Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent tumor types worldwide, especially in Asian countries, and
both incidence and mortality rates of HCC have increased in recent
years (1). Hepatic resection and
transplantation are potential curative treatments for HCC, but only
about 20% of the patients are suitable candidates (2). For the treatment of unresectable HCC,
chemotherapy represents an important tool in the clinical therapy
of HCC. However, severe cytotoxicity induced by most of the
commonly used anticancer drugs on normal cells necessitates the
screening and development of some novel therapeutic reagents with
relatively low side effects (3).
Recently, traditional Chinese medicines have been recognized as a
new source of anticancer drugs and new adjuvant therapy to enhance
the efficacy of chemotherapy and to reduce the cancer
chemotherapy-related side effects (4). In this regard, Chinese traditional
herbs have been of particular interest, due to their relatively low
toxicity as concluded from their extensive clinical usage in the
past. Isolation and screening of the active components from the
herbs possessing anticancer potential appears to be a promising way
of discovering novel therapeutic compounds.
The Chinese herb Scutellaria baicalensis (SB)
is a member of the Lamiaceae or mint family and is known as Chinese
skullcap (common name: Huang-Qin in China) and as Japanese Ogon. SB
has been widely used in traditional Chinese medicine with
multi-properties, such as antitumor, anti-inflammatory,
anti-hypertensive, anti-cardiovascular, antibacterial and antiviral
(4). Diverse phytoestrogen-like
substances mainly including baicalin, baicalein and wogonin have
been isolated from SB. Accumulating studies have demonstrated that
baicalin possibly was the key bioactive ingredient mediating the
anticancer effect of SB (4).
Baicalin exerts strong suppressive effects on many types of cancer
cells and has been documented to be a promising anticancer
candidate (4–7). However, the precise mechanisms
underlying its anticancer effects remain to be elucidated.
Autophagy is a highly conservative intracellular
process for degrading long-lived proteins and cytoplasmic
organelles that consists of several sequential steps:
sequestration, transport to lysosomes, degradation and utilization
of degradation products (8). It is
characterized by the appearance of double- and multi-membrane
cytoplasmic vesicles that engulf cytoplasm and organelles, forming
autophagosomes marked by microtubule-associated protein light chain
3 (LC3) (9). Many key molecules are
involved in this biological process, especially Beclin 1, a
mammalian homolog of yeast Atg6/Vps30 and an essential regulator
that promotes autophagosome formation through mediating the
localization of other autophagy proteins on the pre-autophagosomal
membrane (10). Autophagy plays a
wide variety of roles in physiological and pathological processes,
such as starvation adaptation (11), embryonic development (12), cell survival and death (13), and tumor suppression (14). Recent publications have reported two
seemingly opposite functions of autophagy in tumor progression
(8,15). Based on the ability of autophagy to
promote cell survival in response to metabolic stress, it has been
suggested that autophagy may contribute to tumor development. On
the other hand, autophagy also directly or indirectly induces
autophagic cell death through excessive self-digestion and the
activation of apoptosis and inhibits tumor progression (14). In this context, it is a novel
anticancer strategy to induce autophagic cell death in cancer cells
and this concept has been confirmed with several chemotherapy
agents from traditional Chinese medicine such as berberine
(16,17) and arsenic trioxide (18). However, it has never been
investigated whether baicalin induces autophagy in cancer
cells.
CD147, a glycosylated immunoglobulin superfamily
transmembrane protein, is highly expressed in HCC cell lines and
tumor tissues (19,20). Several studies in vitro have
suggested that CD147 inhibits tumor cell apoptosis (21) and anoikis (22), promotes invasion and metastasis
(19,23,24),
enhances tumor angiogenesis (25),
and conferrs chemoresistance to some drugs (22). These findings indicate that CD147
may serve as a key therapeutic target for HCC. Studies from our
laboratory demonstrated that CD147 may play an important role in
the inhibitory regulation of autophagy and autophagic cell death in
HCC cells (17,26). However, whether CD147 also plays a
role in mediating the anticancer effects of baicalin remains
unclear.
In the present study, we sought to assess the
effects of baicalin on tumor cell growth, autophagy induction and
the possible molecular mechanisms underlying baicalin-induced cell
death in SMMC-7721 cells in vitro. Our results demonstrate
that baicalin induced both autophagic cell death and apoptosis with
downregulation of CD147 expression in SMMC-7721 cells in
vitro. To the best of our knowledge, this is the first study to
investigate whether baicalin induces autophagy and the relationship
between baicalin-induced cell death and CD147 expression.
Materials and methods
Materials
Baicalin was purchased from the China National
Information Infrastructure for Reference Materials (Beijing, China)
and dissolved in normal saline (NS) to 20 mg/ml, pH 7.2, then
vortexed at room temperature for 10 min. This solution was
centrifuged at 5,000 rpm for 10 min to remove any insoluble
ingredients, then the supernatant was passed through a 0.22-μm
pore-size filter (Millipore, Bedford, MA) for sterilization and
diluted in RPMI-1640 medium at a final concentration of 1 mol/l (M)
as a stock solution. The pEGFP-LC3 plasmid was kindly provided by
Professor Xingchun Gou (Institute of Basic Medicine, Xi’an Medical
University, Xi’an, China). All reagents were obtained from common
commercial sources.
Cell culture
SMMC-7721 cells were provided by the Institute of
Cell and Biochemistry, Chinese Academy of Sciences (Shanghai,
China) and cultured in RPMI-1640 medium supplemented with 10%
heat-inactivated (56°C) fetal bovine serum (FBS) and 100 U/ml
penicillin, 100 μg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2. To study baicalin-induced cell
death, SMMC-7721 cells were cultured either with or without
baicalin (treatment group or control group).
MTT assay
Cell viability was determined after treatment with
various concentrations of baicalin and different durations of
exposure to baicalin by the MTT assay as previously described
(27). In brief, SMMC-7721 cells
were seeded at a density of 5×103 cells/well in 96-well
microplates in RPMI-1640 medium, and were cultured for 24 h. Then,
the cells were treated with various concentrations (0, 10, 20, 40,
80, and 160 μM) of baicalin for different times (0, 24, 48 or 72
h). Subsequently, 10 μl of 5 mg/ml MTT (Sigma Chemical Co., USA)
was added to each well for an additional 4-h incubation and the
resulting formazan crystals were dissolved in 100 μl DMSO. Finally
the optical density (OD) at 570 nm was measured using an automatic
microplate reader (Immuno Mini NJ-2300; Inter Med, Tokyo, Japan).
