Introduction
Toll-like receptors (TLRs) recognize a set of
conserved molecular structures called pathogen-associated molecular
patterns, which allow them to sense innate and adaptive immune
responses. Among them, TLR9 is essential for recognition of
microbial CpG DNA or synthetic CpG oligonucleotide analogs
containing a CpG oligodeoxynucleotide (ODN). CpG DNA activates
macrophages, monocytes, and dendritic cells to secrete
proinflammatory cytokines (1,2). The
binding of CpG DNA to TLR9 and subsequent endosomal maturation are
thought to be essential for CpG DNA-driven immunostimulatory
activity (3). After CpG DNA
binding, TLR9 signaling is initiated by recruitment of the adaptor
molecule MyD88 followed by the engagement of interleukin
(IL)-1R-associated kinases and tumor necrosis factor (TNF)-α
receptor (TNFR)-associated factor 6 (4). These complexes activate the IκB kinase
complex and subsequently activate NF-κB-dependent pro-inflammatory
cytokines such as TNF-α and IL-1β (5). TLRs are members of the IL-1R
superfamily and share a common activation pathway through their
Toll/IL-1R signaling domain (6).
Despite this common pathway, TLRs show differences in their rate,
intensity, or efficiency of activation by yet unidentified
mechanisms.
Members of the FoxO subfamily of forkhead
transcription factors include the mammalian ortholog DAF-16, which
regulates longevity in the nematode Caenorhabditis elegans
(7). Mice and humans possess three
highly related FoxO homologs (FoxO1, FoxO3 and FoxO4) with
overlapping expression patterns and transcriptional activities
(8). Suppression of FoxO
transcriptional activity by Akt-mediated phosphorylation leads to
enhanced cell survival (9). In
conditions in which the Akt survival and growth pathway is
activated, FoxO3a is phosphorylated by Akt and exported to the
cytoplasm (10). In contrast,
unphosphorylated FoxO3a proteins are active forms and are located
in the nucleus where they bind to their target gene promoters.
Overexpression of the constitutively activated form of FoxO3a leads
to apoptosis in many cell types (11). Additionally, FoxO3a mediates
apoptosis by activating pro-apoptotic genes such as TNF-related
apoptosis-inducing ligand (TRAIL) (12). Although FoxO3a has generally been
considered an inducer of apoptosis, there is little evidence of TLR
signaling.
In this study, we investigated the role of TRAIL in
TLR9-mediated anti-apoptosis of macrophages. We found that CpG ODN
treatment blocked serum deprivation-mediated apoptosis. We also
found that CpG ODN downregulated TRAIL gene expression. We further
investigated the mechanisms of CpG ODN-induced TRAIL expression via
the TLR9-Akt-FoxO3a signaling pathway.
Materials and methods
Reagents and antibodies
Cell culture reagents were obtained from Life
Technologies (Grand Island, NY, USA). Fetal bovine serum (FBS) was
obtained from Thermo Scientific HyClone (Logan, UT). Chloroquine,
propidium iodide (PI) and β-actin antibody were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Phosphorothioated unmethylated
endotoxin-free CpG ODN (B-class, TCCATGACGTTCCTGATGCT) and
control ODN 1720 (TCCATGAGCTTCCTGATGCT, inactive control for CpG
ODN 1668) were obtained from Genotech (Daejeon, South Korea), and
an RNA reverse transcription-polymerase chain reaction (RT-PCR)
core kit was purchased from Axygen Biosciences (Union City, CA,
USA). Antibodies (Abs) against FoxO3a and Akt were purchased from
Cell Signaling Technology (Beverly, MA, USA). Bafilomycin A1 and
LY294002 were purchased from Calbiochem (San Diego, CA, USA).
Cell culture
The Raw264.7 macrophage cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA). Cells
were grown in Dulbecco's modified Eagle's medium (Invitrogen,
Carlsbad, CA, USA) containing 10% FBS, 2 μM L-glutamine, 10 U/ml
penicillin and 10 μg/ml streptomycin at 37°C in a humidified
atmosphere under 5% CO2. Cells were treated with
synthetic CpG ODN for various times.
