Introduction
Lung cancer remains the deadliest cancer worldwide
despite improvements in diagnostic and therapeutic techniques
(1). The reported incidences of
lung cancer have yet to peak in many parts of world, particularly
in China. The prognosis for lung cancer patients is generally poor,
with an overall 5-year survival rate of approximately 10–15%, and
it has shown little improvement in recent decades (2,3).
Several independent prognostic factors for survival have been
identified: performance status, disease stage, age, gender and the
amount of weight loss (4). However,
the discriminate value of most potential prognostic biologic
markers is insufficient to predict the optimal therapeutic course
for an individual. Thus, it is important to identify biological
markers with predictive values for the survival of patients
undergoing treatment.
Caveolin-1 (cav-1), a major structural component of
specialized plasma membrane invaginations (caveolae), was first
cloned in 1992 by Rothberg et al (5). There has been significant interest in
understanding the structure and function of cav-1. The function of
cav-1 in tumorigenesis remains controversial. In several in
vitro studies, cav-1 was down-regulated in tumor cells isolated
from the breast (6,7), cervix (8), lung (9,10) and
ovary (11), and oncogenic
transformation of cells was associated with a reduction in cav-1
expression (12,13). This evidence indicates that cav-1
may act as a tumor suppressor. Conversely, cav-1 is consistently
up-regulated in bladder cancer (14), esophageal cancer (15) and prostate carcinomas (16), and this up-regulation has been
associated with metastases and poor prognosis in prostate carcinoma
and esophageal squamous cell carcinomas (SCC) (17,18).
These results suggest that cav-1 can function as an oncogene rather
than a tumor suppressor. Taking into consideration these
conflicting in vitro and clinical results, we postulate that
the functions of cav-1 may have different roles in various cancer
cell types.
In primary lung cancer, only a few studies on cav-1
expression have been reported (19–24).
In these studies, only immunohistochemical staining (IHC) was used
to detect cav-1 expression. In SCC of the lung (20), the expression of cav-1 has been
significantly correlated with the advanced pathological stage and
poor prognosis. In a study by Kato, et al (22), cav-1 was found to serve as a tumor
suppressor in lung adenocarcinoma (AC), with the loss of cav-1
regulation resulting in tumor extension and a lack of
differentiation. In lung SCC, cav-1 overexpression may be
correlated with tumor extension. However, the cav-1 mRNA/protein
expression levels in lung tumor tissues (TT) compared to
surrounding normal tissues and the association with
clinicopathological factors in non-small cell lung cancer (NSCLC)
is unknown.
In the present study, we have examined the cav-1
mRNA expression in 136 lung TT and matched tumor-free tissues (TF)
using real-time PCR analysis and the protein expression in 20
paired lung TT and TF by Western blot analysis. In addition, the
protein expression in another set of 115 paraffin-embedded blocks
of NSCLC and 19 non-cancerous lung tissues was detected by IHC. The
clinical significance of the cav-1 mRNA and protein expression
levels detected by IHC with respect to the prognostic value of
NSCLC patients is clarified.
Materials and methods
Patients and tissue specimens
During the period from August 2007 to May 2009, 136
lung cancer patients who received surgery at the Department of
Thoracic Surgery of Nanjing Chest Hospital and the Jinling Hospital
affiliated to the Nanjing University School of Medicine, were
included in this study. TT and matched TF >5 cm distal from the
tumor edge were immediately snap-frozen in liquid nitrogen and then
stored at −80°C until use (protein and RNA isolation). Necrotic or
hemorrhagic tissues were excluded. In addition, a set of 115
paraffin-embedded lung cancers and 19 non-cancer specimens between
2001 and 2003 were collected from the Nanjing Chest Hospital and
the 81 Hospital of PLA. The 19 non-cancerous specimens included 5
pulmonary tuberculosis cases, 4 bronchiectasis cases, 6 lung bullae
cases and 3 inflammatory pseudotumor cases. None of the patients
received any chemotherapy or radiotherapy before surgery.
Clinicopathological data were obtained by medical records in the
archives room. The data included patient age, gender, smoking
condition, tumor site, lymph nodal status and pathological stage.
The postoperative pathological staging was determined according to
the 7th edition of the TNM classification (25). Histological type was determined
according to the classification by the World Health Organization.
Inclusion criteria for this study were surgical complete resection
of the tumor (resection margin microscopically free of tumor
cells); patient survival for more than 3 months after surgery; and
the patient did not die of causes other than lung cancer within 5
years following surgery. Follow-up information was obtained by
phone investigations. The median follow-up of surviving patients at
the time of analysis was 22 months (range, 3–81 months). The date
of the last follow-up was March 21, 2009. The Research Ethics
Committee of the Jinling Hospital affiliated to the Nanjing
University School of Medicine approved this protocol and informed
consent was obtained from all participants.
RNA extraction and cDNA synthesis
Total RNA was isolated from frozen tissue with
TRIzol (Invitrogen) using the manufacturer’s protocol. With random
hexamer primers, 2 μg of RNA was reverse transcribed to cDNA using
the PrimeScript™ first-strand cDNA synthesis kit (Takara),
according to the manufacturer’s protocol.
Real-time PCR
Primers for cav-1 and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) were designed and synthesized by Sangon
Biotech Co., Ltd. The primers were as follows: cav-1, forward,
TTCGCCATTCTCTCTTTCCT, and reverse, CAGCTTCAAAGAGTGGGTCA; GAPDH;
forward, GCACCGTCAAGGCTGAGAAC, and reverse, TGG TGAAGACGCCAGTGGA.
