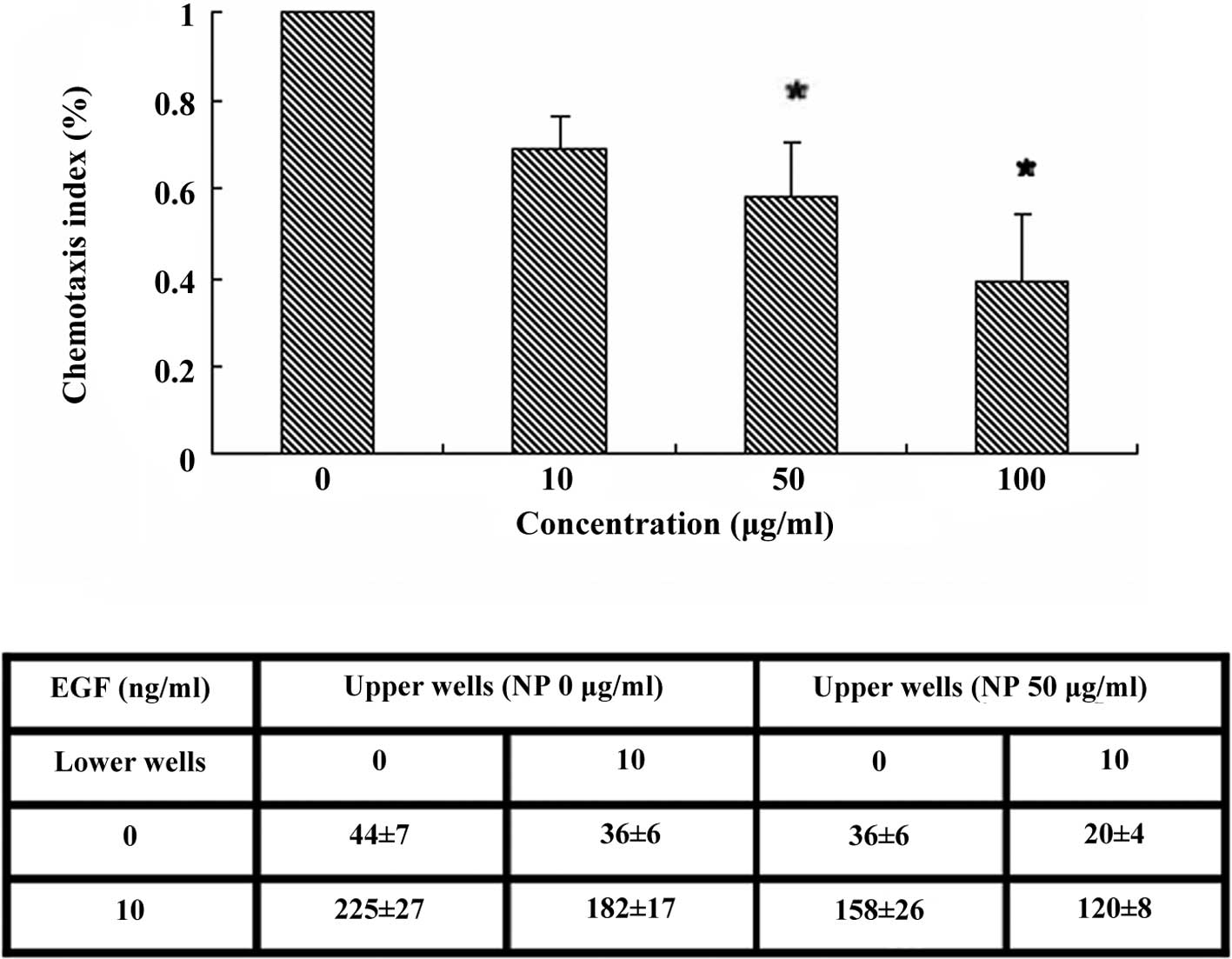
assays. A 48-well EGF-induced chemotaxis model was applied to
perform 3 h cell chemotaxis. The cells treated with 50 and 100
μg/ml of [Gd@C82(OH)22]n
nanoparticles showed decreased chemotactic ability compared to the
control. The low dose of 10 μg/ml did not show a significant
inhibition effect (Fig. 2A). The 50
μg/ml dose of [Gd@C82(OH)22]n
nanoparticles was selected for the experiments mentioned below.
