Introduction
Childhood acute lymphoblastic leukemia (ALL) is the
most common malignancy in humans aged 18 years or younger. In a
number of these children the outcome of treatment is difficult to
predict and is considered to be an individual response of the
patient to chemotherapy. It is likely that the observed clinical
heterogeneity reflects a diverse pathogenesis of leukemia. The
molecular basis of childhood ALL is to a large extent unknown, and
it is likely that significant advances in the treatment of this
malignancy are dependent on a better understanding of the molecular
events that cause the disease (1,2). A
number of investigators have attempted to identify groups of genes,
termed ‘transcriptional signatures’ whose expression can be
directly associated with drug resistance (3).
The immunophenotypic characteristics of precursor
B-cell acute lymphoblastic leukemic cells (clonal expansion of
progenitors of B-cell lymphocytes) are believed to reflect normal
hematopoietic B-cell precursors. However, previous studies showed
that through the simultaneous acquisition of various antigens,
almost all B-precursor-ALL cases display phenotypic aberrations
(4–8). The latter may be associated with
specific genetic abnormalities, and it has been suggested that they
are useful for a better understanding of protein expression
dysregulation (9). Approximately
84% of ALL cases are B-precursor ALL, 14% are T-cell ALL and 2% are
B-cell (B-)ALL (10). At least 32%
of the ALL cases show clonal chromosomal abnormalities (11). The so-called Philadelphia (Ph)
chromosome t(9;22) is present in 4% of pediatric ALL patients and
confers an unfavorable prognosis, particularly when associated with
either a high whole blood cell count (WBC) or slow early response
to initial therapy (12–14).
This study reported a childhood B-ALL case with
unique complex aberrations and six chromosomal breakpoints. In this
case, the array-proven high-resolution multicolor banding (aMCB)
technique was of enormous significance for detecting the genetic
changes.
Materials and methods
Case report
In May 2009, a 14-year-old male patient presented
with a WBC of 123.6×109/l, i.e., 6.1% neutrophils, 65%
lymphocytes, 0.1% eosinophiles, 1.2% monocytes, 5.6% basophiles and
27.6% largely unidentified cells. The platelet count was
205×109/l and hemoglobin 8.7 g/dl. Serum lactate
dehydrogenase (LDH) was 556 U/l (normal: up to 480 U/l), and the
level of serum alkaline phosphates was 191 U/l (normal: up to 141
U/l). A physical examination showed no splenomegaly, but loss of
weight was noted. No response was observed after the application of
two standard protocols for ALL and acute myeloid leukemia (AML).
For 2 months the patient was treated with imatinib (400 mg per
day). However, one month later the patient succumbed to the disease
while undergoing treatment. Cytogenetics and molecular
cytogenetics. Banding cytogenetics using GTG-banding was performed
according to standard procedures (15), and 20 metaphases derived from the
unstimulated bone marrow of the patient were analyzed. Patient
consent for the study was obtained.
Fluorescence in situ hybridization (FISH)
using commercially available probes for BCR/ABL and subtelomeric
for 10p/10q (Abbott/Vysis) were applied according to the
manufacturer’s instructions. High-resolution aMCB, based on
microdissection-derived region-specific libraries for chromosomes
1, 4, 8 and 10, was performed as previously described; method and
MCB probe sets are specified (15,16). A
total of 30 metaphase spreads were analyzed, each using a
fluorescence microscope (AxioImager.Z1 mot, Zeiss) equipped with
appropriate filter sets to discriminate between a maximum of five
fluorochromes and the counterstain 4′,6-diamino-2-phenylindole
(DAPI). Image capturing and processing were carried out using an
Isis mFISH imaging system (MetaSystems, Altlussheim, Germany) for
the evaluation of MCB.
Immunophenotyping
Immunophenotyping of leukemic blasts was carried out
using fluorescein isothiocyanate (FITC)- and phycoerythrin
(PE)-conjugated monoclonal antibodies (Becton-Dickinson, Franklin
Lakes, NJ, USA). Antibodies against the following antigens were
used: CD34, CD45, HLA-Dr, CD117, CD10, CD4, CD7, CD8, CD19, CD38,
as well as cCD13, cMPO, cTdt, cCD2, cCD3, cCD22 and cCD79a
(17). Positivity (<20%), was
considered to be a negative result.