Real-time PCR was performed in triplicate for each sample in a
20-μl reaction mixture, which consisted of template DNA (1 μl) and
primers (0.2 μM), the ROX Reference Dye II (1X), dH2O
(9.0 μl) and SYBR® Premix Ex Taq (1X, SYBR Premix Ex Taq
kit, Takara). PCR was performed on an ABI 7500 real-time PCR system
using the following thermal settings: 1 cycle of 30 sec at 95°C, 45
cycles of 5 sec at 95°C and 34 sec at 60°C. The relative expression
ratio (RR) of the cav-1 gene was calculated based on the Ct
comparative method with the reference gene (GAPDH) in the
sample.
Western blot analysis
For protein analysis, samples were homogenized in
lysis buffer (0.1% SDS, 50 mM Tris-HCl, pH 7.5, 1% NP-40, 150 mM
NaCl, 1 mM Triton X-100, 1 mM EDTA) containing complete protease
inhibitor (PMSF + P8340). Protein concentrations were measured by
the BCA protein assay (Sigma). A total of 100 μg of protein was
separated by 10–15% SDS-PAGE. The protein was then transferred to a
nitrocellulose-membrane and these were saturated by incubating for
2 h with 5% non-fat dry milk in PBS/0.1% Tween-20 at 37°C. The
membranes were incubated with the Mouse IgG monoclonal cav-1
antibody (BD Transduction Laboratories) overnight at 4°C. After 3
washes (5 min each) with PBS/0.1% Tween-20, membranes were
incubated with the anti-rabbit immunoglobulin coupled to peroxidase
(Abcam) at 37°C. After 1 h incubation, the membranes were washed 4
times (5 min each) with PBS/0.1% Tween-20 and the blots were
developed using a chemiluminescence procedure (Amersham,
Bioscence). The polyclonal anti-β-actin antibody served as the
control.
Immunohistochemical staining
Resected specimens were fixed in 10% formalin and
paraffin-embedded blocks were prepared. Five millimeter sections
were cut from the specimens and placed on slides coated with
poly-L-lysine. IHC was performed using the EnVision two-step
immunohistochemical method (EnVision Detection kit, Peroxidase/DAB,
Rabbit/Mouse, Dako, Denmark), according to the manufacturer’s
instructions. In brief, sections were routinely deparaffinized with
xylene and rehydrated in decreasing concentrations of alcohol.
Antigen retrieval was performed by placing the specimen in the EDTA
retrieval agent at pH 8.0 and autoclaved at 12°C for 2 min to allow
fixing. The sections were washed in phosphate-buffered saline (PBS)
buffer (pH 7.6) and the sections were incubated overnight at 4°C in
a moist chamber with the rabbit anti-human cav-1 polyclonal
antibody (1:400, N-20: sc-894, Santa Cruz Biotechnology, Santa
Cruz, USA). After washing the sections in PBS 3 times for 5 min the
sections were treated for 30 min at room temperature in ChemMate
EnVision+/HRP (Dako, Denmark). Subsequently, the sections were
washed with PBS and diaminobenzidine (DAB) coloration was applied,
followed by the application of a DAB solution (ChemMate
EnVision+/DAB) until the color developed. Staining was monitored
under bright-field microscopy and the reaction was stopped by
washing with distilled water. The sections were then counterstained
with hematoxylin, dehydrated in increasing concentrations of
alcohol and coverslipped with neutral Gummi.
The slides were independently reviewed by 2 of the
authors (P.Z. and X.-K.S.) who had no knowledge of the patients
clinicopathological status. If discrepancies existed between the 2
reviewers, a consensus judgment was reached through discussion. The
proportion of staining tumor cells in each selected field was
determined by counting individual tumor cells at 4 randomly
selected high magnification (x400) fields using light microscopy
(Model CX31RTSF, Olympus, Tokyo, Japan). The immunoreactivities
were graded as (−), (+), (++) and (+++) according to the percentage
of positive tumor cells identified: (−) represents 0 or <5%
tumor cells; (+) represents 5–25% tumor cells; (++) represents
25–50% tumor cells; and (+++) represents the strongest staining
with >50% tumor cells present. The immunoreactivity of cav-1 was
normally localized to fibroblasts, type I pneumocytes and
endothelial cells of blood vessels in all tissue specimens
examined, which served as an internal quality control in the
immunohistochemistry analysis. The same above protocol without the
primary antibody was used as a negative control. High expression of
cav-1 was artificially defined if ≥50% of the tumor cells (+++)
showed granular staining at the cell membrane and in the
cytoplasm.
Statistical analysis
The difference in the level of expression of cav-1
mRNA and protein in TT and paired TF specimens was performed using
the paired t-test. Associations between cav-1 protein expression
and the clinicopathological characteristics were analyzed using the
Mann-Whitney U-test. Overall survival (OS) was calculated from the
day of surgery to the date of the last follow-up or the date of
death. Survival at the last follow-up date was censored. The
postoperative survival curves were calculated using the
Kaplan-Meier method and differences in the survival rates were
analyzed using the log-rank test. All statistical procedures were
performed using SPSS (Version 16.0 SPSS Inc., Chicago). Values of
P<0.05 were considered as statistically significant.