Fluorescence-activated cell sorting
(FACS) analysis
To quantify apoptotic nuclei, cells were fixed in
ethanol, stained with 50 μg/ml PI and RNase A for 30 min at room
temperature followed by washing, and the samples were processed by
flow cytometry using a FACSCalibur apparatus (BD Biosciences,
Franklin Lakes, NJ, USA). The results are shown as a histogram with
sub-G1 positive cells considered the apoptotic cells.
Western blot analysis
The cells were washed with cold-PBS, trypsinized,
and pelleted at 700 × g. Cell pellets were resuspended in lysis
buffer comprised of 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 150 mM
NaCl, 0.5% Nonidet P-40, 1 mM PMSF, and a protease inhibitor
cocktail. The preparations were then cleared by centrifugation, and
the supernatants were saved as cell lysates. Proteins were
separated by 8% reducing sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and immunoblotted in 20% methanol, 25 mM Tris, and
192 mM glycine onto nitrocellulose membranes. The membranes were
then blocked with 5% non-fat dry milk in TTBS (25 mM Tris-HCl, 150
mM NaCl, and 0.2% Tween-20) and incubated with primary Ab for 4 h.
Subsequently, membranes were washed, incubated for 1 h with
secondary Ab conjugated to horseradish peroxidase, rewashed, and
finally developed using an enhanced chemiluminescence system
(Amersham, Buckinghamshire, UK).
Real-time RT-PCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen). Total RNA (1 μg) was used as a template to
make first strand cDNA by oligo-dT priming using a reverse
transcriptase system (Promega, Madison, WI, USA). Real-time RT-PCR
was performed using a LightCycler 1.5 (Roche Diagnostics, Almere,
The Netherlands) with SYBR-Green I as the florescent dye, according
to the manufacturer's instructions. The synthetic gene-specific
primer sets used for PCR were: i) TRAIL forward primer,
5′-CCTCTCGGAAAGGGCATTC-3′, and reverse primer,
5′-TCCTGCTCGATGACCAGCT-3′, which amplified 70 bp of the mouse TRAIL
cDNA; ii) β-actin forward primer, 5′-AGAGGGAAATCGTGCGTGAC-3′, and
reverse primer, 5′-CAATAGTGATGACCTGGCCGT-3′, which amplified 137 bp
of the mouse β-actin cDNA. Cycling conditions were 95°C for 10 min,
followed by 45 cycles of 95°C for 10 sec, 62°C for 5 sec, and 72°C
for 6 sec. Target genes were normalized to β-actin for
quantification.
Knock-down of FoxO3a using small
interfering RNA (siRNA)
Oligonucleotides corresponding to the mouse FoxO3a
siRNA sequence 5′-UGAUGAUCCACCAAGAGCUCUUGCC-3′ were purchased from
Invitrogen. A control siRNA was also purchased and used. For
transfection, 2×106 Raw264.7 cells were resuspended in a
nucleoporator buffer (Lonza, Allendale, NJ, USA) with 200 pmole
siRNA. Cells were nucleoporated according to the manufacturer's
protocol, and the above genes were knocked down for 24 h.
FoxO3a and Akt overexpression
A vector encoding a FoxO3a protein
(pLenti6/V5-D-TOPO-FoxO3a) was generously provided by Dr Kim
(Yeungnam University, South Korea) for cell protein expression. Akt
was subcloned into the pEGFP-C1 mammalian expression vector. Cells
were transfected with the control vector, wild-type FoxO3a, or Akt
cDNA for 24 h, and fresh medium was added. Cells transfected with
the cDNA were cloned by serial dilution in a 96-well plate in a
culture medium with selecting antibiotics to obtain a stable cell
line. Sub-culturing was continued for 4 weeks, and then wells
representing a single colony were selected and expression was
confirmed using the protein level as determined by Western blot
analysis.