Apart from the chemotaxis assay, we also carried out
a chemokinesis assay that presents random cell motility stimulated
in a gradient-independent manner (8). Chemokinesis was also impaired in the
cells treated with 50 μg/ml of
[Gd@C82(OH)22]n nanoparticles,
which may have accounted for the decrease in chemotaxis (Fig. 2B). The change in cell proliferation,
however, did not interfere with the chemotaxis of LN-229 cells, as
it only took <3 h to complete the chemotaxis assay in this
study, shorter than the doubling time.
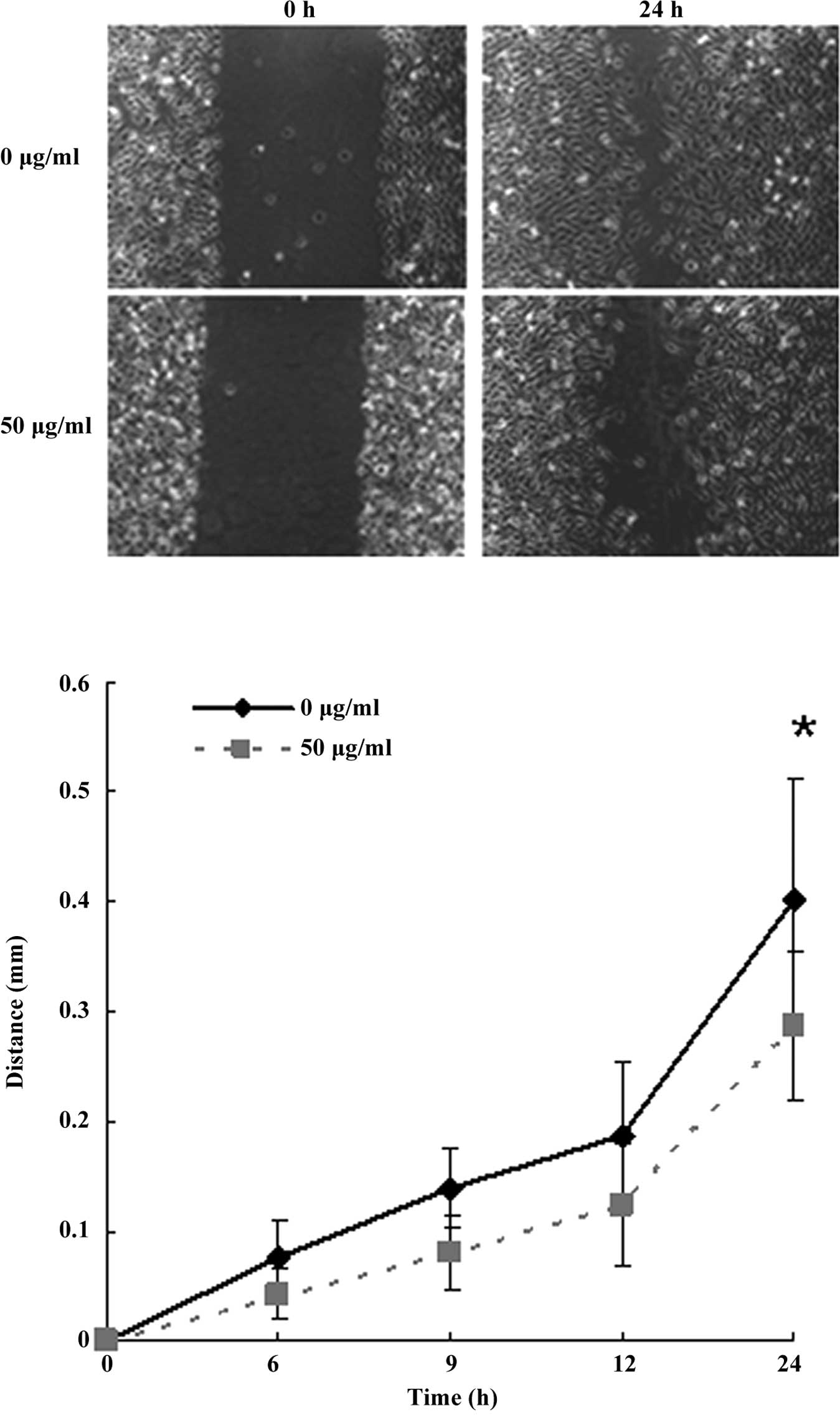
A scratch assay was used as an in vitro assay
for wound healing and directional movement (13). When a scratch was created in the
fluent monolayer cells, the
[Gd@C82(OH)22]n
nanoparticle-treated (50 μg/ml) LN-229 cells required a longer time
to fill the gap, further supporting a defect in the migration
ability (Fig. 3A and B).