Results
Characteristics of the NSCLC cases
The 136 patients consisted of 100 males and 36
females, aged 17–80-years-old (mean 59.7-years-old). According to
the classification of the World Health Organization (WHO), the
specimens were classified into 74 (54.4%) AC, 44 (32.4%) SCC, and
18 (13.2%) others (large cell carcinomas, adenosquamous carcinomas
and carcinoid). The average tumor size was 4.42±1.35 cm (range: 1–9
cm). Thirty-five cases involved tumors ≤3 cm in size, whereas 101
cases involved tumors >3 cm in size. There were 61 negative
cases and 75 positive cases for lymph node metastases. The patient
characteristics are presented in Tables
I and II.
 | Table IClinicopathological factors of lung
cancer and the association with caveolin-1 mRNA expression in 136
NSCLC patients. |
Table I
Clinicopathological factors of lung
cancer and the association with caveolin-1 mRNA expression in 136
NSCLC patients.
| | Cav-1 mRNA
expression |
|---|
| |
|
|---|
| n (%) | Low | High | P-value |
|---|
| Number of
patients | 136 | 104 | 32 | |
| Gender | | | | 0.656 |
| Male | 100 (73.5) | 75 | 25 | |
| Female | 36 (26.5) | 29 | 7 | |
| Age | | | | 0.194 |
| <60 years | 65 (47.8) | 46 | 19 | |
| ≥60 years | 71 (52.2) | 58 | 13 | |
| Size of tumor | | | | 0.558 |
| ≤3 cm | 35 (25.7) | 25 | 10 | |
| >3 cm | 101 (74.3) | 79 | 22 | |
| Histology | | | | 0.041a |
| Squamous cell
carcinoma | 44 (32.4) | 38 | 6 | |
|
Adenocarcinoma | 74 (54.4) | 52 | 22 | |
| Other | 18 (13.2) | 14 | 4 | |
| Lymph node
metastasis (pN) | | | | 0.062 |
| N0 | 61 (44.9) | 51 | 10 | |
| N1–3 | 75 (55.1) | 53 | 22 | |
| p-TNM stages | | | | 0.076 |
| I | 54 (39.7) | 44 | 10 | |
| II | 27 (19.9) | 19 | 8 | |
| III | 52 (38.2) | 38 | 14 | |
| IV | 3 (2.2) | 3 | 0 | |
 | Table IIClinicopathological characteristics
of lung cancer and the association with caveolin-1 protein
expression in 115 NSCLC patients. |
Table II
Clinicopathological characteristics
of lung cancer and the association with caveolin-1 protein
expression in 115 NSCLC patients.
| | Caveolin-1 protein
expression |
|---|
| |
|
|---|
| n (%) | Low | High | P-value |
|---|
| Number of
patients | 115 (100) | 55 | 60 | |
| Gender | | | | 0.865 |
| Male | 87 (75.7) | 42 | 45 | |
| Female | 28 (24.3) | 13 | 15 | |
| Age | | | | 0.452 |
| <60 years | 44 (38.3) | 23 | 21 | |
| ≥60 years | 71 (61.7) | 32 | 39 | |
| Smoking | | | | 0.187 |
| Non-smoker | 47 (40.9) | 19 | 28 | |
| Smoker | 68 (59.1) | 36 | 32 | |
| Size of tumor | | | | 0.124 |
| ≤3 cm | 32 (27.8) | 19 | 13 | |
| >3 cm | 83 (72.2) | 36 | 47 | |
| Histology | | | | 0.596 |
| Squamous cell
carcinoma | 40 (34.8) | 17 | 23 | |
|
Adenocarcinoma | 63 (54.8) | 31 | 32 | |
| Other | 12 (10.4) | 7 | 5 | |
| N-stage | | | | 0.673 |
| N0 | 42 (36.5) | 19 | 23 | |
| N1 + N2 | 73 (63.5) | 36 | 37 | |
| p-TNM stages | | | | 0.432 |
| I | 36 (31.3) | 15 | 21 | |
| II | 27 (23.5) | 11 | 16 | |
| III | 41 (35.7) | 22 | 19 | |
| IV | 11 (9.6) | 7 | 4 | |
Expression of caveolin-1 mRNA and protein
in NSCLC paired tissues
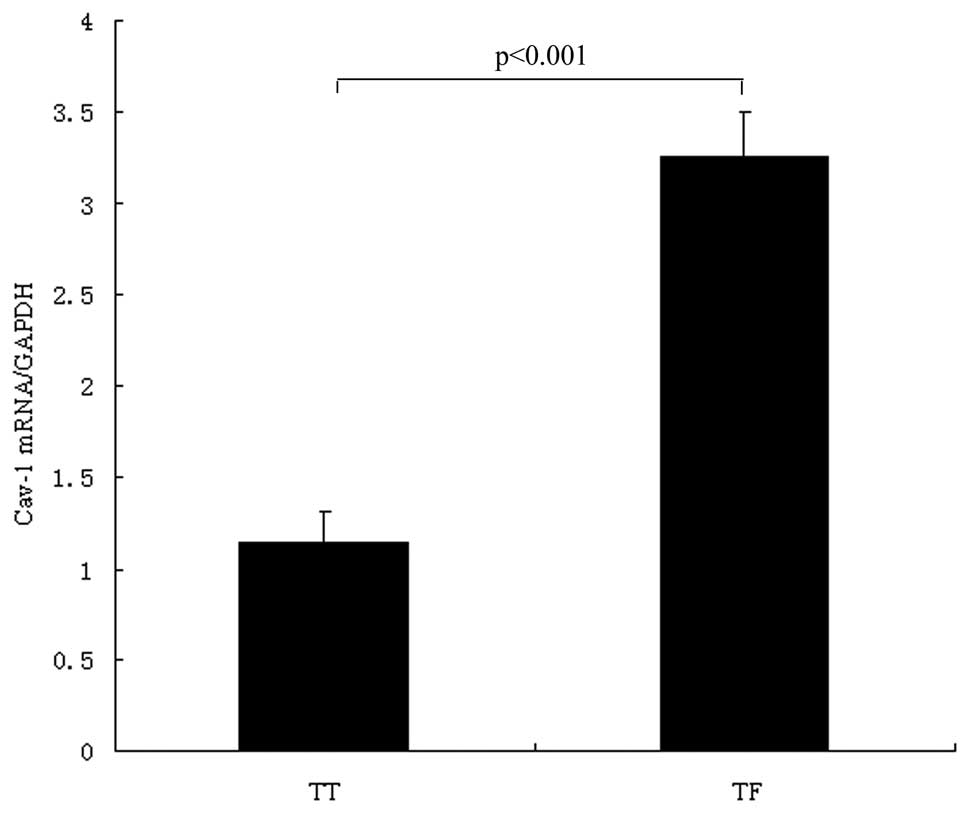
We detected the mRNA levels of cav-1 in 136 paired
specimens with lung TT and TF by SYBR-Green real-time PCR. The
relative mRNA level of the cav-1 gene (cav-1 per GAPDH, mean ± SD)
showed significant differences between lung cancer tissue
(1.146±0.167) and the surrounding normal lung tissue (3.254±0.248).
