Introduction
Salivary adenoid cystic carcinoma (SACC) is one of
the most frequent malignancies of the salivary glands, constituting
approximately 18% of all salivary gland malignancies (1). It has a protracted clinical course
with diffuse invasion, local recurrences, late distant metastases,
and poor response to classical chemotherapies (2). A prominent feature of SACC is its high
affinity for basement membrane-rich tissues, such as nerves and
blood vessels (3). The infiltration
of major nerves and blood vessels in SACC could prevent complete
surgical resection and lead to a worse prognosis (4,5).
The special proclivity of SACC cells to invade
nerves and endothelial sheaths may be related to their high
chemotactic response to the extracellular matrix (ECM) (3,6).
Degradation of the ECM by matrix metalloproteinases (MMPs) is a
crucial step in tumor invasion and metastasis (7). In many tumors, expression of MMPs is
mainly regulated by tumor-stroma interactions via a tumor
cell-associated protein and extracellular MMP inducer (EMMPRIN,
CD147), which is a transmembrane glycoprotein that belongs to the
immunoglobulin superfamily (8).
EMMPRIN has been found to promote tumor invasion and metastasis by
mediating the expression of MMPs within the tumor microenvironment
(9,10). In our recent immunohistochemical
study, we found that EMMPRIN expression in SACC was
positively-associated with tumor perineural and perivascular
invasion, and that MMP-2 and MMP-9 were expressed both in the tumor
and stromal compartments (11).
Thus, we hypothesized that EMMPRIN may be a key mediator in the
invasion of SACC through functionally mediating the expression of
MMPs in the surrounding stromal cells and tumor cells.
To examine our hypothesis, we evaluated the effects
of blocking EMMPRIN by its antibody on human highly metastatic SACC
cells (SACC-LM cells) when cultured alone or co-cultured with
fibroblasts. In the present study, we performed an in vitro
assay for invasive behavior using modified Boyden chambers to
explore the role of EMMPRIN in the invasion of SACC. We also
examined the tumor cell adhesion and MMPs expression activity to
explore the possible mechanism of EMMPRIN in the invasion of
SACC.
Materials and methods
Cell culture
Salivary adenoid cystic carcinoma cells with high
metastatic potential (SACC-LM) and low metastatic potential
(SACC-83) were provided by the College of Stomatology, Beijing
University (12,13). Human embryonic pulmonary fibroblasts
were purchased from the Chinese Academy of Medical Sciences. All
cells were cultured in DMEM (Gibco, USA) supplemented with 10% FBS
(Gibco) at 37°C in a humidified atmosphere of 5%
CO2.
Western blot analysis of EMMPRIN
The cellular proteins were separated by 10% SDS-PAGE
and transferred onto PVDF membranes (Pall Corporation).
Non-specific binding sites on the membrane were blocked in 5% skim
milk for 1 h at room temperature. The membranes were then incubated
with a 1:500 dilution of mouse anti-human EMMPRIN (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) or β-actin antibody (Sigma,
USA) for 2 h at 37°C and followed by a reaction with goat
anti-mouse IgG (Sigma) for 1 h at room temperature. After washing,
the membranes were treated with enhanced chemiluminescence reagent
(Santa Cruz Biotechnology) and exposed on Kodak X-ray film.
Blockage of EMMPRIN
Tumor cells (1×106) were collected in an
Eppendorf tube containing 500 μl serum-free DMEM. The blockage of
EMMPRIN was carried out with 5 μg monoclonal anti-EMMPRIN/CD147
antibody (Santa Cruz Biotechnology) under gentle agitation for 1 h.
The cells were subsequently washed with PBS and used for further
assay. As a control, the anti-EMMPRIN/CD147 antibody was replaced
with PBS.
Immunofluorescence and flow
cytometry
Immunofluorescence and flow cytometry were performed
to determine the blocking efficiency of anti-EMMPRIN/CD147 on
EMMPRIN expression in tumor cells. Briefly, tumor cells of the
EMMPRIN blockage group and control group were both stained with
R-phycoerythrin (RPE)-labeled anti-CD147/EMMPRIN antibody (Serotec)
for 30 min at 4°C. After washing with PBS, half of the cells were
smeared on a slide and observed using immunofluorescence microscopy
(Olympus), and the other half of the cells were subjected to flow
cytometric analysis using a FACSCalibur flow cytometer and the
CellQuest software (Becton-Dickinson).