The percentage of cell viability inhibition was calculated
according to the following formula: [(OD value of the control cells
- OD value of the treated cells)/OD value of the control cells] ×
100%. The viability of the control cells, from the untreated
cultures, was defined as 100%.
Autophagy assay with LC3 dots
SMMC-7721 cells were transfected with pEGFP-LC3 for
24 h and then cultured with different concentration of baicalin (0,
40, 80 or 160 μM). Exogenous enhanced GFP (EGFP)-fused
microtubule-associated protein light chain 3 (EGFP-LC3) is a
specific autophagy marker widely used in autophagy research
(28). When autophagy is
stimulated, the distribution pattern of GFP-LC3 changes from a
diffuse cytoplasmic pattern to a punctate pattern that labels
pre-autophagosomal and autophagosomal membranes (29). Autophagy was analyzed using an
Olympus BX60 fluorescence microscope (Olympus, Tokyo, Japan). The
percentage of cells with EGFP-LC3 punctate dots was determined as
previously described (26).
Briefly, a minimum of 100 cells from each sample was counted in
three independent experiments. The percentage of cells showing
EGFP-LC3 punctate dots was calculated by dividing the number of
cells showing punctate dots by the number of cells counted.
Transmission electron microscope (TEM)
analysis
A TEM analysis was performed as previously described
(26). Briefly, after culture in
the absence or presence of 160 μM baicalin for 48 h, SMMC-7721
cells were fixed with 3% glutaraldehyde in 0.2 M phosphate buffer
(pH 7.3) for 4 h at 4°C, then post-fixed with 1% osmium tetroxide
and 0.5% tannic acid for 1 h at 4°C followed by washing three times
with 0.1 M phosphate buffer (pH 7.3). Next, cells were dehydrated
and embedded in Epon812 (Electron Microscopy Sciences, Fort
Washington, PA, USA). Finally, sections were counterstained with
uranyl acetate and lead citrate, and examined using a JEM-2000EX
transmission Electron Microscope (Jeol Ltd., Tokyo, Japan).
Immunofluorescence staining
SMMC-7721 cells were stained for Beclin 1 (Abcam
Co., USA) by immunofluorescence followin the protocol as detailed
previously with the following modifications (30). Briefly, cells on coverslips were
fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.1%
Triton X-100 for 10 min, washed three times (PBS containing 0.01%
Triton X-100 and 10% FBS), followed by incubation with the primary
antibody (anti-human Beclin 1) overnight at 4°C, washed, and
incubated with protein-blocking solution. Subsequently, cells were
incubated for 1 h at room temperature with a secondary antibody
(goat-anti mouse IgG) that was conjugated to fluorescein
isothiocyanate (FITC, green fluorescence) (Molecular Probes Inc.,
Eugene, Oregon, USA) and then washed. Finally, images were captured
using an Olympus BX60 (Olympus Optical Co., Ltd., Tokyo, Japan)
fluorescence microscope.
Western blot analysis
Beclin 1 and CD147 protein expression in SMMC-7721
cells was detected by Western blotting performed as previously
described (25). Briefly, cells
were washed twice with ice-cold PBS. Cell samples were lysed with
RIPA buffer (Pierce Biotechnology, Inc., USA) for 45 min on ice and
protein concentrations were measured using the BCA kit (Pierce
Biotechnology, Inc.). Equal amounts of protein (10 μg) were
separated by 10% SDS-PAGE and electrophoretically transferred to
polyvinylidene difluoride membranes (Millipore, Bedford, MA) using
a mini trans-blot apparatus (Bio-Rad Laboratories). Membranes were
blocked with PBS-0.05% Tween-20 containing 5% non-fat dry milk for
1 h and incubated with monoclonal mouse anti-human Beclin 1 and
CD147 or tubulin antibody (Palo Alto, CA, USA) for 2 h at room
temperature. Membranes were then washed three times with PBS-0.05%
Tween-20 and incubated with HRP-labeled goat anti-mouse antibody
(Carlsbad, CA, USA) at a 1:10,000 dilution for 1 h. Blots were
developed using an enhanced chemiluminescence kit (Pierce
Biotechnology, Inc.). Each experiment was repeated at least three
times.
Trypan blue dye exclusion assay
Cell viability of SMMC-7721 cells was assessed by
the trypan blue exclusion assay as previously described (31). Briefly, SMMC-7721 cells were
pretreated with 0, 40, 80 or 160 μM baicalin for 48 h in the
absence or presence of the autophagy specific inhibitor 3-MA (100
μM) (Sigma Chemical Co.) and/or the caspase-3 specific inhibitor
Zyloxy-Asp-Glu-Val-Asp fluoromethyl ketone (z-DEVD-fmk) (100 nM)
(Beyotime Institute of Biotechnology, China). Cells were washed
with PBS, trypsinized, and collected by centrifugation, and then
resuspended in 200 μl PBS. After mixing with 200 μl of 0.8% trypan
blue, the cells were counted using a hemocytometer. The number of
dead cells with disrupted membranes (blue cells) per 200 cells was
counted in triplicates. Cell death was expressed as the mean
percentage of blue cells/total cells.
Statistical analysis
All statistical analyses were performed using the
SPSS 16.0 statistical package for Microsoft Windows (SPSS, Chicago,
IL). Statistical significance was determined using a Student’s
t-test. All tests in this study were two-sided and P<0.05 was
considered statistically significant.