The paired t-test showed that cav-1 in TT was significantly lower
than in TF (P<0.001) (Fig. 1).
In summary, the cav-1 mRNA level is down-regulated in tumor samples
of NSCLC.
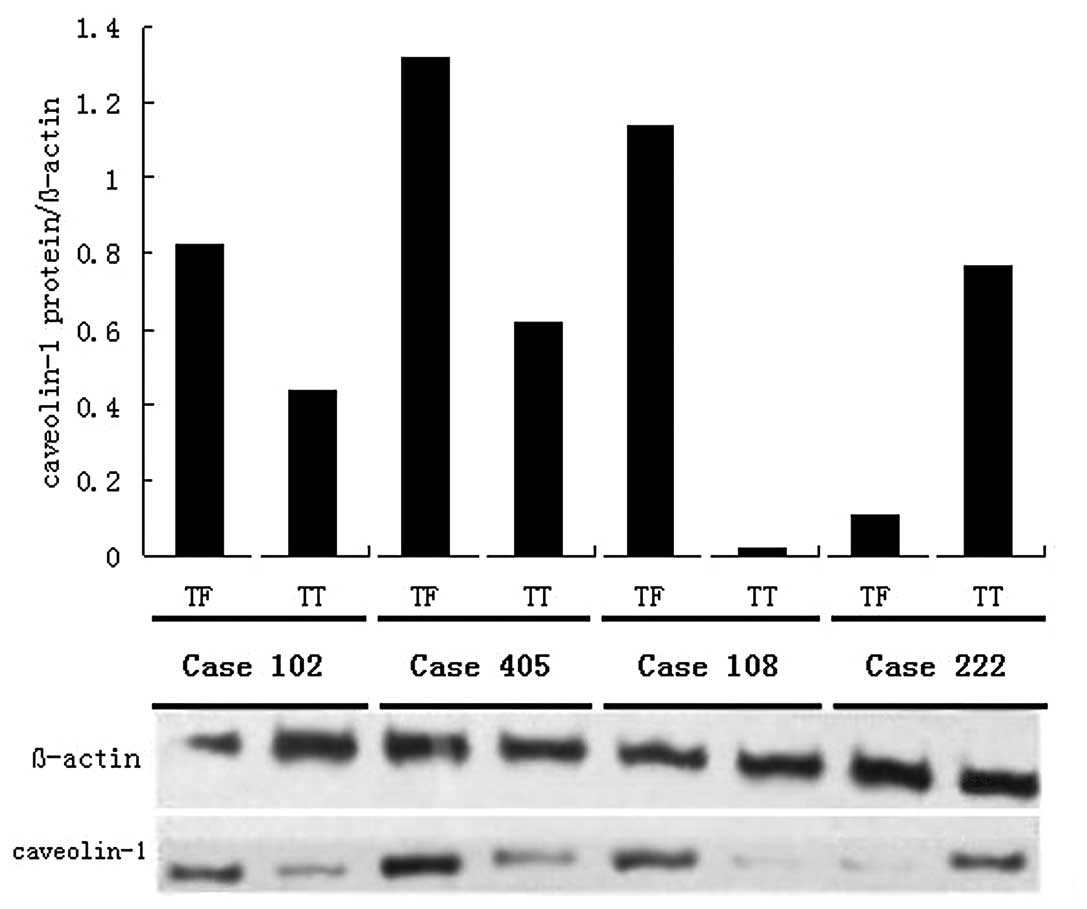
In addition, 20 paired samples were analyzed by
Western blot analysis. We confirmed that the protein expression of
cav-1 was also down-regulated in TT. The protein level of cav-1
(cav-1 per β-actin, mean ± SD) was 0.56±0.39 in TT and 0.87±0.51 in
TF, respectively. The paired t-test showed that cav-1 in TT was
significantly lower than in TF (P=0.002), and 4 representative
cases are shown in Fig. 2.