The process of cell movement can be divided into
three stages. The first includes the reorganization of the actin
work at its leading edge. The second involves adherence to the
substrate at the leading edge and the consequent deadherence. The
third stage involves the contractile force required by the action
of the acto-myosin network to pull the cell forward (14). Therefore, adhesion is critical for
cell movement. This study investigated the manner in which
[Gd@C82(OH)22]n nanoparticles
affect cell adhesion in LN-229 cells. Following treatment with
[Gd@C82(OH)22]n nanoparticles for
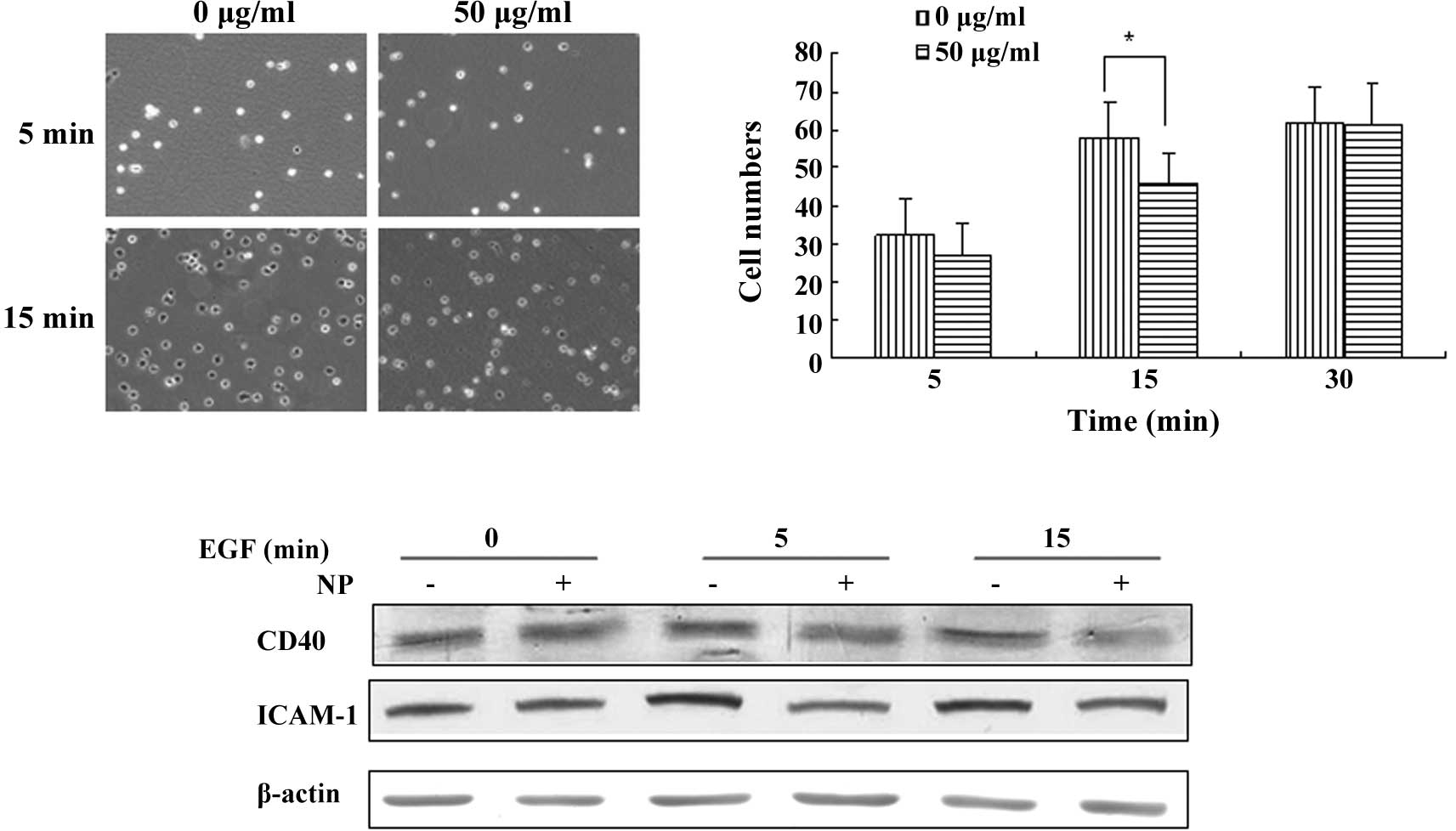
24 h, the adhesion ability of control and treated LN-229 cells were
compared. At 5 min of adhesion assay, the adhesion ability of
LN-229 cells showed a similar level in the absence or presence of a
50 μg/ml treatment of
[Gd@C82(OH)22]n. At 15 min,
nanoparticle treatment inhibited the adhesion ability of LN-229
cells (Fig. 4A and B).