Adhesion assays
For adhesion assays of tumor cells to the ECM, the
96-well plates were coated with basement membrane Matrigel (BD
Biosciences, USA) at a concentration of 5 mg/ml and incubated at
4°C overnight. Then, tumor cells (2×104/well) suspended
in serum-free DMEM were added to the wells and incubated at 37°C
for 45 min. After removing the medium and non-attached cells, 0.2%
crystal violet was added for 10 min and 5% SDS/50% ethanol was
added for 20 min. Finally, the plate was read at 540 nm.
Gelatin zymography analysis
Gelatin zymography was performed to assess the
effect of EMMPRIN on gelatinase activity as previously described
(14). Cells of each group were
continuously cultured or co-cultured with equal fibroblasts in
serum-free DMEM for 24 h. Conditioned medium was separated by
SDS-PAGE under non-reducing conditions using 8% separating gel
containing 0.1% gelatin (Sigma). The gels were incubated in a 2.5%
Triton X-100 solution at room temperature with gentle agitation to
remove SDS and then were soaked in reaction buffer (50 mmol/l
Tris-HCl, 200 mmol/l NaCl, 10 mmol/l CaCl2, pH 7.5) at
37°C for 24 h. After reaction, the gels were stained for 6 h with
staining solution and were destained for about 30 min.
Gelatinolytic activity of MMPs was visualized as a clear band
against a dark background of stained gelatin.
In vitro invasion assay
The invasion activity of tumor cells in vitro
were demonstrated in modified Boyden chambers (15). Transwell invasion chambers
containing polycarbonate filters (pore size, 8 μm) were coated on
the upper surface with basement membrane Matrigel
(Becton-Dickinson). Tumor cells (1×105) alone or
together with equal numbers of fibroblasts in 200 μl serum-free
DMEM were placed in the upper chamber. The lower chamber was filled
with 600 μl conditioned medium (incubating fibroblasts in
serum-free DMEM medium for 24 h) as chemoattractant. After 24-h
incubation, the cells on the upper surface of the filter were