Results
Antiproliferative effect of baicalin on
SMMC-7721 cells
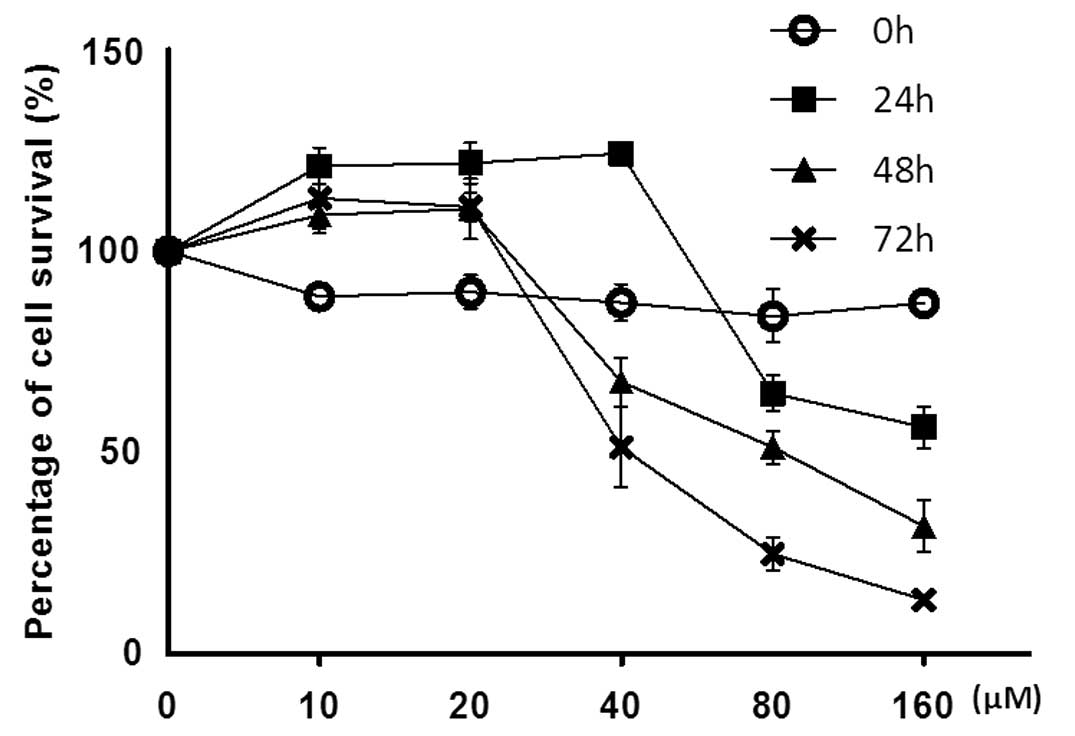
We first examined the effect of baicalin on the
survival of SMMC-7721 cells using the MTT assay. Our results
indicated that baicalin displayed strong inhibitory effect on the
growth of SMMC-7721 cells in a dose and time-dependent manner
(Fig. 1), and the IC50
value of baicalin was determined to be 40 μM for 72 h or 80 μM for
48 h (Fig. 1). Apparently, at a
concentration as high as 160 μM, baicalin provoked the strongest
inhibitory effect on SMMC-7721 cells, with morphological
alterations characteristic of cell death observed under a light
microscope (data not shown). However, at a low dose range (10 and
20 μM), baicalin failed to elicit a significant inhibitory effect
on SMMC-7721 cells vitality. Thus, a baicalin concentration ≥40 μM
was used for subsequent experiments.
Autophagy induced by baicalin in
SMMC-7721 cells
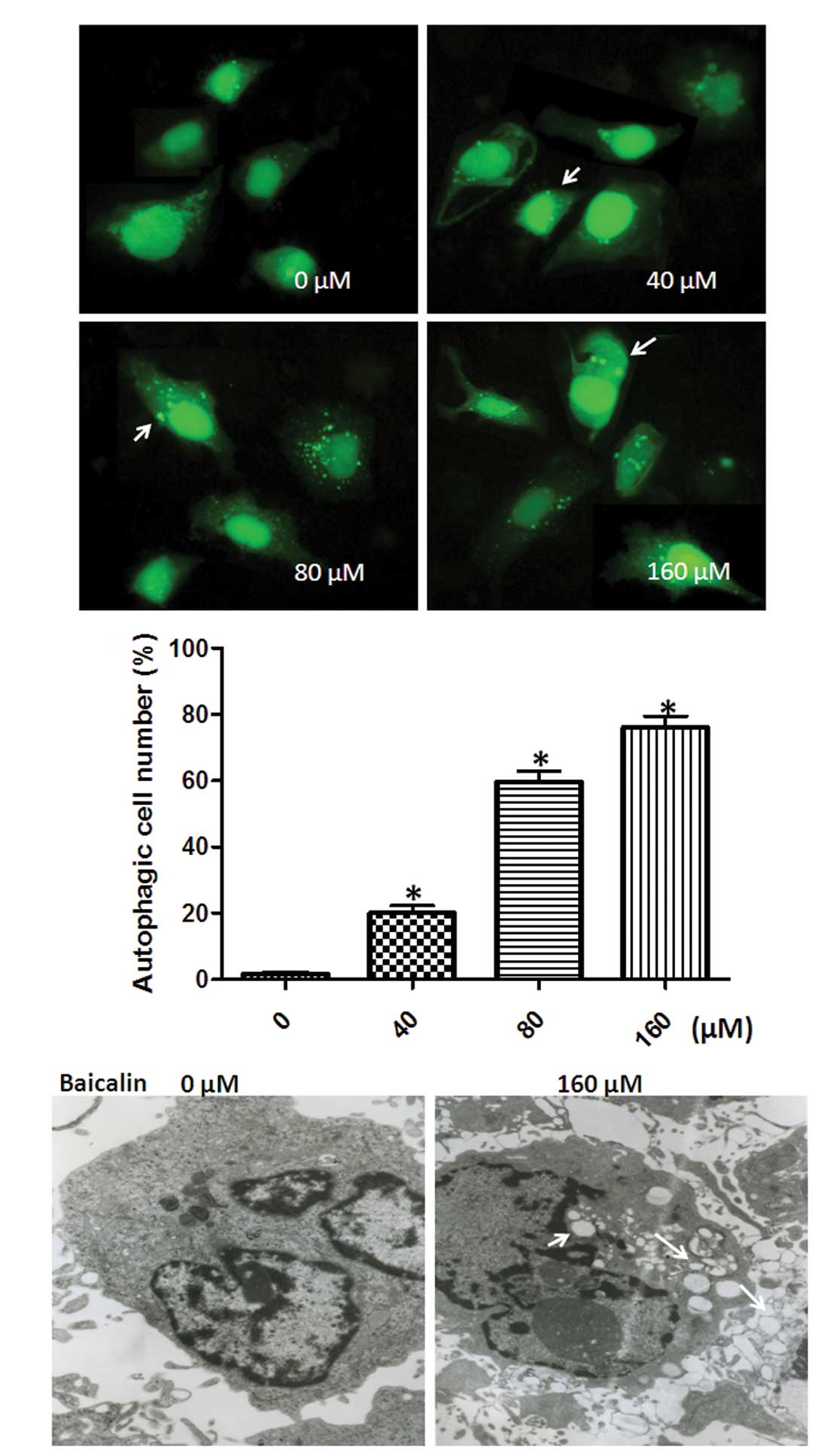
To determine whether baicalin could induce
autophagy, the SMMC-7721 cells were transfected with a plasmid
expressing an pEGFP-LC3 fusion protein and then exposed to
different concentrations of baicalin (40, 80 or 160 μM) for 48 h.