Results
CpG ODN blocks TRAIL expression and
apoptosis using serum starvation
We first investigated the effect of CpG ODN in serum
starvation-induced apoptosis. As expected, CpG ODN had a
significant inhibitory effect on sub-G1 cell accumulation following
serum starvation (Fig. 1A). The
change of TRAIL mRNA expression using serum starvation in Raw264.7
cells was further examined. While serum starvation induced a
dramatic increase in TRAIL expression in a time-dependent manner,
CpG ODN strongly inhibited this response (Fig. 1B). We studied whether TLR9 mediates
the inhibition of TRAIL expression and apoptosis. Pretreatment with
bafilomycin A1 or chloroquine recovered TRAIL expression reduced by
CpG ODN (Fig. 1C). To confirm the
role of TRAIL in apoptosis following serum starvation, we treated
Raw264.7 cells with the neutralizing TRAIL antibody or a
combination with CpG ODN. Apoptosis partially but significantly
decreased in the TRAIL neutralizing antibody-treated cells, and
this effect was very similar to that of the CpG ODN treated cells.
However, no additional effect was observed with the combination of
CpG ODN and the TRAIL neutralizing antibody (Fig. 1D). These results suggest that CpG
ODN plays a role in serum starvation-induced apoptosis by
inhibiting TRAIL expression.
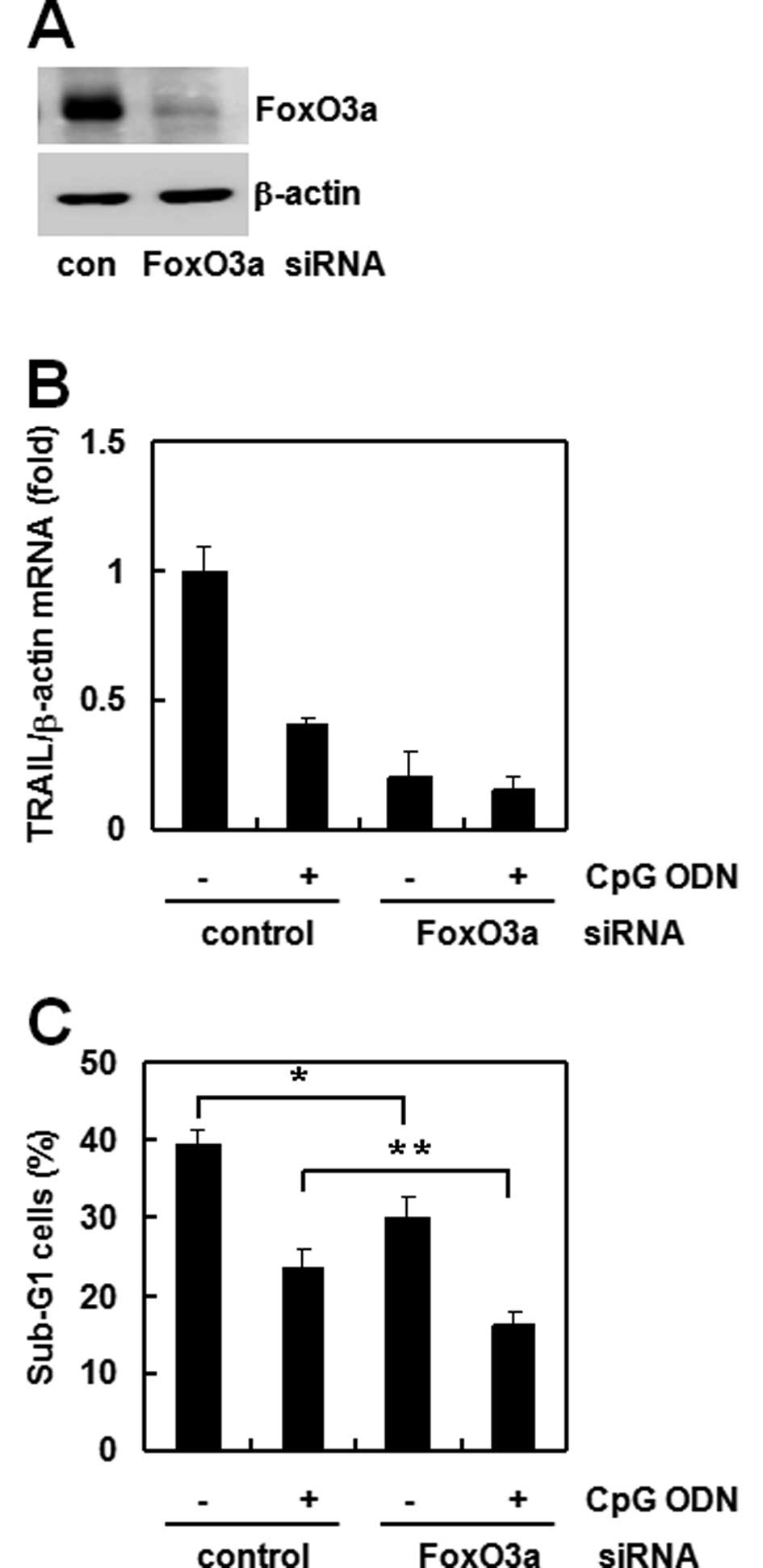
FoxO3a is involved in CpG ODN-regulated
TRAIL expression
FoxO3a is a well known transcription factor for
TRAIL regulation (13). Therefore,
we assessed the involvement of FoxO3a in CpG ODN-mediated TRAIL
downregulation using siRNA or an overexpression technique.