removed with a cotton swab. The cells that had invaded the Matrigel
and reached the lower surface of the filter were fixed in methanol,
stained with H&E, and counted under a magnification of ×400. We
chose five fields and counted the number of the invaded cells.
Statistical analysis
All experiments were performed in triplicate and the
data were calculated as means ± SD. Data analysis was performed by
SPSS 16.0 software (USA). Multiple groups were analyzed by one-way
ANOVA tests. Single group data was assessed using the Student’s
t-test. P-values <0.05 were considered statistically
significant.
Results
EMMPRIN expression in SACC-LM and SACC-83
cells
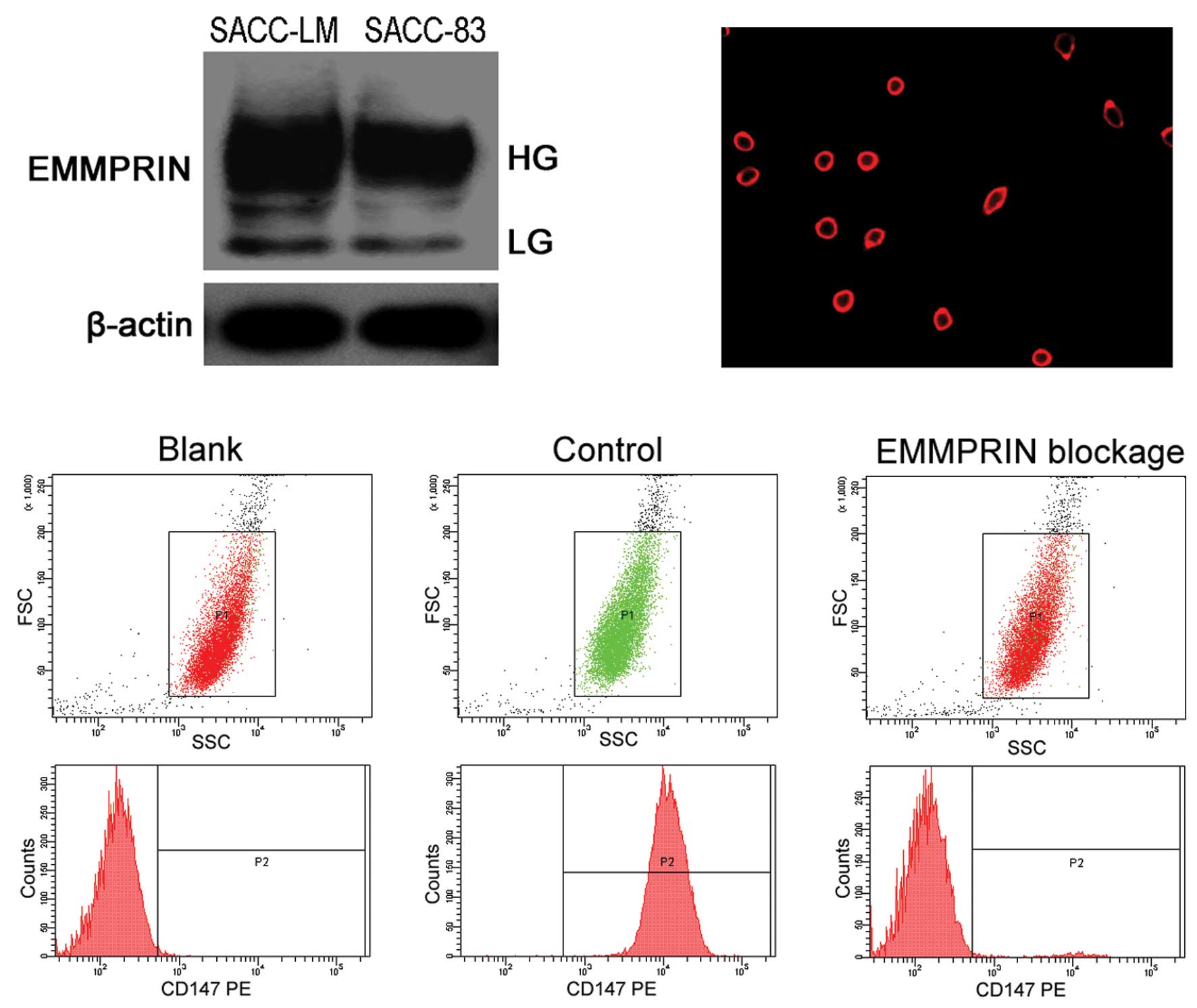
To compare the levels of EMMPRIN expression among
two SACC cell lines, we utilized Western blot analysis and
demonstrated that EMMPRIN expression was significantly increased in
SACC-LM cells in comparison to SACC-83 cells (Fig. 1A). Thus, the SACC-LM cell line was
chosen for further experiments.
Blocking of EMMPRIN by its antibody in
SACC-LM cells
We first observed positive immunolabeling of EMMPRIN
mainly on the membrane of control SACC-LM cells (Fig. 1B), and no positive immunolabeling of
EMMPRIN on EMMPRIN-blocked SACC-LM cells by immunofluorescence.
Then, we evaluated the blocking efficiency of the
anti-EMMPRIN/CD147 antibody by flow cytometry (Fig. 1C). RPE-labeled anti-CD147/EMMPRIN
antibody presented a gated positive binding rate of 95.87±2.25% to
control SACC-LM cells. However, after SACC-LM cells were incubated
with anti-EMMPRIN/CD147 antibody, RPE-labeled anti-CD147/EMMPRIN
antibody presented only a rate of 1.48±0.47% to EMMPRIN blocked
SACC-LM cells, which were close to 0.70±0.38% of the blank control
SACC-LM cells. These results suggested that the anti-EMMPRIN/CD147
antibody effectively bound to and blocked the EMMPRIN molecule on
the membrane of SACC-LM cells with a relatively high affinity.