Results
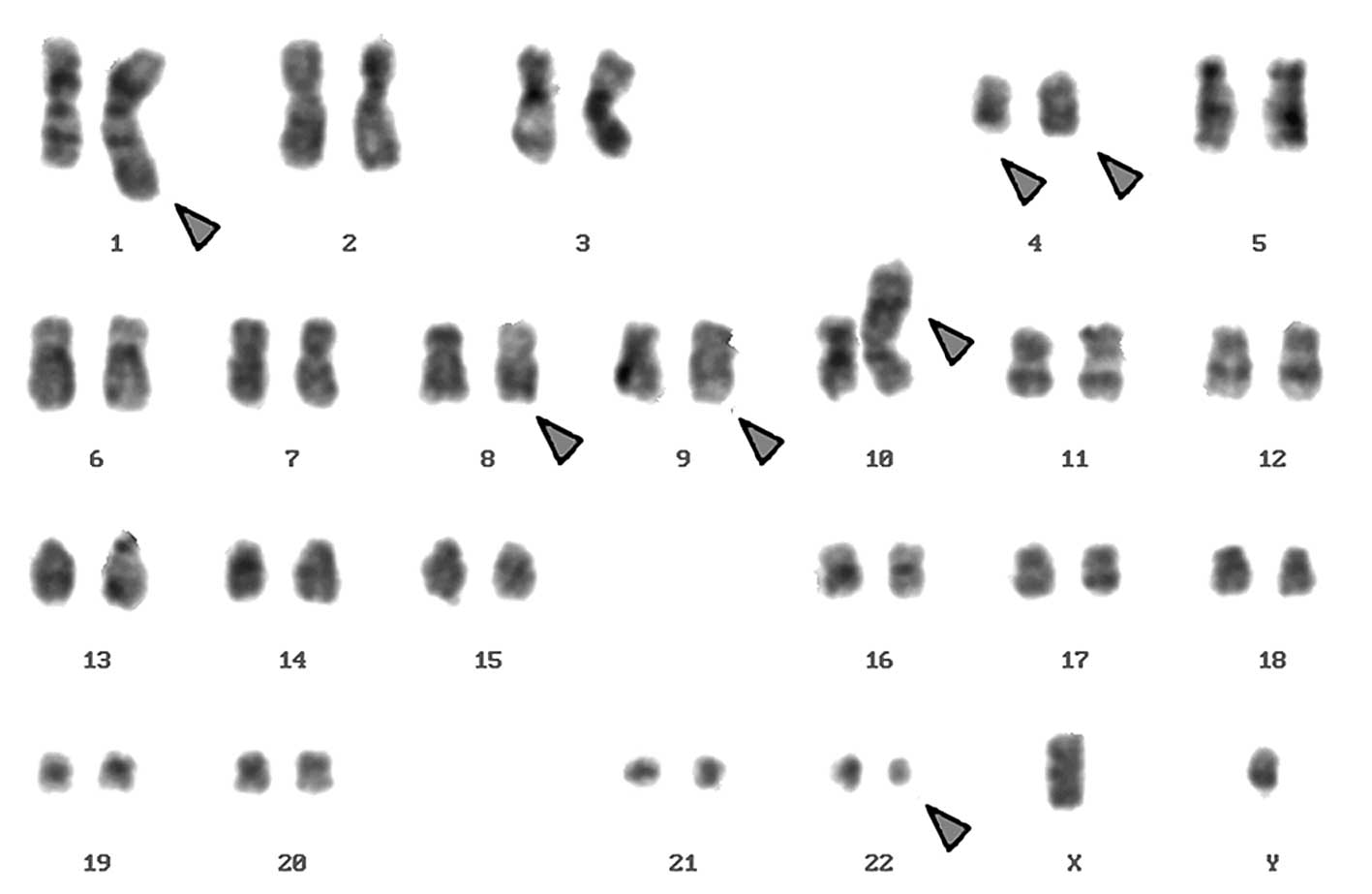
Karyotyping was carried out after the initiation of
chemotherapy treatment, showing certain karyotypic changes. A
complex karyotype 46,XY/46,XY,der(1),del(4),der(4),t(9;22), der(10) was determined in the GTG-banding
(Fig. 1) and was further studied by
molecular cytogenetics (Figs.