The formation of EGFP-LC3 punctate dots is a widely used marker for
autophagy, which can be easily monitored by a fluorescence
microscope (32). As expected, in
normal condition without baicalin treatment, EGFP-LC3 fluorescence
was largely diffused throughout the cytoplasm with few dots
denoting basal autophagosome formation. However, the number of
EGFP-LC3 dots rapidly increased within 24-h exposure to baicalin
(40, 80 or 160 μM), indicating that autophagy was induced. The
percentage of autophagic cells with more than three GFP-LC3 puncta
(GFP-LC3 positive) was 20.4% at 40 μM, 61.2% at 80 μM, and 79.7% at
160 μM baicalin, respectively, indicating that autophagy was
induced by baicalin in a dose-dependent manner (Fig. 2A and B).
To further confirm the effect of baicalin on
autophagy, we evaluated the level of baicalin-induced autophagy in
SMMC-7721 cells using a TEM method, which is currently the standard
method for monitoring autophagy. As shown in Fig. 2C, autophagic vacuoles (white arrows)
were observed in SMMC-7721 cells treated with 160 μM baicalin. The
results suggest that a significantly higher level of autophagy
occurred in SMMC-7721 cells treated with 160 μM baicalin compared
with that in the control (0 μM baicalin). Moreover, we observed
that cell nuclei had collapsed and disintegrated in cancer cells
treated with baicalin (white arrowheads) (Fig. 2C), which indicated that baicalin
also induced apoptosis and was in line with results of previous
studies (33,34). Taken together, all these results
clearly demonstrated that baicalin induced autophagy as well as
apoptosis in SMMC-7721 cells.
Baicalin upregulates autophagy-related
protein Beclin 1 expression
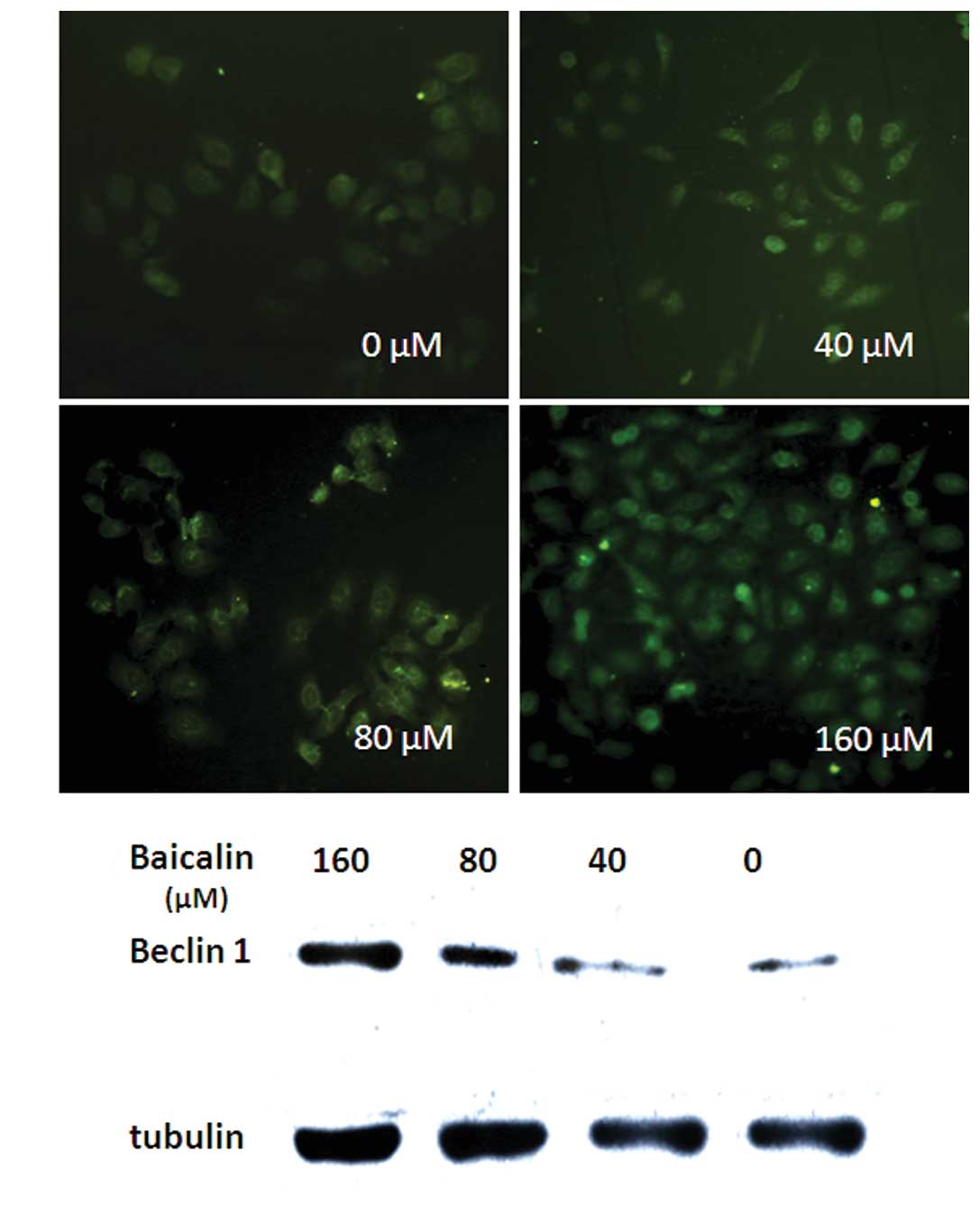
To investigate the mechanisms by which baicalin
induces autophagy, we detected the protein levels of Beclin 1, the
key regulator of autophagy which has already been shown to be
essential for the occurrence of autophagy (30). Following the treatment on SMMC-7721
cells with different concentrations of baicalin (40, 80 or 160 μM)
for 48 h, we performed the immunofluorescence staining and Western
blot analysis to evaluate the expression level of Beclin 1.