Detection of caveolin-1 in NSCLC tissues
and non-cancerous lung tissues
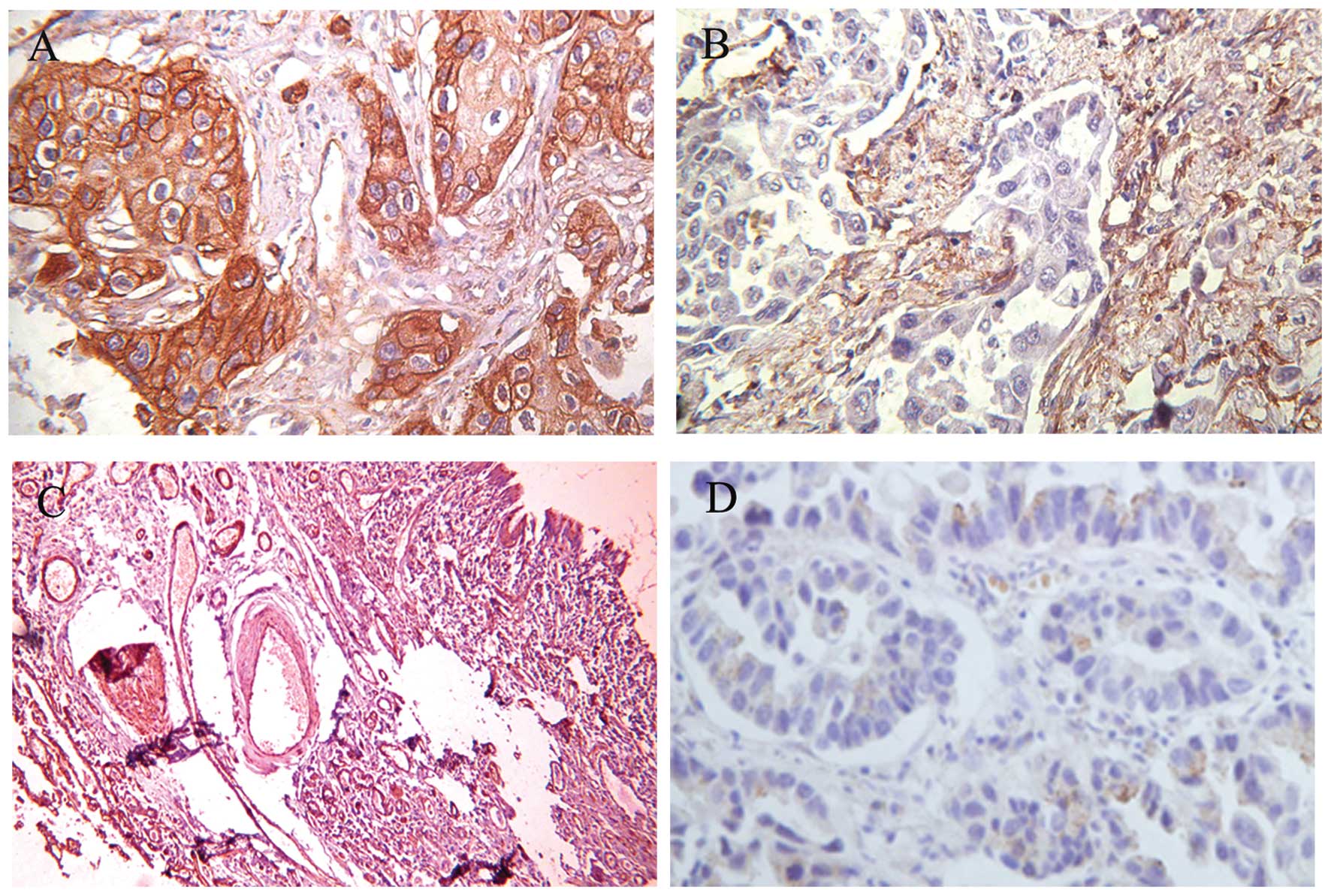
Immunohistochemistry was also performed to determine
the expression and subcellular localization of the cav-1 protein in
the 115 paraffin-embedded lung cancer specimens and 19
non-cancerous lung specimens. The positive immunoreactivity of
cav-1 was localized in the membrane and the intracytoplasm of the
lung AC (Fig. 3A). The majority of
the cells in cancer or non-cancerous cases, including fibroblasts
(Fig. 3B), type I pneumocytes,
bronchial epithelium and smooth muscle cells (Fig. 3C), showed positive staining for
cav-1. Negative expression of cav-1 is presented in Fig. 3D. Cav-1 overexpression was found in
60 of the 115 (52.2%) NSCLC patients, 23 of the 40 SCC (57.5%) and
32 cases of the 63 AC (50.8%). In addition, 15 of the 19
non-cancerous cases (78.9%) exhibited high expression levels of
cav-1. Furthermore, the incidence of high cav-1 expression was
significantly lower in NSCLC cases than non-cancerous cases (52.2%
vs. 78.9%, P<0.05) (Table
III).
 | Table IIICaveolin-1 expression in NSCLC and
non-cancerous tissues. |
Table III
Caveolin-1 expression in NSCLC and
non-cancerous tissues.
| | Caveolin-1 | |
|---|
| |
| |
|---|
| n | Low | High | P-value |
|---|
| NSCLC | 115 | 55 | 60 | 0.029 |
| Non-cancerous | 19 | 4 | 15 | |
Relationship between the expression
status of caveolin-1 mRNA and clinicopathological factors
To determine the significance of cav-1 mRNA
expression in NSCLC, all patients were divided into 2 groups
according to the relative expression levels (comparative to the
TF). Analyzing these results in relation to the different
histotypes, 22 of 74 (29.7%) lung AC, and 6 of 38 (15.8%) lung SCC
showed up-regulated cav-1 mRNA expression and the difference by
statistical analysis was significant (P=0.041). However, there was
no significant correlation between the expression status of cav-1
mRNA and other clinicopathologic factors, such as gender, age, the
size of the tumor, lymph node metastasis (pN) and p-TNM stages
(P>0.05). The relationship between cav-1 mRNA expression and
clinicopathologic factors is summarized in Table I.