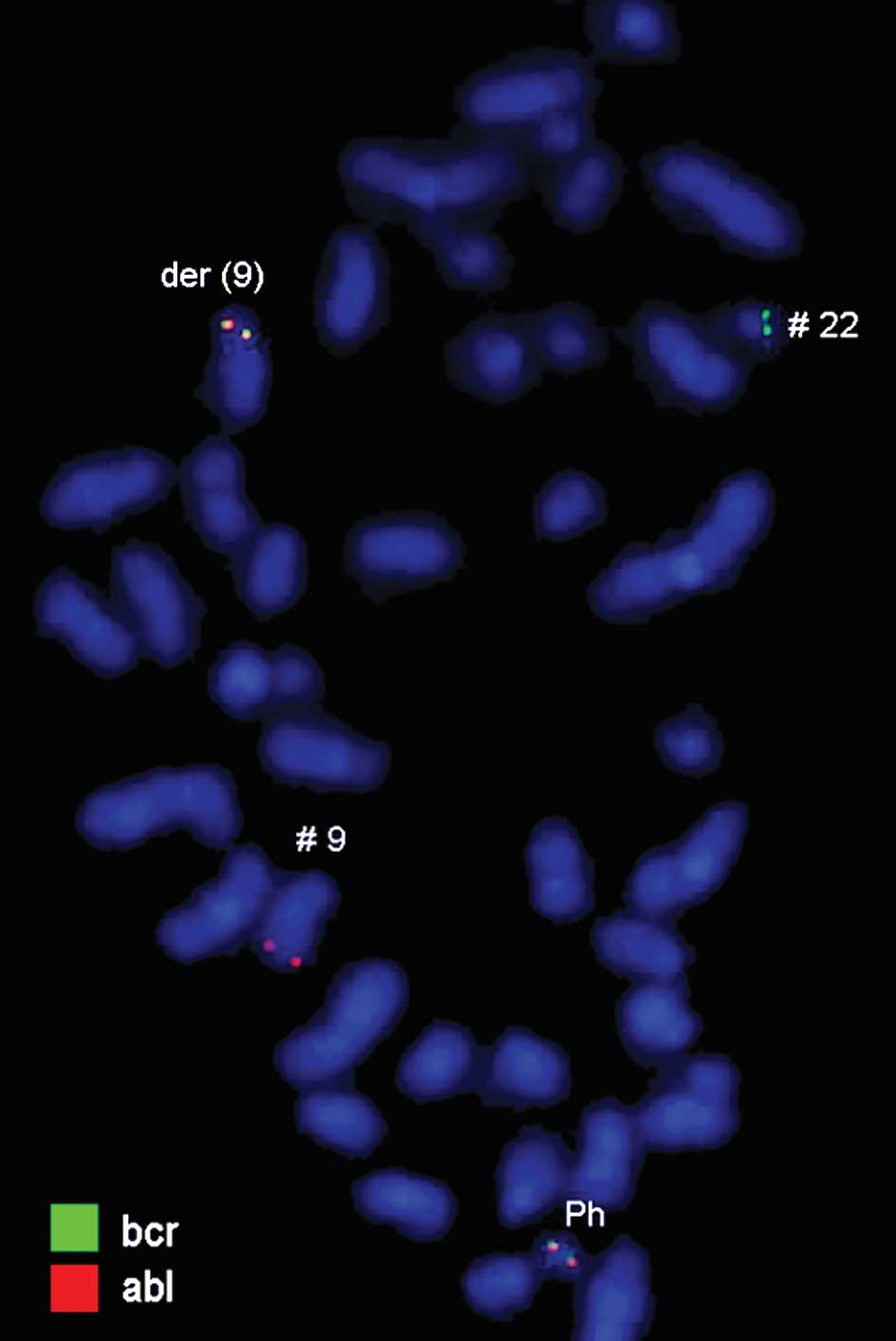
2–4). Dual-color-FISH using a
commercially available probe specific for BCR and ABL showed that
the typical Ph chromosome with BCR/ABL translocation was present.
The application of subtelomeric probes for 10p and 10q showed
normal signals of subtelomeric 10p/10q on chromosome 10. However,
only subtelomeric region 10q was present on the derivative
chromosome 10, while subtelomeric region 10p was detected on the
derivative chromosome 1 (Fig. 2).
Thus, aMCB, using probes for the corresponding chromosomes involved
according to GTG-banding, was performed (16). The result obtained was:
46,XY[4]/46,XY,der(1)t(1;4;10)(1pter->1q42::4q21->4q35::10p15.3-10pter),del(4)(q24),t(9;22)(q34;q11.2),der(10)t(8;4;10)(8qter->8q22::4q21->4q35::10p15.3->10qter)
(16).