Immunofluorescence staining showed that Beclin 1 abundance was
remarkably higher in SMMC-7721 cells treated with baicalin compared
to control in a dose-dependent manner (Fig. 3A). A similar result was obtained by
Western blot analysis (Fig. 3B).
These findings suggest that autophagy induced by baicalin in
SMMC-7721 cells is, at least in part, Beclin 1-dependent.
Baicalin induces both autophagic cell
death and apoptosis
Autophagy in cancer cells has been demonstrated to
play a dual role in cell survival and cell death (15). To further determine the functional
role of autophagy induced by baicalin in cancer cell survival or
death and the relationship between autophagy and apoptosis, we
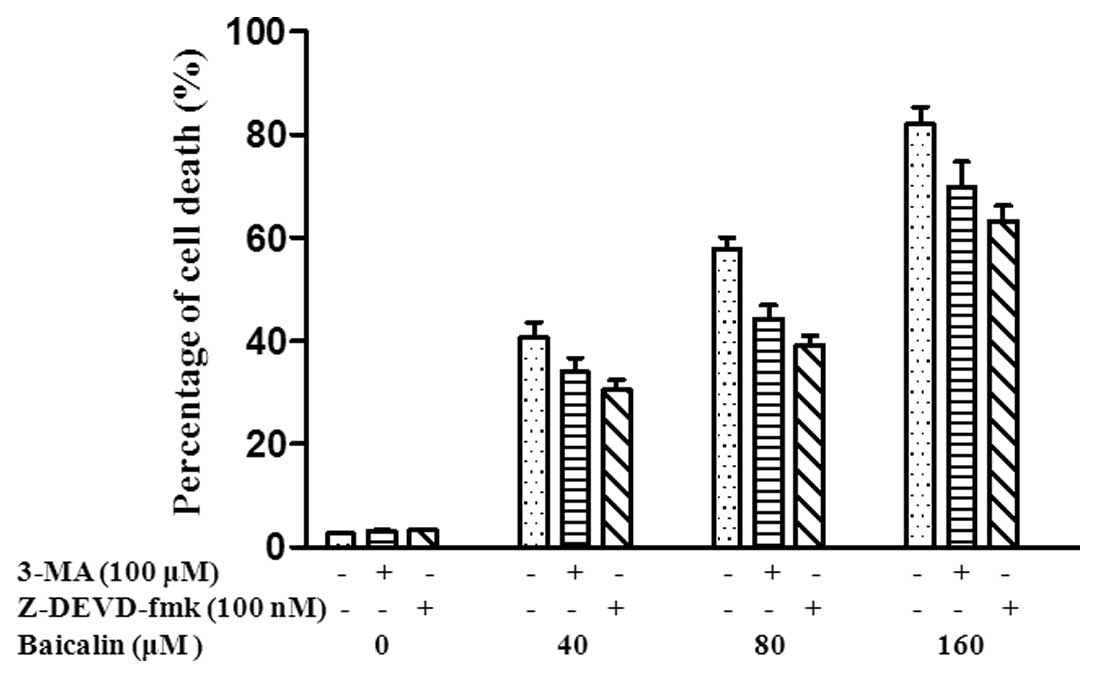
investigated baicalin-induced cell death in SMMC-7721 cells using a
Trypan blue exclusion assay. 3-Methyladenine (3-MA), a known
inhibitor of autophagy, was used to inhibit autophagy and prevent
autophagic cell death induced alone (35). z-DEVD-fmk, a caspase-3-specific
inhibitor of apoptosis, inhibits only apoptosis with no other types
of cell death (36). SMMC-7721
cells were treated with 3-MA (100 μM), z-DEVD-fmk (100 nM) or 3-MA
(0 μM) + z-DEVD-fmk (0 nM), and then cells were treated with 40, 80
and 160 μM baicalin. The percentage of cell death was 33.9, 30.5
and 40.6%, respectively in SMMC-7721 cells treated with 40 μM
baicalin. The percentage of cell death was 44.3, 39.1 and 57.7%,
respectively in SMMC-7721 cells treated with 80 μM baicalin and
69.9, 63.3 and 82.1%, respectively in SMMC-7721 cells treated with
160 μM baicalin (Fig. 4).
Collectively, our results demonstrate that cell death is inhibited
by both 3-MA and z-DEVD-fmk, respectively, which further suggests
that baicalin-induced cell death including both autophagic cell
death and apoptosis.