Transfection of FoxO3a siRNA effectively decreased protein
expression in Raw264.7 cells (Fig.
2A). Then, we compared the CpG ODN effect between control and
FoxO3a siRNA cells. TRAIL expression was reduced dramatically in
FoxO3a siRNA cells compared to that of control siRNA cells.
Treatment with CpG ODN reduced TRAIL expression (Fig. 2B) and sub-G1 accumulation in both
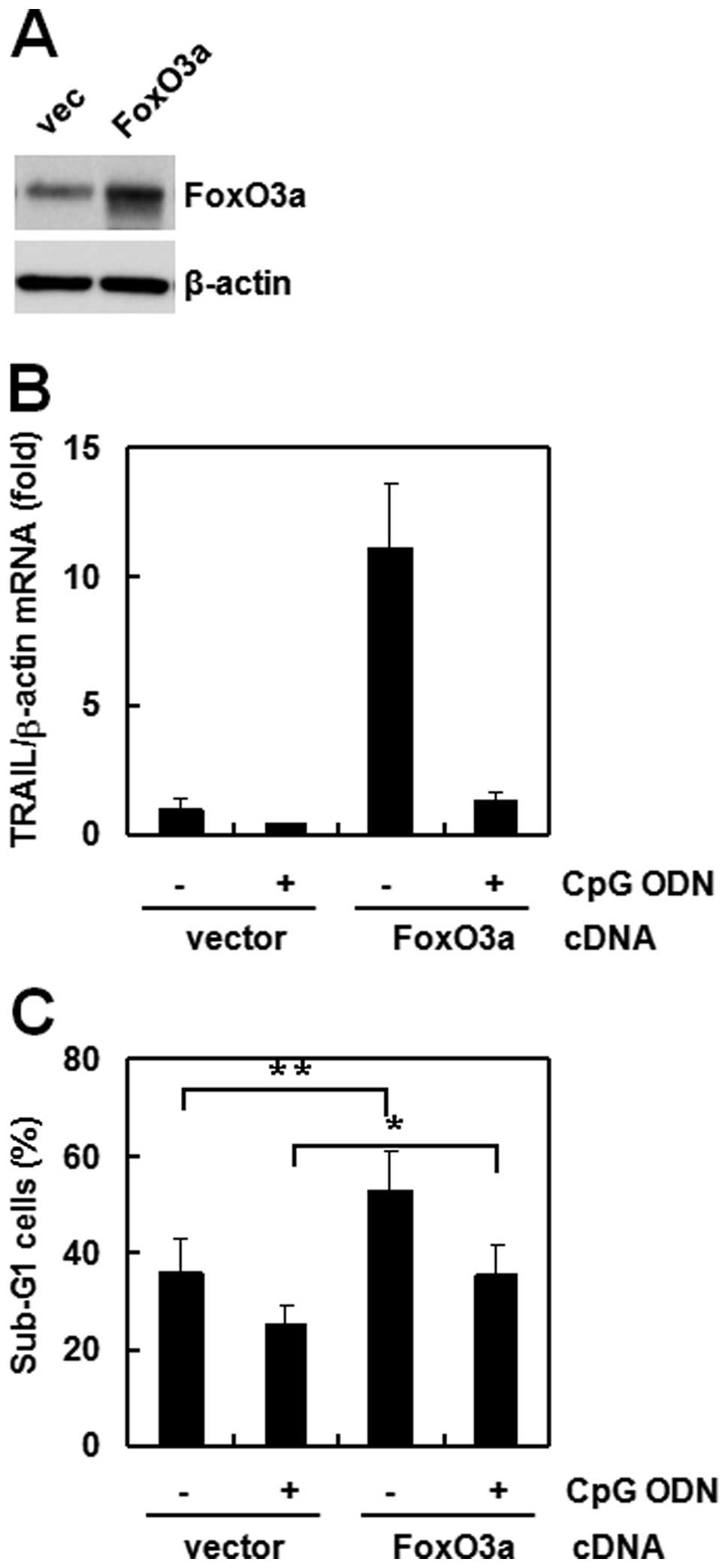
siRNA cells (Fig. 2C). To confirm
the role of FoxO3a, we overexpressed the gene in the same cell
lines and investigated the effect of CpG ODN. TRAIL expression
increased strongly in FoxO3a overexpressed cells compared to that
of empty-vector transfected cells (Fig.
3A). However, CpG ODN treatment decreased TRAIL expression
(Fig. 3B) and sub-G1 accumulation
(Fig. 3C) in both types of
transfected cells. These results suggest that FoxO3a directly
regulates TRAIL expression and that the anti-apoptosis effect of
CpG ODN occurs through TRAIL.
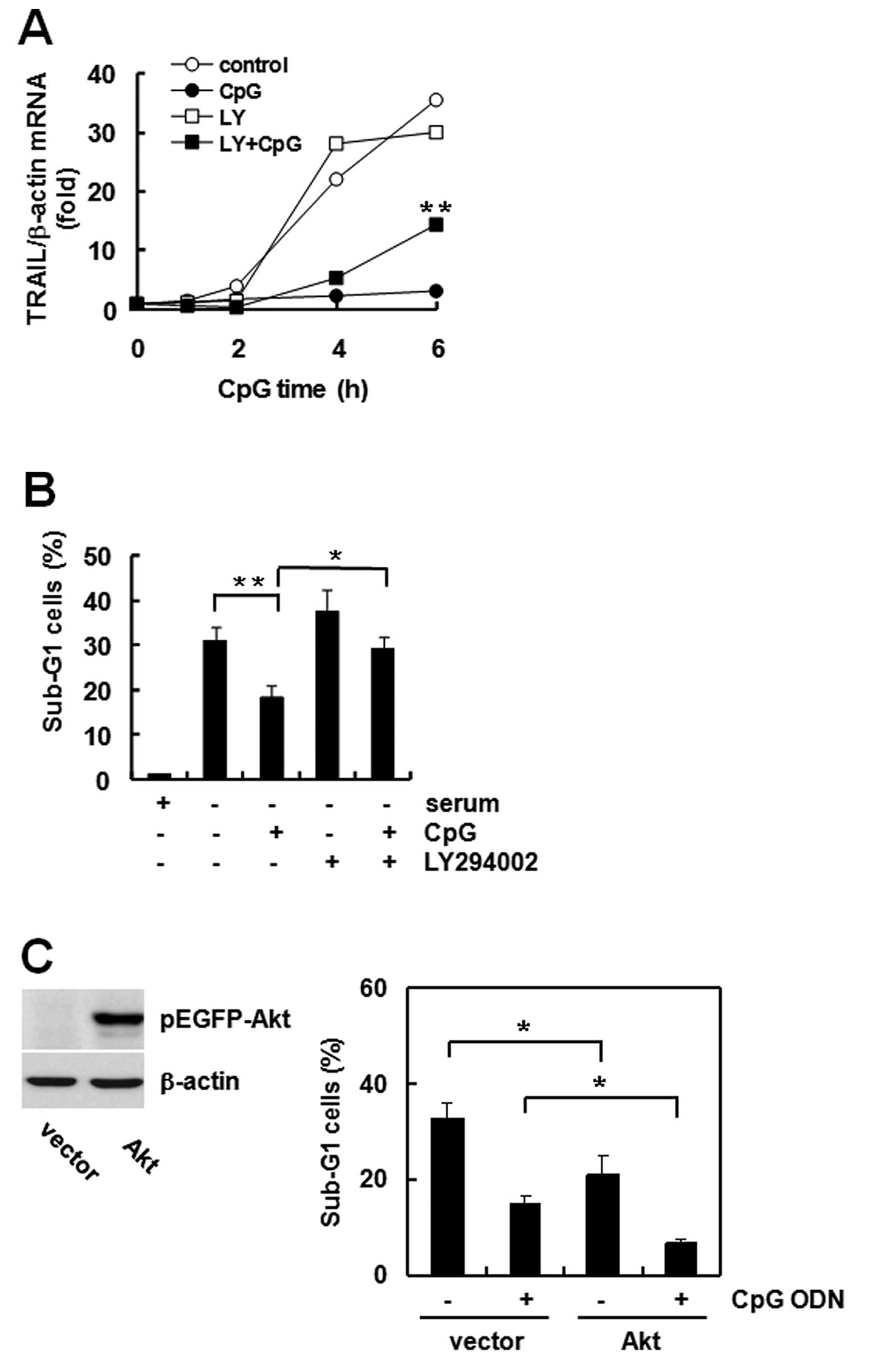
The Akt-FoxO3a pathway is involved in
regulating TRAIL expression and apoptosis
Akt is a well known apoptosis regulatory kinase, and
FoxO3a phosphorylation by Akt leads to inactivation of its