Effect of EMMPRIN on the adhesion
activity of SACC-LM cells to the ECM
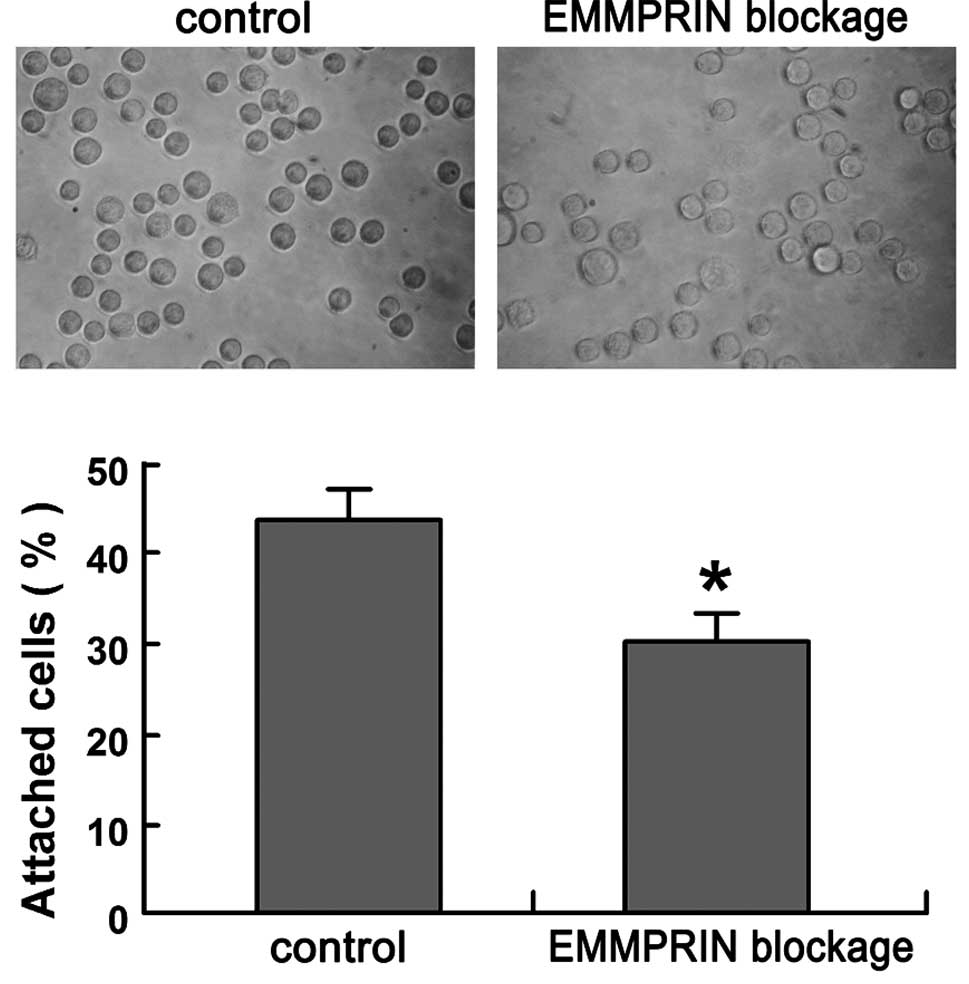
To examine the effects of EMMPRIN blockage on
SACC-LM cell adhesion activity, adhesion assays of SACC-LM cells to
the ECM were performed. After 45 min of incubation, a significant
decrease in the amount of cells attached to the Matrigel-coated
plates was observed in EMMPRIN blocked SACC-LM cells (30.26±2.86%),
as compared to that in control SACC-LM cells (44.34±3.21%)
(P<0.01; Fig. 2).
Effect of EMMPRIN on gelatinase activity
in SACC-LM cells
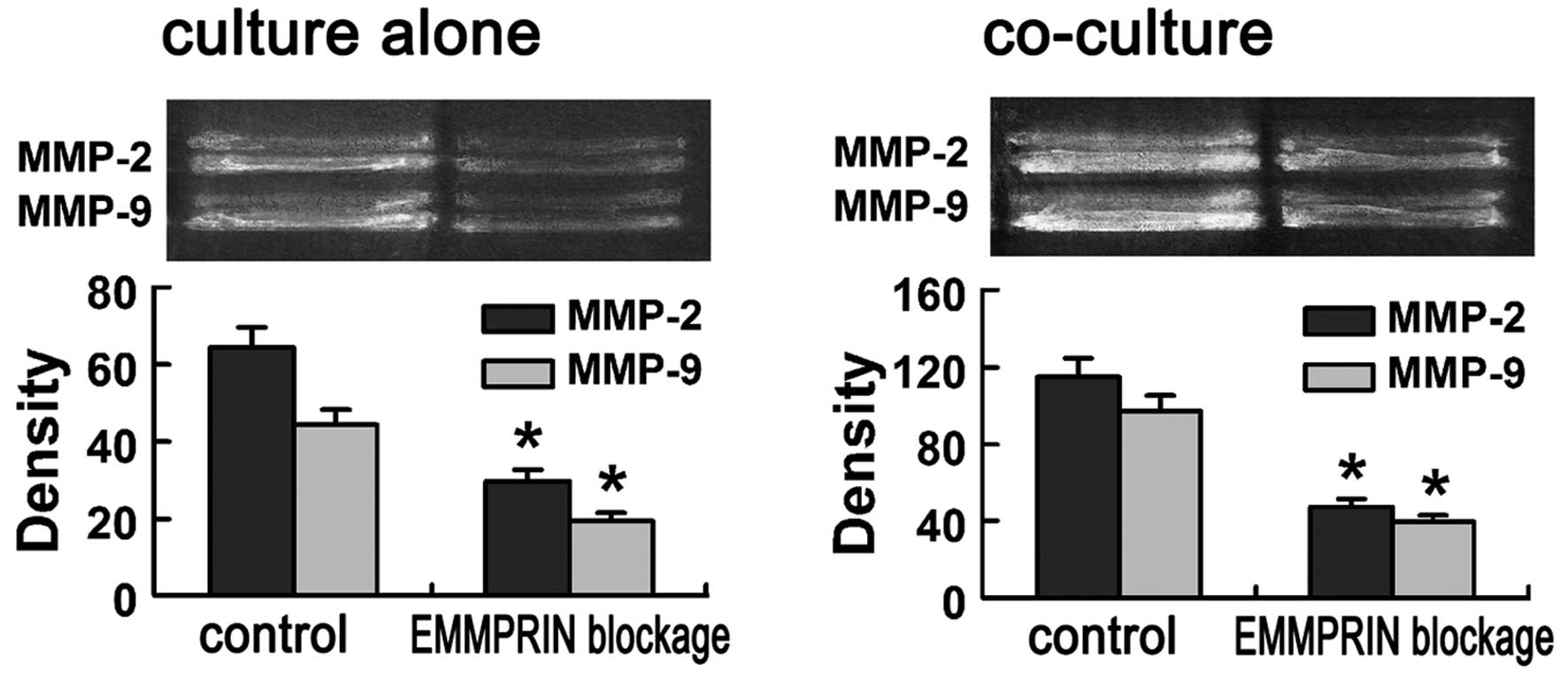
Since MMPs play critical roles in tumor cells
invasion, we examined the effect of blocking EMMPRIN by its
antibody on the enzyme activity of MMP-2 and MMP-9 using gelatin
zymography. The gelatinase activity of both MMP-2 and MMP-9 were
found to be reduced markedly in EMMPRIN blocked SACC-LM cells
compared to that in control SACC-LM cells (P<0.01; Fig. 3A).
To mimic the in vivo tumor-stroma interaction
in the local microenvironment, SACC-LM cells were co-cultured with
(1:1) human fibroblasts and exhibited an enhanced gelatinase