Relationship between the protein
expression status of caveolin-1 and clinicopathological
factors
We further evaluated the significance of cav-1
protein expression in NSCLC patients. The patients were divided
into 2 groups according to the IHC evaluation criteria. The cav-1
protein expression levels and the association with
clinicopathological variables are listed in Table II. There were no significant
correlations observed between cav-1 expression and gender, age,
histological type and the size of the tumor, pathological N-stage
and pathological TNM-stage (P>0.05). We next performed a
stratified analysis by histological type to evaluate the
significance of the cav-1 protein expression in different
histological types of NSCLC patients. Interestingly, a significant
difference between high expression of cav-1 and poorer N-stage
(P=0.032) and higher pathological TNM-stage (P=0.012) were only
found in lung AC patients. However in lung SCC patients, there was
no significant association between cav-1 protein expression and any
other clinicopathological characteristics. Thus, the experimental
data indicate that lung AC patients with high cav-1 protein
expression levels show poorer N-stage and pathological
TNM-stage.
Survival analysis on caveolin-1 protein
expression
In our study, there was inadequate clinical
follow-up time for the NSCLC patients in which cav-1 mRNA was
detected. Thus, we only analyzed the prognostic significance of
cav-1 protein expression in the 115 paraffin-embedded NSCLC cases
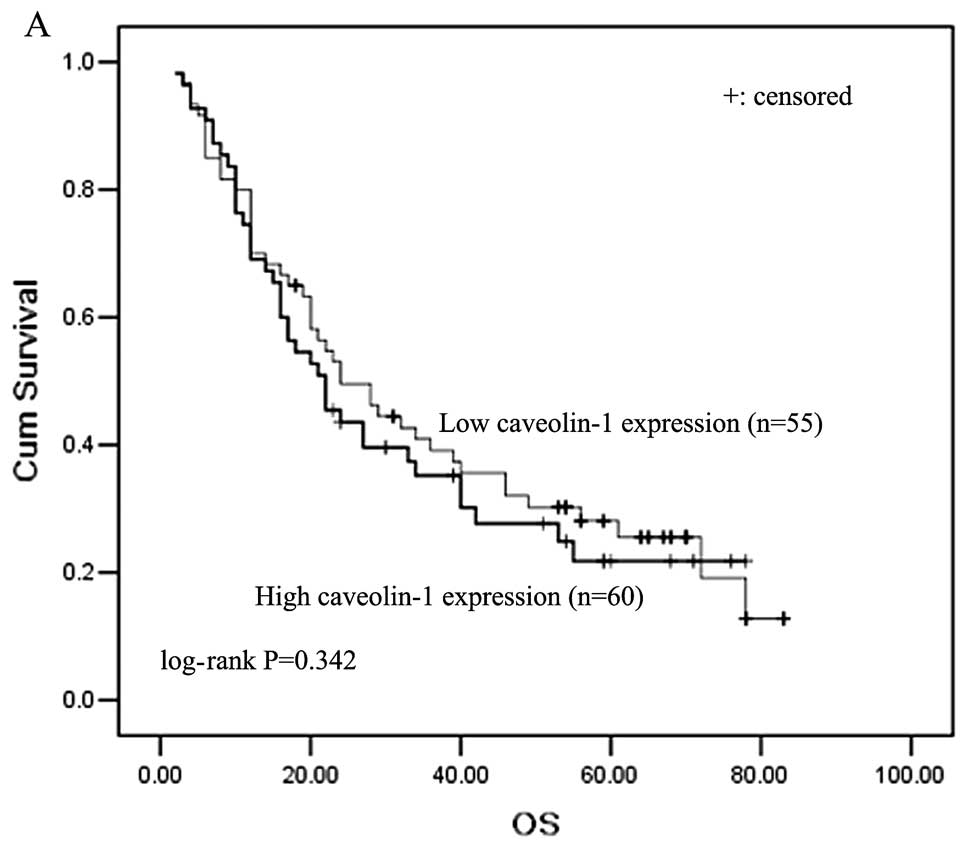
using IHC. The 5-year overall survival rate of all 115 patients was
41.3%. From Kaplan-Meier survival curves, we observed that patients
with high cav-1 expression survived a shorter survival time than
patients with low cav-1 levels (5-year survival rates, 22.3 and
29.2%, respectively); however, the result was not statistically
significant (P=0.342, log-rank test, Fig. 4A). When stratified analysis by
histological type was carried out, we found that lung AC patients
with higher cav-1 expression showed significantly shorter survival
than those with lower cav-1 expression, which indicated
significantly poorer survival for higher cav-1 expression (P=0.032,
log-rank test; Fig. 4B). However,
in lung SCC patients, cav-1 was not a prognostic marker for overall
survival.
Discussion
This is the first study to extensively investigate
the cav-1 mRNA and protein levels in paired TT and TF tissues, and
their association with clinicopathological features. In this study,
we showed that the levels of cav-1 mRNA and protein expression were
significantly lower in lung TT than in matched TF tissue. Using
IHC, the protein expression of cav-1 was significantly lower in
NSCLC cases than in non-cancerous lung tissues. Up-regulation of
cav-1 mRNA expression was found in lung AC more than lung SCC. We
showed that the higher cav-1 protein expression closely correlated
with poorer N-stage (P=0.032) and higher pathological TNM stage
(P=0.012) in lung AC patients; however, no significant association
between cav-1 protein expression and any other clinicopathological
characteristics was found in lung SCC. Moreover, the most important
point revealed in this study was that lung AC patients with higher
cav-1 expression levels showed significantly shorter survival than
those with lower cav-1 expression levels.