Identification of cell death-related
genes that regulate autophagy and apoptosis
Recent studies have uncovered significant
interactions between autophagic and apoptotic signaling pathways
(37,38). It has been reported that autophagy
and apoptosis may be linked to each other and occur simultaneously
or sequentially in cancer cells (39). To better understand the relationship
between autophagy and apoptosis in SMMC-7721 cells treated with
baicalin, we investigated whether some known cell death genes
related both to autophagy and apoptosis, were involved in
baicalin-induced cell death. Previous studies have suggested that
CD147 inhibited apoptosis (21) and
starvation-induced autophagic cell death (26), and was related with cell cycle
arrest in SMMC-7721 cells (32). We
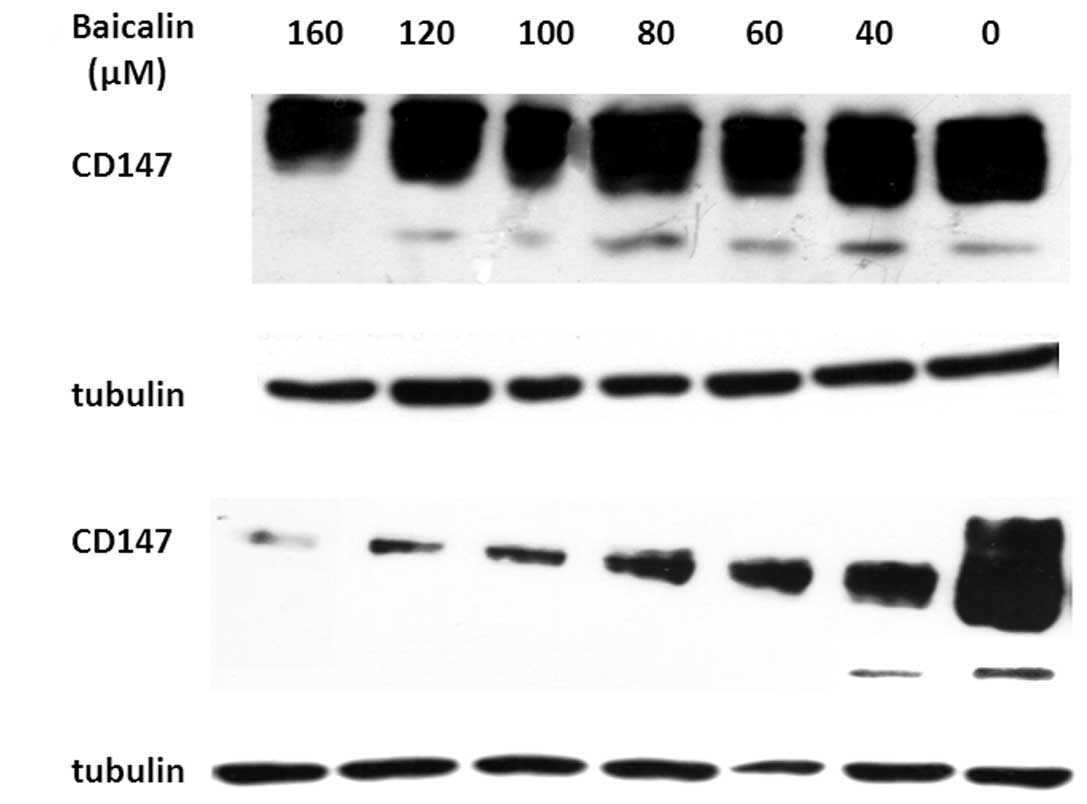
thus investigated CD147 expression levels in SMMC-7721 cells
treated with different concentrations of baicalin (40, 60, 80, 100,
120 or 160 μM). Western blotting results showed that CD147
expression level was significantly downregulated in SMMC-7721 cells
treated with different concentrations of baicalin (40, 60, 80, 100,
120 and 160 μM) for 24 h (Fig. 5A)
and 48 h (Fig. 5B) compared to the
controls (cells without baicalin treatment) in a dose-dependent
manner at protein levels. The data suggest that downregulation of
CD147 expression may be involved in both baicalin-induced autophagy
and apoptosis.
Discussion
Currently, chemotherapy using plant-derived
anticancer drugs such as paclitaxel (40), vinorelbine (41), or vincristine (42) has been proven to be effective and
safe in many clinical settings. It was shown that these products
enhance cell growth inhibition, induce apoptosis or cell cycle
arrest in several cancer cell lines and are highly effective and
safe in clinical trials. Noticeably, about 75% of plant-derived
drugs used today in the clinic originate from traditional medicines
(43). Our previous result showed
that berberine isolated from the Chinese herb Scutellaria
baicalensis (SB) Georgi coule induced both apoptosis and
autophagy in the HCC lines HepG2 and SMMC-7721 (17).
In the present study, we investigated the anticancer
effects of baicalin, another ingredient isolated from SB, on HCC
SMMC-7721 cells in vitro. Our results showed that baicalin
exerted a significant antiproliferative effect on SMMC-7721 cells,
in line with previous studies which demonstrated the
antiproliferative effect of baicalin in other cancer cell lines
(PC-3, DU145, LNCaP, MCF-7, HL-60, and NB4) (33,34,44,45).
Our next attempt was to find out the underlying
mechanisms of baicalin on SMMC-7721 cell proliferation inhibition.
Previous studies indicated that baicalin induced apoptosis and cell
cycle arrest in many types of cancers which contributed to cell
growth inhibition (33,34,44,45).
However, whether baicalin induced other types of cell death such as
autophagic cell death in cancer cells remains unknown. The present
study is the first to provide compelling experimental evidence that
baicalin induced autophagy in SMMC-7721 cells. This finding sheds
new light on the pharmacological function of baicalin. Cancer is
one of the first diseases genetically linked to autophagy
malfunction (8,46). The ATG gene Beclin 1, as an
important autophagy-associated signaling molecule, is
mono-allelically deleted in 40–75% of human malignancies such as
liver, lung, breast, ovarian, and prostate cancer (14). The restoration of Beclin 1
expression inhibits tumor cell proliferation and tumor growth
(47). Moreover, upregulation of
Beclin 1 expression mediates anticancer agent-induced autophagy
(48). In the present study, we
demonstrated that the expression of Beclin 1 was upregulated by
baicalin in a dose-dependent fashion and positively associated with
the level of autophagy, indicating that upregulation of Beclin 1
expression may be one of the major mechanisms of baicalin-induced
autophagy. The detailed mechanisms underlying the
inter-relationships between autophagy, cell survival and cell death
are largely unknown. Many studies showed that autophagy
augmentation may be effective in preventing tumour formation and
progression, whereas autophagy inhibition may be helpful in
promoting tumour regression (8,46).
Currently, many studies have summarized the essential connections
between defects in autophagy regulation or execution and cancer,
and suggested that autophagy was a true tumour suppressor pathway
(49). Several chemotherapy agents
such as berberine (16,17) and arsenic trioxide (18) have been demonstrated to induce
autophagic cell death in cancer cells and thus exert anticancer
activity. Therefore, in the present study we determined the
functional role of autophagy induced by baicalin in SMMC-7721 cell
survival or death. We further investigated whether baicalin could
induce autophagic death in SMMC-7721 cells, defining a novel role
of baicalin as an antitumor agent. Our results indicated that
alongside apoptosis, baicalin also induced autophagic cell death in
SMMC-7721 cells. These results suggest that induction of autophagy,
as well as apoptosis, by baicalin may represent a novel mechanism
by which baicalin inhibits tumor cell growth and modulates tumor
progression.