transcriptional activity (14).
Previously, we observed that CpG ODN phosphorylates Akt for
activation and phosphorylates FoxO3a for inactivation (15). Therefore, we investigated the role
of Akt in TRAIL expression and apoptosis by CpG ODN. While CpG ODN
blocked serum starvation-induced TRAIL expression, LY294002
pretreatment partially and significantly recovered the reduced
TRAIL expression (Fig. 4A).
Apoptosis increased following LY294002 treatment (Fig. 4B). To confirm the role of Akt in
apoptosis, we transfected the GFP vector or GFP-tagged Akt cDNA and
compared the effect of CpG ODN. Akt expression increased in Akt
cDNA transfected cells. Akt transfection itself decreased the
sub-G1 population compared to that of vector-transfected cells, and
the decrease was deepened by CpG ODN (Fig. 4C). These results suggest that Akt is
a very important regulator of CpG ODN-mediated TRAIL expression and
anti-apoptosis.
Discussion
TLRs including TLR9 play a central role in innate
immunity by mediating pathogen recognition (16). Previous studies have demonstrated a
role for TLR9 in mediating the effects of cell survival including
macrophages. We also observed that treating macrophages with TLR9
agonists strongly obviated apoptosis. Many groups have investigated
the regulatory proteins involved in cell survival, but the specific
proteins involved in the response to a TLR9 agonist have not been
clarified. CpG ODN promotes cell survival via Hsp70 upregulation to
increase Bcl-xL (17). Furthermore,
Hsp90β is also involved in the TLR9 anti-apoptotic effect (18). Therefore, we examined the effect of
a TLR9 agonist on the cellular levels of the many proteins
participating in apoptotic pathways. Among the possible regulatory
factors, our results identified TRAIL as an important regulator of
macrophages in apoptosis.
Our results demonstrate that CpG ODN strongly
downregulated TRAIL expression in macrophages via the
TLR9-dependent pathway. TRAIL is involved in apoptosis signaling
pathways, specifically by modulating immune system function
(19). Interestingly, TRAIL
receptor-mediated apoptosis is inhibited by FLIP, through
suppression of either recruitment of procaspase-8 by FADD or
autocatalytic activation of caspase-8 (20,21).
Therefore, our results suggest that TRAIL is suppressed after CpG
ODN treatment, and that these responses could interfere with serum
deprivation-induced apoptosis.