CD40, a TNF-R-related cell surface receptor, was
reported to be expressed in glioma cells in vitro and in
vivo (15). CD40 up-regulates
the expression of ICAM-1 and plays a role in cell adhesion
(16,17). The protein level of CD40 and ICAM-1
was detected upon [Gd@C82(OH)22]n
treatment. We found that LN-229 cells treated with 50 μg/ml of
[Gd@C82(OH)22]n nanoparticles
exhibited a decreased expression level of CD40 and ICAM-1
responding to a 5- and 15-min EGF stimulation, which was consistent
with the decreased adhesion ability.
Discussion
The rapid development of nanotechnology and the
discovery of its desirable applications has allowed for a wide
variety of nanoparticles to provide a broad range of opportunities
in multidisciplinary fields, such as medicine, therapeutics,
clinical diagnosis (18), disease
treatment and physiological and immunological mechanisms (19). By using novel nanoparticles to
identify and treat cancer, nano-medicine has the potential to
specifically treat cancer cells while leaving healthy cells intact
in the human body (20).
Manufactured nanoparticles, such as carbon black and fullerene (the
third allotrope of carbon), are widely used due to their desirable
properties in the medical field (5). Our previous studies demonstrated that
Gadolinium fullerene compounds, Gd@C82, composed of a
Gd3+ ion trapped in an 82-carbon fullerene cage, are
able to effectively inhibit tumor growth without detectable
cytotoxicity in vitro and toxic side effects in vivo
(2,4,5,21).
Based on this function, we hypothesized that Gd@C82(OH)x
influences the migration and adhesion of tumor cells.
Directed cell migration and polarity are crucial in
various biological processes, including wound healing and immune
responses, as well as in pathological phenomena, such as tumor
invasion and metastasis (9–12). In the present study, the chemotaxis
and scratch assays showed that the
[Gd@C82(OH)22]n
nanoparticle-treated LN-229 cells exhibited a significantly
decreased migration ability. However, what role the nanoparticle
plays in migration remains to be elucidated, and further studies
are necessary.
ICAM-1 is one of the critical adhesion proteins of
cancer cells and is present constitutively on the cell surface
(16,22). The investigation of CD40 and ICAM-1
interaction showed that CD40 activation up-regulates ICAM-1
expression with the concomitant enhanced adhesion of tumor cells,
and this process is mediated via the p38 MAPK signal transduction
pathway (16). In the present
study, glioblastoma cells treated with
[Gd@C82(OH)22]n nanoparticles
exhibited CD40 and ICAM-1 down-regulation followed by decreased
adhesive ability. This suggests that the
[Gd@C82(OH)22]n nanoparticle
function on cell adhesion is mediated by CD40/ICAM-1 signaling.
We found that the
[Gd@C82(OH)22]n nanoparticle is a
type of nano-material that exerts an anti-tumor effect. Our results
suggest that [Gd@C82(OH)22]n
nanoparticles, due to their unique physical size and surface
chemical properties, can target multiple molecular processes
simultaneously and, therefore, are promising for the development of
more effective tumor chemotherapy.
Acknowledgements
This study was supported by the China 973 Program
(2006CB705600 and 2010CB933900), the 863 Program (2007AA021802),
the National Scientific Foundation of China (30700253 and
30800355), and the Tianjin Commission of Science and Technology
(2008ZCKFSH04800).