Cav-1, the principal structural protein in caveolae,
has been recognized as a key player in the regulation of several
signal transduction molecules, such as the HARS protein, epidermal
growth factor (EGF) receptor, Src family tyrosine kinase, protein
kinase C, transforming growth factor b/SMAD and the
Wnt/β-catenin/lef-1 pathway (26–30).
Cav-1 is an interesting molecule because of its apparent
paradoxical biological functions in malignant tumors. Two previous
studies showed that the expression of the cav-1 gene was
down-regulated in lung cancer cells compared to normal bronchial
epithelial cells. Decreased expression of cav-1 has also been found
(9–10) in a variety of cancer cell lines of
breast carcinoma, colon carcinoma, uterine cervical carcinoma and
ovary (6–8,11).
Conversely, in an in vivo study, overexpression of cav-1 was
also observed in many human cancer tissues: bladder cancer
(14), esophageal cancer (15), and prostate carcinomas (16). In our study, we used real-time PCR
and Western blotting assays to detect the expression of cav-1 mRNA
and protein in lung cancer tissues and matched TF tissues. The
protein expression of cav-1 was significantly lower in NSCLC cases
than in non-cancerous lung tissues. Our results indicate that the
levels of cav-1 mRNA and protein expression were significantly
decreased in lung TT when compared to matched TF tissue.
In total, 4 clinical studies (19–23)
demonstrated the clinicopathological variables and the prognostic
significance of cav-1 in resected NSCLC. Wikman and colleagues
(23) investigated the cav-1
protein levels with IHC in all histological types of NSCLC and
revealed that patients with high cav-1 showed no correlation with
the disease outcome when all histological types were analyzed as 1
group or separately. In contrast, 3 other studies documented
conflicting results. In two studies (20,21)
the expression of cav-1 was associated with poor prognosis of
patients with lung SCC and lung pleomorphic carcinoma. Ho et
al, (19) revealed that
up-regulated cav-1 could accentuate the metastasis capability of
lung AD, and was an independent functional predictor of poor
survival in lung AD. In our study, we showed that high protein
expression of cav-1 was significantly correlated with poor survival
in lung AC patients.
Cav-1 has been shown in in vitro studies to
be down-regulated in various tumor cells including NSCLC (9,10). In
our in vivo study, the results indicate that the levels of
cav-1 mRNA and protein expression were significantly decreased in
lung TT compared to matched TF tissue, and we confirmed the concept
that cav-1 is down-regulated in NSCLC. Previous studies (31) have reported the cav-1 expression was
present in alveolar epithelial type I (ATI) lung cells, but absent
in its progenitor, the alveolar epithelial type II (ATII) cells.
However, in the process of cell culturing, cav-1 expression
increased with the signal at 192 h post-seeding being up to 50-fold
greater than at 60 h. In addition, the study by Ho et al,
(19) revealed that re-introduction
of cav-1 expression into less invasive lung carcinoma cells caused
an increase in cell invasive ability. These results, along with our
observation that high expression of the cav-1 protein correlated
with pN and poor over survival in lung AC patients, supports the
concept that the function of cav-1 varies in the development and
progression of lung cancer. In the deterioration and progression of
lung cancer, cav-1 can be up-regulated, which may contribute to
tumor invasion and metastasis.
In summary, we have shown that the levels of cav-1
mRNA and protein expression were significantly lower in lung TT
than in matched TF tissue. Up-regulation of cav-1 mRNA expression
was found in lung AC more than lung SCC. The higher cav-1 protein
expression closely correlated with poorer N-stage and higher
pathological TNM-stage in lung AC patients. Moreover, the most
important point in this study was the observation that lung AC
patients with higher cav-1 expression showed significantly shorter
survival than those with lower cav-1 expression.
Acknowledgements
This study was supported by a grant from the Major
Program of Nanjing Medical Science and Technique Development
Foundation (Molecular Predictor of Personalized Therapy for Chinese
Patients with Non-small Cell Lung Cancer) (L.-K.Y.).
References
|
1
|
Alberg AJ and Samet JM: Epidemiology of
lung cancer. Chest. 123:21–49. 2003. View Article : Google Scholar
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alberg AJ, Ford JG and Samet JM:
Epidemiology of lung cancer: ACCP evidence-based clinical practice
guidelines. Chest. 132:29S–55S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paesmans M, Sculier JP, Libert P, et al:
Prognostic factors for survival in advanced non-small-cell lung
cancer: univariate and multivariate analyses including recursive
partitioning and amalgamation algorithms in 1,052 patients. The
European Lung Cancer Working Party. J Clin Oncol. 13:1221–1230.
1995.
|
|
5
|
Rothberg KG, Heuser JE, Donzell WC, et al:
Caveolin, a protein component of caveolae membrane coats. Cell.
68:673–682. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sloan EK, Stanley KL and Anderson RL:
Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene.
23:7893–7897. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Williams TM, Medina F, Badano I, et al:
Caveolin-1 gene disruption promotes mammary tumorigenesis and
dramatically enhances lung metastasis in vivo: role of Cav-1 in
cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion.
J Biol Chem. 279:51630–51646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Razani B, Altschuler Y, Zhu L, et al:
Caveolin-1 expression is down-regulated in cells transformed by the
human papilloma virus in a p53-dependent manner. Replacement of
caveolin-1 expression suppresses HPV-mediated cell transformation.