Immunophenotyping of leukemic blasts was performed
using FITC- and PE-conjugated monoclonal antibodies. The blasts
stained positively with CD10 (88%), CD19 (86%), CD79a (80%), CD34
(86%), CD45 (95%) and HLA-DR (49%). Based on these findings and
using the criteria of the European Group for the Immunological
Characterization of Acute Leukemia (EGIC), the patient was
diagnosed as having common precursor B-cell acute lymphoblastic
leukemia.
Discussion
According to the literature, 32% of ALL cases
demonstrate an abnormal karyotype, either in chromosome number
(ploidy) or in structural changes such as translocations,
inversions, or deletions (11–12).
Not infrequently, chromosomes studied in ALL exhibit a poor
morphology, tend not to spread well, and appear fuzzy with
indistinct margins, making banding studies challenging or even
impossible (12). Nonetheless, it
is known that in ALL the most common chromosomal changes are
t(12;21), t(9;22), t(4;11) and del(6q) followed by t(8;14), t(1;19)
and del(9p) (18). Among the
specific chromosome translocations identified and causally linked
to leukemogenesis, BCR/ABL gene rearrangements are one of the best
characterized rearrangements (19,20).
It was shown that among B-precursor-ALL patients, this
translocation is present in approximately 20–30% of adult cases
while it is rarely detected in children (21–27),
as found in the present patient. In this case, we described unique
complex translocations associated with B-ALL, including the
involvement of two chromosomes 4 in complex translocations, in
addition to the t(9;22)(q34;q11.2). The lymphatic marker CD10 was
also expressed. The patient did not respond to the standard
chemotherapy protocols suggested by large prospective studies on
childhood ALL (30,31), and the cytogenetic investigation
after six months of chemotherapy treatment showed the
afore-mentioned chromosomal abnormalities.
B-lineage, defined by the expression of CD19,
HLA-DR, CD10 (cALLa) and other B-cell-associated antigens are
observed in 80–85% of childhood ALL. Approximately 80% of
B-precursor ALL cases express the cALLa, CD10 antigen (18). Thus, the observed immunophenotype of
the reported case was appropriate to this group and aided in the
identification of the type of malignancy present.
One study reported that the pattern of expression of
a set of genes can determine resistance to common chemotherapeutic
agents (3). This study identified
45 genes differentially expressed between resistant and sensitive
ALL samples whose expression pattern was significantly related to
treatment response (28). The 45
genes were involved in the regulation of transcription, cellular
transport and cell cycle maintenance. Using patterns of gene
expression, the authors were able to distinguish a subgroup of ALL
tumors with cross-chemoresistance and unfavorable outcome from
those which exhibited only single-drug resistance. The same study
also showed that transcriptional regulation of key apoptosis genes
can be linked to cellular drug resistance and prognosis in
pediatric B-lineage ALL (29). The
present case may reflect the same manner of drug-resistance due to
the large number of chromosome breaks and aberrations involved.
Therefore, the observed complex karyotype, along
with the Ph chromosome is a poor prognostic factor in B-ALL
patients, since no response was observed after the application of
two standard treatment protocols for ALL and AML.
Acknowledgements
We would like to thank Professor Ibrahim Othman, the
Director General of the Atomic Energy Commission of Syria (AECS)
and Dr Nizar MirAli, Head of the Molecular Biology and
Biotechnology Department, for their support. This study was
partially supported by the AECS, by the Stefan-Morsch-Stiftung,
Monika-Kutzner-Stiftung and the DAAD (D/07/09624).
References
|
1
|
Endo C, Oda M, Nishiuchi R and Seino Y:
Persistence of TEL-AML1 transcript in acute lymphoblastic leukemia
in long-term remission. Pediatr Int. 45:275–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McLean TW, Ringold S, Neuberg D, Stegmaier
K, Tantravahi R, Ritz J, Koeffler HP, Takeuchi S, Janssen JW, Seriu
T, Bartram CR, Sallan SE, Gilliland DG and Golub TR: TEL-AML1
dimerizes and is associated with a favorable outcome in childhood
acute lymphoblastic leukemia. Blood. 88:4252–4258. 1996.PubMed/NCBI
|
|
3
|
Holleman A, Cheok MH, den Boer ML, Yang W,
Veerman AJ, Kazemier KM, Pei D, Cheng C, Pui CH, Relling MV,
Janka-Schaub GE, Pieters R and Evans WE: Gene-expression patterns
in drug-resistant acute lymphoblastic leukemia cells and response
to treatment. N Engl J Med. 351:533–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ong ST and Larson RA: Current management
of acute lymphoblastic leukemia in adults. Oncology. 9:433–442.