References
|
1
|
Kroto HW, Heath JR, O’Brien SC, Curl RF
and Smalley RE: C60: Buckminsterfullerene. Nature. 318:162–163.
1985. View
Article : Google Scholar
|
|
2
|
Wang J, Chen C, Li B, et al: Antioxidative
function and biodistribution of
[Gd@C82(OH)22]n nanoparticles in
tumor-bearing mice. Biochem Pharmacol. 71:872–881. 2006.
|
|
3
|
Cagle DW, Kennel SJ, Mirzadeh S, Alford JM
and Wilson LJ: In vivo studies of fullerene-based materials using
endohedral metallofullerene radiotracers. Proc Natl Acad Sci USA.
96:5182–5187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen C, Xing G, Wang J, et al:
Multihydroxylated [Gd@C82(OH)22]n
nanoparticles: antineoplastic activity of high efficiency and low
toxicity. Nano Lett. 5:2050–2057. 2005.
|
|
5
|
Anderson SA, Lee KK and Frank JA:
Gadolinium-fullerenol as a paramagnetic contrast agent for cellular
imaging. Invest Radiol. 41:332–338. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mikawa M, Kato H, Okumura M, et al:
Paramagnetic water-soluble metallofullerenes having the highest
relaxivity for MRI contrast agents. Bioconjug Chem. 12:510–514.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bolskar RD, Benedetto AF, Husebo LO, et
al: First soluble M@C60 derivatives provide enhanced access to
metallofullerenes and permit in vivo evaluation of
Gd@C60[C(COOH)2]10 as a MRI contrast agent. J
Am Chem Soc. 125:5471–5478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Gu F, She C, et al: Reduction of
Akt2 inhibits migration and invasion of glioma cells. Int J Cancer.
125:585–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo H, Gu F, Li W, et al: Reduction of
protein kinase c zeta inhibits migration and invasion of human
glioblastoma cells. J Neurochem. 109:203–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang J, Chen K, Gong W, Dunlop NM and
Wang JM: G-protein coupled chemoattractant receptors and cancer.
Front Biosci. 13:3352–3363. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurosaka S and Kashina A: Cell biology of
embryonic migration. Birth Defects Res C Embryo Today. 84:102–122.
2008. View Article : Google Scholar
|
|
12
|
Rorth P: Collective cell migration. Annu
Rev Cell Dev Biol. 25:407–429. 2009. View Article : Google Scholar
|
|
13
|
Etienne-Manneville S and Hall A:
Integrin-mediated activation of Cdc42 controls cell polarity in
migrating astrocytes through PKCzeta. Cell. 106:489–498. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lefranc F, Brotchi J and Kiss R: Possible
future issues in the treatment of glioblastomas: special emphasis
on cell migration and the resistance of migrating glioblastoma
cells to apoptosis. J Clin Oncol. 23:2411–2422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wischhusen J, Schneider D, Mittelbronn M,
et al: Death receptor-mediated apoptosis in human malignant glioma
cells: modulation by the CD40/CD40L system. J Neuroimmunol.
162:28–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H and Nord EP: CD40/CD154 ligation
induces mononuclear cell adhesion to human renal proximal tubule
cells via increased ICAM-1 expression. Am J Physiol Renal Physiol.
289:F145–F153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sumimoto S and Mayumi M: [Role of
LFA-1/ICAM-1-dependent cell adhesion in CD40-mediated inhibition of
anti-IgM antibody-induced B-cell death]. Nippon Rinsho.
54:1779–1783. 1996.
|
|
18
|
Jain KK: Nanotechnology in clinical
laboratory diagnostics. Clin Chim Acta. 358:37–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juillerat-Jeanneret L and Schmitt F:
Chemical modification of therapeutic drugs or drug vector systems
to achieve targeted therapy: looking for the grail. Med Res Rev.
27:574–590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
LaRocque J, Bharali DJ and Mousa SA:
Cancer detection and treatment: the role of nanomedicines. Mol
Biotechnol. 42:358–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin JJ, Lao F, Meng J, et al: Inhibition
of tumor growth by endohedral metallofullerenol nanoparticles
optimized as reactive oxygen species scavenger. Mol Pharmacol.
74:1132–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roebuck KA and Finnegan A: Regulation of
intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc
Biol. 66:876–888. 1999.PubMed/NCBI
|