Biochemistry. 39:13916–13924. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Racine C, Belanger M, Hirabayashi H, et
al: Reduction of caveolin 1 gene expression in lung carcinoma cell
lines. Biochem Biophys Res Commun. 255:580–586. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sunaga N, Miyajima K, Suzuki M, et al:
Different roles for caveolin-1 in the development of non-small cell
lung cancer versus small cell lung cancer. Cancer Res.
64:4277–4285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiechen K, Diatchenko L, Agoulnik A, et
al: Caveolin-1 is down-regulated in human ovarian carcinoma and
acts as a candidate tumor suppressor gene. Am J Pathol.
159:1635–1643. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galbiati F, Volonte D, Engelman JA, et al:
Targeted downregulation of caveolin-1 is sufficient to drive cell
transformation and hyperactivate the p42/44 MAP kinase cascade.
EMBO J. 17:6633–6648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koleske AJ, Baltimore D and Lisanti MP:
Reduction of caveolin and caveolae in oncogenically transformed
cells. Proc Natl Acad Sci USA. 92:1381–1385. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fong A, Garcia E, Gwynn L, et al:
Expression of caveolin-1 and caveolin-2 in urothelial carcinoma of
the urinary bladder correlates with tumor grade and squamous
differentiation. Am J Clin Pathol. 120:93–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu YC, Lam KY, Law S, et al: Profiling of
differentially expressed cancer-related genes in esophageal
squamous cell carcinoma (ESCC) using human cancer cDNA arrays:
overexpression of oncogene MET correlates with tumor
differentiation in ESCC. Clin Cancer Res. 7:3519–3525. 2001.
|
|
16
|
Li L, Yang G, Ebara S, et al: Caveolin-1
mediates testosterone stimulate survival/clonal growth and promotes
metastatic activities in prostate cancer cells. Cancer Res.
61:4386–4392. 2001.PubMed/NCBI
|
|
17
|
Kato K, Hida Y, Miyamoto M, et al:
Overexpression of caveolin-1 in esophageal squamous cell carcinoma
correlates with lymph node metastasis and pathologic stage. Cancer.
94:929–933. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang G, Truong LD, Wheeler TM, et al:
Caveolin-1 expression in clinically confined human prostate cancer:
a novel prognostic marker. Cancer Res. 59:5719–5723.
1999.PubMed/NCBI
|
|
19
|
Ho CC, Huang PH, Huang HY, et al:
Up-regulated caveolin-1 accentuates the metastasis capability of
lung adenocarcinoma by inducing filopodia formation. Am J Pathol.
161:1647–1656. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoo SH, Park YS, Kim HR, et al: Expression
of caveolin-1 is associated with poor prognosis of patients with
squamous cell carcinoma of the lung. Lung Cancer. 42:195–202. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moon KC, Lee GK, Yoo SH, et al: Expression
of caveolin-1 in pleomorphic carcinoma of the lung is correlated
with a poor prognosis. Anticancer Res. 25:4631–4637.
2005.PubMed/NCBI
|
|
22
|
Kato T, Miyamoto M, Kato K, et al:
Difference of caveolin-1 expression pattern in human lung
neoplastic tissue. Atypical adenomatous hyperplasia, adenocarcinoma
and squamous cell carcinoma. Cancer Lett. 214:121–128. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wikman H, Seppänen JK, Sarhadi VK, et al:
Caveolins as tumour markers in lung cancer detected by combined use
of cDNA and tissue microarrays. J Pathol. 203:584–593. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ho CC, Kuo SH, Huang PH, et al: Caveolin-1
expression is significantly associated with drug resistance and
poor prognosis in advanced non-small cell lung cancer patients
treated with gemcitabine-based chemotherapy. Lung Cancer.
59:105–110. 2008. View Article : Google Scholar
|
|
25
|
Groome PA, Bolejack V, Crowley JJ, et al;
IASLC International Staging Committee; Cancer Research and
Biostatistics; Observers to the Committee; Participating
Institutions. The IASLC Lung Cancer Staging Project: validation of
the proposals for revision of the T, N and M descriptors and
consequent stage groupings in the forthcoming (seventh) edition of
the TNM classification of malignant tumours. J Thorac Oncol.
2:694–705. 2007. View Article : Google Scholar
|
|
26
|
Li S, Couet J and Lisanti MP: Src tyrosine
kinases, Galpha subunits, and H-Ras share a common
membrane-anchored scaffolding protein, caveolin: caveolin binding
negatively regulates the auto-activation of Src tyrosine kinases. J
Biol Chem. 271:29182–29190. 1996. View Article : Google Scholar
|
|
27
|
Oka N, Yamamoto M, Schwencke C, et al:
Caveolin interaction with protein kinase C: isoenzyme-dependent
regulation of kinase activity by the caveolin scaffolding domain
peptide. J Biol Chem. 272:33416–33421. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Couet J, Sargiacomo M and Lisanti MP:
Interaction of a receptor tyrosine kinase, EGF-R, with caveolins:
caveolin binding negatively regulates tyrosine and serine/threonine
kinase activities. J Biol Chem. 272:30429–30438. 1997. View Article : Google Scholar
|
|
29
|
Razani B, Zhang XL, Bitzer M, et al:
Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD
signaling through an interaction with the TGF-beta type I receptor.
J Biol Chem. 276:6727–6738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galbiati F, Volonte D, Brown AM, et al:
Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by
recruiting beta-catenin to caveolae membrane domains. J Biol Chem.
275:23368–23377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Campbell L, Hollins AJ, Al-Eid A, et al:
Caveolin-1 expression and caveolae biogenesis during cell
transdifferentiation in lung alveolar epithelial primary cultures.
Biochem Biophys Res Commun. 262:744–751. 1999. View Article : Google Scholar : PubMed/NCBI
|