NF-κB is a transcription factor that potentially
affects the expression of many genes and may favor cell survival by
upregulating gene products with anti-apoptotic properties or
downregulating pro-apoptotic factors (22). It has been suggested that activating
NF-κB via the TLR2 signaling pathway and the subsequent induction
of gene expression can protect cells from FasL-induced apoptosis
(23). Recognition of TLR9 by CpG
ODN is also followed by NF-κB activation (3). Despite this, the expression of several
apoptosis-regulating genes is controlled by other transcription
factors including FoxO (24).
Previous studies have suggested that activating Akt
negatively regulates FoxO transcription factors (9,25), and
that the direct phosphorylation of Akt inhibits FoxO3a
transcriptional activation (26).
Additionally, the PI3K/Akt pathway is very important for the
anti-apoptosis effects of CpG ODN (27). Furthermore, because Akt activity
prevents the induction of apoptosis by cytokines, growth factors,
and cellular stress (28), we
determined the effect of the Akt pathway on FoxO3a activation,
TRAIL expression, and apoptosis. Use of the pharmacological
inhibitor LY294002 enabled us to assess the role of the Akt
signaling pathway in the regulation of FoxO3a phosphorylation. Our
data show that inhibiting Akt resulted in a significant increase in
TRAIL gene expression in these cells. Furthermore, direct evidence
was obtained by wild-type FoxO3a overexpression or using siRNA. Our
data demonstrate that CpG ODN treatment increased TRAIL expression
in FoxO3a overexpressing cells compared to vector control cells.
FoxO3a siRNA cells demonstrated an opposite result compared to that
of overexpressing cells. Together, these data strongly support the
conclusion that FoxO3a is a transcription factor for TRAIL
regulation in response to CpG ODN.
CpG ODN protects B-cells and macrophages against
apoptosis (17,29). Based on this result, we confirmed
that engaging TLR9 protected against serum deprivation-induced
apoptosis. Furthermore, we showed that CpG ODN induced an increase
in FoxO3a phosphorylation. This protective effect was controlled by
decreased TRAIL expression in CpG ODN-stimulated macrophages. The
anti-apoptotic effects of CpG ODN stimulation require participation
of the Akt-FoxO3a signaling pathway. Taken together, these results
suggest that TLR9 triggers the FoxO3a transcription factor through
Akt signaling, and that the regulation of TRAIL by CpG ODN may
contribute to the anti-apoptotic effect.
Acknowledgements
This research was supported by Yeungnam University
research grants in 2010 (210-A-356-007).
Abbreviations:
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
CpG ODN
|
CpG oligodeoxynucleotide
|
|
TLR9
|
toll-like receptor 9
|
|
FoxO
|
forkhead transcription factors of the
O class
|
References
|
1
|
Ivanov S, Dragoi AM, Wang X, et al: A
novel role for HMGB1 in TLR9-mediated inflammatory responses to
CpG-DNA. Blood. 110:1970–1981. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanjuan MA, Rao N, Lai KT, et al:
CpG-induced tyrosine phosphorylation occurs via a TLR9-independent
mechanism and is required for cytokine secretion. J Cell Biol.
172:1057–1068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeshita F, Gursel I, Ishii KJ, Suzuki K,
Gursel M and Klinman DM: Signal transduction pathways mediated by
the interaction of CpG DNA with Toll-like receptor 9. Semin
Immunol. 16:17–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawai T, Sato S, Ishii KJ, et al:
Interferon-alpha induction through Toll-like receptors involves a
direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol.
5:1061–1068. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bagchi A, Herrup EA, Warren HS, et al:
MyD88-dependent and MyD88-independent pathways in synergy, priming,
and tolerance between TLR agonists. J Immunol. 178:1164–1171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bulek K, Swaidani S, Qin J, et al: The
essential role of single Ig IL-1 receptor-related molecule/Toll
IL-1R8 in regulation of Th2 immune response. J Immunol.