1995.PubMed/NCBI
|
|
5
|
Campana D and Pui CH: Detection of minimal
residual disease in acute leukemia: methodologic advanced and
clinical significance. Blood. 85:1416–1434. 1995.PubMed/NCBI
|
|
6
|
Ciudad J, San Miguel JF, López-Berges MC,
Vidriales B, Valverde B, Ocqueteau M, Mateos G, Caballero MD,
Hernández J, Moro MJ, Mateos MV and Orfao A: Prognostic value of
immunophenotypic detection of minimal residual disease in acute
lymphoblastic leukemia. J Clin Oncol. 16:3774–3781. 1998.PubMed/NCBI
|
|
7
|
Orfao A, Schmitz G, Brando B,
Ruiz-Arguelles A, Basso G, Braylan R, Rothe G, Lacombe F, Lanza F,
Papa S, Lucio P and San Miguel JF: Clinically useful information
provided by the flow cytometric immunophenotyping of hematological
malignancies: current status and future directions. Clin Chem.
45:1708–1717. 1999.
|
|
8
|
Orfao A, Ciudad J, Almeida J and San
Miguel JF: Residual disease detection of leukemia.
Immunophenotyping. Stewart CC and Nicholson JKA: Wiley-Liss; New
York: pp. 239–259. 2000
|
|
9
|
Tabernero MD, Bortoluci AM, Alaejos I,
López-Berges MC, Rasillo A, García-Sanz R, García M, Sayagués JM,
González M, Mateo G, San Miguel JF and Orfao A: Adult precursor
B-ALL with BCR/ABL gene rearrangements displays a unique
immunophenotype based on the pattern of CD10, CD34, CD13 and CD38
expression. Leukemia. 15:406–414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Camitta BM, Pullen J and Murphy S: Biology
and treatment of acute lymphocytic leukemia in children. Oncology.
24:83–91. 1997.PubMed/NCBI
|
|
11
|
Martinez-Climent JA: Molecular
cytogenetics of childhood hematological malignancies. Leukemia.
11:1999–2021. 1997. View Article : Google Scholar
|
|
12
|
Hann I, Vora A, Harrison G, Harrison C,
Eden O, Hill F, Gibson B and Richards S: UK Medical Research
Council’s Working Party on Childhood Leukaemia: Determinants of
outcome after intensified therapy of childhood lymphoblastic
leukaemia: results from Medical Research Council United Kingdom
Acute Lymphoblastic Leukaemia XI Protocol. Br J Haematol.
113:103–114. 2001.
|
|
13
|
Aricò M, Valsecchi MG, Camitta B, Schrappe
M, Chessells J, Baruchel A, Gaynon P, Silverman L, Janka-Schaub G,
Kamps W, Pui CH and Masera G: Outcome of treatment in children with
Philadelphia chromosome-positive acute lymphoblastic leukemia. N
Eng J Med. 342:998–1006. 2000.
|
|
14
|
Schrappe M, Aricò M, Harbott J, Biondi A,
Zimmermann M, Conter V, Reiter A, Valsecchi MG, Gadner H, Basso G,
Bartram CR, Lampert F, Riehm H and Masera G: Philadelphia
chromosome-positive (Ph+) childhood acute lymphoblastic
leukemia: good initial steroid response allows early prediction of
a favorable treatment outcome. Blood. 92:2730–2741. 1998.
|
|
15
|
Liehr T, Heller A, Starke H, Rubtsov N,
Trifonov V, Mrasek K, Weise A, Kuechler A and Claussen U:
Microdissection-based high resolution multicolor banding for all 24
human chromosomes. Int J Mol Med. 9:335–339. 2002.PubMed/NCBI
|
|
16
|
Weise A, Mrasek K, Fickelscher I, Claussen
U, Cheung SW, Cai WW, Liehr T and Kosyakova N: Molecular definition
of high-resolution multicolor banding probes: first within the
human DNA sequence anchored FISH banding probe set. J Histochem
Cytochem. 56:487–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mesquita DR, Córdoba JC, Magalhães IQ,
Córdoba MS, Oliveira JRC, Gonçalves A, Ferrari I and Martins-de-Sá
C: Molecular and chromosomal mutations among children with
B-lineage lymphoblastic leukemia in Brazil’s Federal District.