Apoptosis (Type I) and autophagic cell death (Type
II) are the two types of programmed cell death, whereas the
boundary between Type I and II has never been completely clear and
perhaps does not exist, and different types overlap. Autophagy and
apoptosis have been reported to be linked to each other and occur
simultaneously or sequentially in cancer cells (39). Recently, several laboratories have
reported that molecules previously defined as intermediaries in the
activation of apoptosis also function as intermediaries in the
activation of autophagy (37,50),
suggesting these molecules may be involved in the modulation of
both apoptosis and autophagy simultaneously. Targeting these
molecules can be more effective than to target molecules modulating
apoptosis or autophagy alone in cancer treatment. Thus,
characterization of molecules that involve in the modulation of
both apoptosis and autophagy has been actively pursued (51).
CD147 is the most commonly highly expressed gene in
human cancers, mainly functions as a cellular adhesion molecule
inducing the secretion of matrix metalloproteinases (MMPs)
(19). Increased MMP expression was
associated with tumor invasion and metastasis (19). Aside from stimulating MMPs
production, CD147 also stimulates the production of vascular
endothelial growth factor (VEGF) which serves as a major regulator
of the angiogenic process in tumor formation (52). It has been reported that CD147 was
associated with the cell cycle, inhibited cancer cell apoptosis
(21) and anoikis (22). Recent studies have further addressed
that CD147 inhibited autophagy in SMMC-7721 cells with an
involvement of Beclin 1 downregulation (26). These findings open the possibility
that CD147 may be an important modulator involved in both apoptosis
and autophagy. Given the fact observed in our present study that
cell death induced by baicalin in SMMC-7721 cells included both
apoptosis and autophagy. We evaluated the difference of the CD147
expression levels in SMMC-7721 cells treated with different
concentrations of baicalin. As expected, the results indicated that
CD147 expression levels were markedly downregulated by baicalin
accompanied with the occurrence of apoptosis and autophagy. Based
on these results, it is reasonable to hypothesize that
baicalin-induced both apoptosis and autophagy in SMMC-7721 cells,
at least in part, by downregulating CD147 expression and CD147 may
serve as an important modulator of cross-talk between apoptosis and
autophagy. However, further experiments are needed to verify the
mechanisms by which baicalin regulates the expression of the CD147
and induce the cell death including apoptosis and autophagy.
In conclusion, our results show for the first time
that baicalin induces autophagic cell death as well as apoptosis in
SMMC-7721 cells, at least in part, through a pathway involving
downregulation of CD147. These results suggest a novel mechanism
underlying the pharmacological effects of baicalin and provide new
insights into the function of CD147 during tumor progression.
Acknowledgements
The authors thank Professor Xingchun Gou (Institute
of Basic Medicine, Xi’an Medical University, Xi’an, China) for
valuable comments and providing pEGFP-LC3.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Kassahun WT, Fangmann J, Harms J, Hauss J
and Bartels M: Liver resection and transplantation in the
management of hepatocellular carcinoma: a review. Exp Clin
Transplant. 4:549–558. 2006.PubMed/NCBI
|
|
3
|
Wang N, Tang LJ, Zhu GQ, et al: Apoptosis
induced by baicalin involving up-regulation of P53 and bax in MCF-7
cells. J Asian Nat Prod Res. 10:1129–1135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li-Weber M: New therapeutic aspects of
flavones: the anticancer properties of Scutellaria and its
main active constituents Wogonin, Baicalein and Baicalin. Cancer
Treat Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ikemoto S, Sugimura K, Yoshida N, et al:
Antitumor effects of Scutellariae radix and its components
baicalein, baicalin, and wogonin on bladder cancer cell lines.
Urology. 55:951–955. 2000.
|
|
6
|
Goh D, Lee YH and Ong ES: Inhibitory
effects of a chemically standardized extract from Scutellaria
barbata in human colon cancer cell lines, LoVo. J Agric Food
Chem. 53:8197–8204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Y and Yao S: Effect of baicalin on
invasion and metastasis of human hepatocellular line BEL-7402 in
vitro. Acta Academiae Medicinae Militaris Tertiae. 6:332006.
|
|
8
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar
|
|
9
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Y and Klionsky DJ: Physiological
functions of Atg6/Beclin 1: a unique autophagy-related protein.
Cell Res. 17:839–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krustev LP: Cell autophagy of the liver in
starvation and undernutrition. Bibl Nutr Dieta. 23:145–154.
1976.PubMed/NCBI
|
|
12
|
Qu X, Zou Z, Sun Q, et al: Autophagy
gene-dependent clearance of apoptotic cells during embryonic
development. Cell. 128:931–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takagi H, Matsui Y and Sadoshima J: The
role of autophagy in mediating cell survival and death during
ischemia and reperfusion in the heart. Antioxid Redox Signal.
9:1373–1382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dalby KN, Tekedereli I, Lopez-Berestein G
and Ozpolat B: Targeting the prodeath and prosurvival functions of
autophagy as novel therapeutic strategies in cancer. Autophagy.
6:322–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang N, Feng Y, Zhu M, et al: Berberine
induces autophagic cell death and mitochondrial apoptosis in liver
cancer cells: the cellular mechanism. J Cell Biochem.
111:1426–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou Q, Tang X, Liu H, et al: Berberine
induces cell death in human hepatoma cells in vitro by
downregulating CD147. Cancer Sci. 102:1287–1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanzawa T, Zhang L, Xiao L, Germano IM,
Kondo Y and Kondo S: Arsenic trioxide induces autophagic cell death
in malignant glioma cells by upregulation of mitochondrial cell
death protein BNIP3. Oncogene. 24:980–991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Xu HY, Zhang Q, et al: HAb18G/CD147
functions in invasion and metastasis of hepatocellular carcinoma.