182:2601–2609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SS, Kennedy S, Tolonen AC and Ruvkun
G: DAF-16 target genes that control C. elegans life-span and
metabolism. Science. 300:644–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paik JH, Kollipara R, Chu G, et al: FoxOs
are lineage-restricted redundant tumor suppressors and regulate
endothelial cell homeostasis. Cell. 128:309–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Wang Y and Zhu WG: Applications of
post-translational modifications of FoxO family proteins in
biological functions. J Mol Cell Biol. 3:276–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barthelemy C, Henderson CE and Pettmann B:
Foxo3a induces motoneuron death through the Fas pathway in
cooperation with JNK. BMC Neurosci. 5:482004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui M, Huang Y, Zhao Y and Zheng J:
Transcription factor FOXO3a mediates apoptosis in HIV-1-infected
macrophages. J Immunol. 180:898–906. 2008. View Article : Google Scholar
|
|
12
|
Modur V, Nagarajan R, Evers BM and
Milbrandt J: FOXO proteins regulate tumor necrosis factor-related
apoptosis inducing ligand expression. Implications for PTEN
mutation in prostate cancer. J Biol Chem. 277:47928–47937. 2002.
View Article : Google Scholar
|
|
13
|
Sakoe Y, Sakoe K, Kirito K, Ozawa K and
Komatsu N: FOXO3A as a key molecule for all-trans retinoic
acid-induced granulocytic differentiation and apoptosis in acute
promyelocytic leukemia. Blood. 115:3787–3795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan H, Song L, Cai J, et al: Sphingosine
kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to
apoptosis resistance in glioma cells. PLoS One. 6:e199462011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim EJ, Park DW, Lee JG, et al: Toll-like
receptor 9-mediated inhibition of apoptosis occurs through
suppression of FoxO3a activity and induction of FLIP expression.
Exp Mol Med. 42:712–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
17
|
Kuo CC, Liang SM and Liang CM: CpG-B
oligodeoxynucleotide promotes cell survival via up-regulation of
Hsp70 to increase Bcl-xL and to decrease apoptosis-inducing factor
translocation. J Biol Chem. 281:38200–38207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo CC, Liang CM, Lai CY and Liang SM:
Involvement of heat shock protein (Hsp)90 beta but not Hsp90 alpha
in antiapoptotic effect of CpG-B oligodeoxynucleotide. J Immunol.
178:6100–6108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitsiades N, Mitsiades CS, Poulaki V,
Anderson KC and Treon SP: Intracellular regulation of tumor
necrosis factor-related apoptosis-inducing ligand-induced apoptosis
in human multiple myeloma cells. Blood. 99:2162–2171. 2002.
View Article : Google Scholar
|
|
21
|
Xiao CW, Asselin E and Tsang BK: Nuclear
factor kappaB-mediated induction of Flice-like inhibitory protein
prevents tumor necrosis factor alpha-induced apoptosis in rat
granulosa cells. Biol Reprod. 67:436–441. 2002. View Article : Google Scholar
|
|
22
|
Kerbauy DM, Lesnikov V, Abbasi N, Seal S,
Scott B and Deeg HJ: NF-kappaB and FLIP in arsenic trioxide
(ATO)-induced apoptosis in myelodysplastic syndromes (MDSs). Blood.
106:3917–3925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loeuillet C, Martinon F, Perez C, Munoz M,
Thome M and Meylan PR: Mycobacterium tuberculosis subverts
innate immunity to evade specific effectors. J Immunol.
177:6245–6255. 2006. View Article : Google Scholar
|
|
24
|
Fu Z and Tindall DJ: FOXOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khatri S, Yepiskoposyan H, Gallo CA,
Tandon P and Plas DR: FOXO3a regulates glycolysis via
transcriptional control of tumor suppressor TSC1. J Biol Chem.
285:15960–15965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dragoi AM, Fu X, Ivanov S, et al:
DNA-PKcs, but not TLR9, is required for activation of Akt by
CpG-DNA. EMBO J. 24:779–789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Francois S, El Benna J, Dang PM, Pedruzzi
E, Gougerot-Pocidalo MA and Elbim C: Inhibition of neutrophil
apoptosis by TLR agonists in whole blood: involvement of the
phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways,
leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J
Immunol. 174:3633–3642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krieg AM: CpG motifs in bacterial DNA and
their immune effects. Annu Rev Immunol. 20:709–760. 2002.
View Article : Google Scholar : PubMed/NCBI
|