Genet Mol Res. 8:345–353. 2009.
|
|
18
|
Reaman GH, Sposto R, Sensel MG, Lange BJ,
Feusner JH, Heerema NA, Leonard M, Holmes EJ, Sather HN,
Pendergrass TW, Johnstone HS, O’Brien RT, Steinherz PG, Zeltzer PM,
Gaynon PS, Trigg ME and Uckun FM: Treatment outcome and prognostic
factors for infants with acute lymphoblastic leukemia treated on
two consecutive trials of the Children’s Cancer Group. J Clin
Oncol. 17:445–455. 1999.
|
|
19
|
Rowley JD: Molecular genetics in acute
leukaemia. Leukemia. 14:513–517. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sánchez-García I and Grütz G: The
tumorigenic activity of the BCR-ABL oncogenes is mediated by BCL-2.
Proc Natl Acad Sci USA. 92:5287–5291. 1995.
|
|
21
|
Rieder H, Bonwetsch C, Janssen LA, Maurer
J, Janssen JW, Schwartz S, Ludwig WD, Gassman W, Bartram CR, Thiel
E, Loffler H, Gokbuget N, Hollzer D and Fonatsch C: High rate of
chromosome abnormalities detected by fluorescence in situ
hybridization using BCR and ABL probes in adult acute lymphoblastic
leukemia. Leukemia. 12:1473–1481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Copelan EA and McGuire EA: The biology and
treatment of acute lymphoblastic leukemia in adults. Blood.
85:1151–1168. 1995.PubMed/NCBI
|
|
23
|
Secker-Walker LM, Craig JM, Hawkins JM and
Hoffbrand AV: Philadelphia positive acute lymphoblastic leukaemia
in adults – age distribution, BCR breakpoint and prognostic
significance. Leukemia. 5:196–199. 1991.
|
|
24
|
Annino L, Ferrari A, Cedrone M, Giona F,
Lo Coco F, Meloni G, Arcese W and Mandelli F: Adult
Philadelphia-chromosome-positive acute lymphoblastic leukaemia:
experience of treatments during a 10-year period. Leukemia.
8:664–667. 1994.
|
|
25
|
Secker-Walker LM, Pentrice HG, Durrant J,
Richards S, Hall E and Harrison G: Cytogenetics adds independent
prognostic information in adults with acute lymphoblastic leukaemia
on MRC trial UKALL XA. Br J Haematol. 96:601–610. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pui CH, Crist WM and Look T: Biology and
clinical significance of cytogenetic abnormalities in childhood
acute lymphoblastic leukaemia. Blood. 76:1449–1463. 1990.PubMed/NCBI
|
|
27
|
Pui CH and Evans WE: Acute lymphoblastic
leukemia. N Engl J Med. 339:605–615. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lugthart S, Cheok MH, den Boer ML, Yang W,
Holleman A, Cheng C, Pui CH, Relling MV, Janka-Schaub GE, Pieters R
and Evans WE: Identification of genes associated with chemotherapy
crossresistance and treatment response in childhood acute
lymphoblastic leukemia. Cancer Cell. 7:375–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holleman A, den Boer ML, de Menezes RX,
Cheok MH, Cheng C, Kazemier KM, Janka-Schaub GE, Göbel U, Graubner
UB, Evans WE and Pieters R: The expression of 70 apoptosis genes in
relation to lineage, genetic subtype, cellular drug resistance, and
outcome in childhood acute lymphoblastic leukemia. Blood.
107:769–776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nyvold C, Madsen HO, Ryder LP, Seyfarth J,
Svejgaard A, Clausen N, Wesenberg F, Jonsson OG, Forestier E and
Schmiegelow K; Nordic Society for Pediatric Hematology and
Oncology. Precise quantification of minimal residual disease at day
29 allows identification of children with acute lymphoblastic
leukemia and an excellent outcome. Blood. 99:1253–1258. 2002.
View Article : Google Scholar
|
|
31
|
Lal A, Kwan E, Haber M, Norris MD and
Marshall GM: Detection of minimal residual disease in peripheral
blood prior to clinical relapse of childhood acute lymphoblastic
leukaemia using PCR. Mol Cell Probes. 15:99–103. 2001. View Article : Google Scholar : PubMed/NCBI
|