Mol Cancer Res. 5:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Zhou J, Ku XM, et al: Expression
of CD147 as a significantly unfavorable prognostic factor in
hepatocellular carcinoma. Eur J Cancer Prev. 16:196–202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuang YH, Chen X, Su J, et al: RNA
interference targeting the CD147 induces apoptosis of multi-drug
resistant cancer cells related to XIAP depletion. Cancer Lett.
276:189–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang JM, O’Neill P, Jin W, et al:
Extracellular matrix metalloproteinase inducer (CD147) confers
resistance of breast cancer cells to Anoikis through inhibition of
Bim. J Biol Chem. 281:9719–9727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai J, Dou K, Wang C, et al: The
interaction of HAb18G/CD147 with integrin α6β1 and its implications
for the invasion potential of human hepatoma cells. BMC Cancer.
9:3372009.
|
|
24
|
Tang J, Wu YM, Zhao P, Yang XM, Jiang JL
and Chen ZN: Overexpression of HAb18G/CD147 promotes invasion and
metastasis via α3β1 integrin mediated FAK-paxillin and FAK-PI3K-Ca
2+ pathways. Cell Mol Life Sci. 65:2933–2942.
2008.PubMed/NCBI
|
|
25
|
Chen Y, Zhang H, Gou X, Horikawa Y, Xing J
and Chen Z: Upregulation of HAb18G/CD147 in activated human
umbilical vein endothelial cells enhances the angiogenesis. Cancer
Lett. 278:113–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gou X, Ru Q, Zhang H, et al: HAb18G/CD147
inhibits starvation-induced autophagy in human hepatoma cell
SMMC7721 with an involvement of Beclin 1 down-regulation. Cancer
Sci. 100:837–843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan SL, Wei YQ, Wang XJ, Xiao F, Li SF
and Zhang J: Growth inhibition and apoptosis induction of
tanshinone II-A on human hepatocellular carcinoma cells. World J
Gastroenterol. 10:2024–2028. 2004.PubMed/NCBI
|
|
28
|
Kabeya Y, Mizushima N, Ueno T, et al: LC3,
a mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu X, Yu J, Bhagat G, et al: Promotion of
tumorigenesis by heterozygous disruption of the beclin 1 autophagy
gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang XH, Yu J, Brown K and Levine B:
Beclin 1 contains a leucine-rich nuclear export signal that is
required for its autophagy and tumor suppressor function. Cancer
Res. 61:3443–3449. 2001.PubMed/NCBI
|
|
31
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol Appendix 3: Appendix 3B. 2001.
View Article : Google Scholar
|
|
32
|
Balgi AD, Fonseca BD, Donohue E, et al:
Screen for chemical modulators of autophagy reveals novel
therapeutic inhibitors of mTORC1 signaling. PLoS One. 4:e71242009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikezoe T, Chen SS, Heber D, Taguchi H and
Koeffler HP: Baicalin is a major component of PC-SPES which
inhibits the proliferation of human cancer cells via apoptosis and
cell cycle arrest. Prostate. 49:285–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan FL, Choi HL, Chen ZY, Chan PSF and
Huang Y: Induction of apoptosis in prostate cancer cell lines by a
flavonoid, baicalin. Cancer Lett. 160:219–228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Knoblach SM, Alroy DA, Nikolaeva M, Cernak
I, Stoica BA and Faden AI: Caspase inhibitor z-DEVD-fmk attenuates
calpain and necrotic cell death in vitro and after traumatic brain
injury. J Cereb Blood Flow Metab. 24:1119–1132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pattingre S, Tassa A, Qu X, et al: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yousefi S, Perozzo R, Schmid I, et al:
Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis.
Nat Cell Biol. 8:1124–1132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
Role of autophagy in cancer development and response to therapy.
Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gonzalez-Angulo AM, Walters RS, Carpenter
JRJ, et al: Paclitaxel chemotherapy in a pregnant patient with
bilateral breast cancer. Clin Breast Cancer. 5:317–319. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Romero A, Rabinovich MG, Vallejo CT, et
al: Vinorelbine as first-line chemotherapy for metastatic breast
carcinoma. J Clin Oncol. 12:336–341. 1994.PubMed/NCBI
|
|
42
|
Holland JF, Scharlau C, Gailani S, et al:
Vincristine treatment of advanced cancer: a cooperative study of
392 cases. Cancer Res. 33:1258–1264. 1973.PubMed/NCBI
|
|
43
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007.PubMed/NCBI
|
|
44
|
Franek KJ, Zengtong Z, Zhang WD and Chen
WY: In vitro studies of baicalin alone or in combination
with Salvia miltiorrhiza extract as a potential anti-cancer
agent. Int J Oncol. 26:217–224. 2005.
|
|
45
|
Xu XF, Cai BL, Guan SM, et al: Baicalin
induces human mucoepidermoid carcinoma Mc3 cells apoptosis in vitro
and in vivo. Invest New Drugs. 29:637–645. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liang XH, Jackson S, Seaman M, et al:
Induction of autophagy and inhibition of tumorigenesis by beclin 1.
Nature. 402:672–675. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li DD, Wang LL, Deng R, et al: The pivotal
role of c-Jun NH2-terminal kinase-mediated Beclin 1
expression during anticancer agents-induced autophagy in cancer
cells. Oncogene. 28:886–898. 2008.
|
|
49
|
Takahashi Y, Coppola D, Matsushita N, et
al: Bif-1 interacts with Beclin 1 through UVRAG and regulates
autophagy and tumorigenesis. Nat Cell Biol. 9:1142–1151. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pattingre S and Levine B: Bcl-2 inhibition
of autophagy: a new route to cancer? Cancer Res. 66:2885–2828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun PH, Zhu LM, Qiao MM, et al: The XAF1
tumor suppressor induces autophagic cell death via upregulation of
Beclin-1 and inhibition of Akt pathway. Cancer Lett. 28:170–180.
2011.PubMed/NCBI
|
|
52
|
Tang Y, Nakada MT, Kesavan P, et al:
Extracellular matrix metalloproteinase inducer stimulates tumor
angiogenesis by elevating vascular endothelial cell growth factor
and matrix metalloproteinases. Cancer Res. 65:3193–3199. 2005.
|