activity, that was 1.78±0.45-fold (MMP-2), and 2.35±0.53-fold
(MMP-9) higher than that when cultured alone (Fig. 3). When co-cultured with human
fibroblasts, the gelatinase activity of both MMP-2 and MMP-9 were
still found to be reduced markedly in the EMMPRIN-blocked group
compared with that in control group (P<0.01; Fig. 3B).
In vitro invasion assay
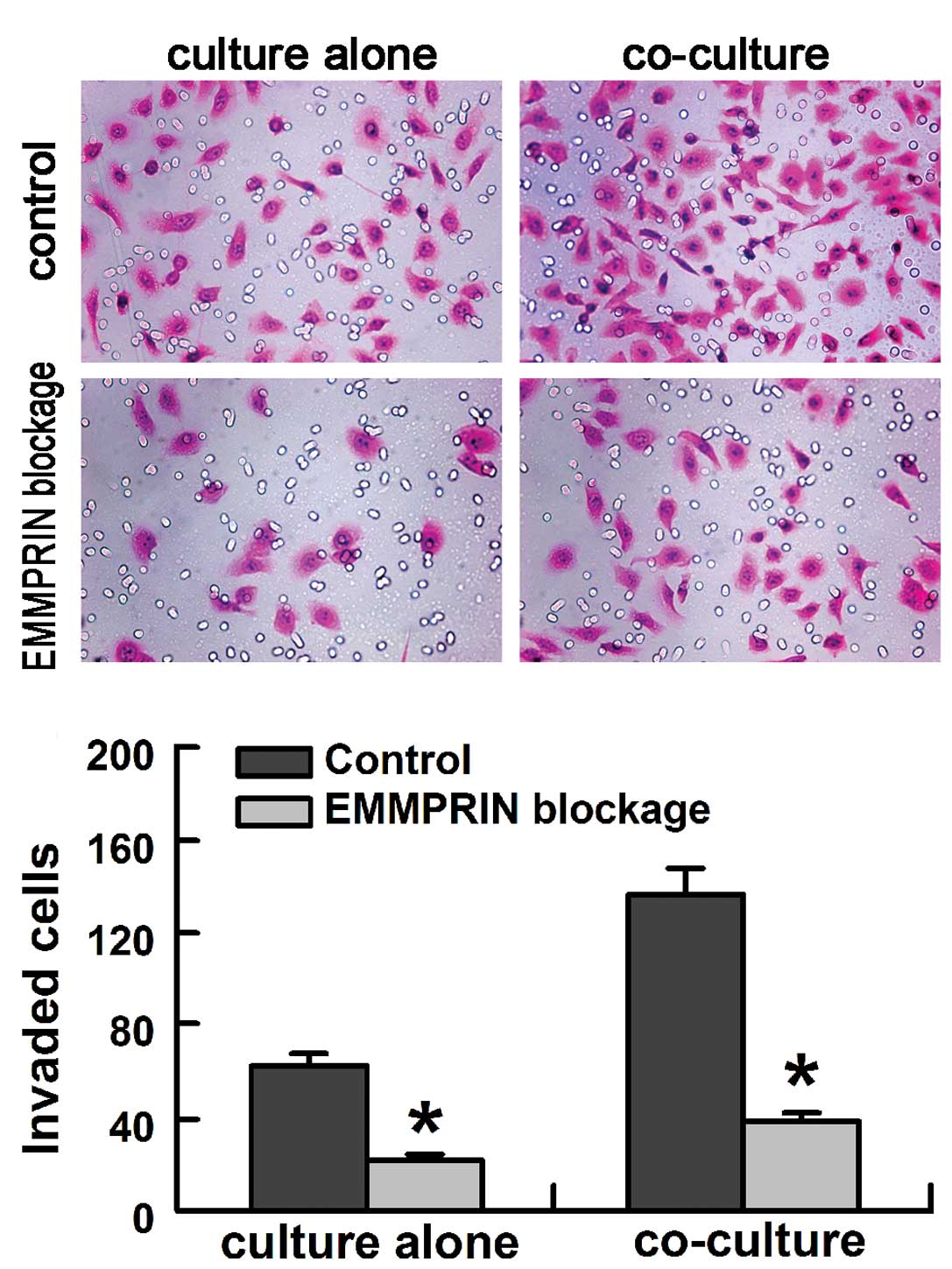
The role of EMMPRIN on the invasion activity of
SACC-LM cells was evaluated in vitro using the modified
Boyden chambers. The EMMPRIN-blocked SACC-LM cells exhibited much
lower invasion activity compared with that in control SACC-LM cells
when cultured alone or co-cultured with fibroblasts (Fig. 4A). When co-cultured with human
fibroblasts, SACC-LM cells exhibited an enhanced invasion activity,
that was 2.21±0.68-fold (SACC-LM cells group), and 1.71±0.54-fold
(EMMPRIN-blocked SACC-LM cells group) higher than that when
cultured alone (Fig. 4). Blocking
EMMPRIN significantly inhibited the invasion activity of SACC-LM
cells by 63.5±2.54% when cultured alone and by 71.6±2.83% when
co-cultured with fibroblasts (P<0.01; Fig. 4B).
Discussion
SACC is an invasive tumor with a special tendency to
involve nerves and endothelial sheaths. EMMPRIN, also called CD147
or basigin, is a cellular adhesion molecule involved in cell-cell
and cell-ECM interactions (8–10).
EMMPRIN is highly expressed on the surface of various malignant
tumor cells compared with their normal counterparts (16,17)
and is involved in tumor invasion and metastasis (14,18).
Our previous study found that overexpression of EMMPRIN was a
prognostic factor for patients with SACC and was positively
associated with the perineural and perivascular invasion of SACC
(11). In the present study, we
demonstrated that blocking EMMPRIN expression by its antibody could
effectively inhibit SACC-LM cell adhesion and invasion activity
in vitro.
Adhesion of tumor cells to surrounding tissues is
the first step for tumor invasion and is highly dependent upon an
increased production of proteases by the tumor cells. As an
adhesion molecule, EMMPRIN has been reported to bind to a variety
of cell types, including endothelial cells and fibroblasts, as well
as the ECM (19). Matrigel is a
basement membrane preparation containing almost all of the ECM
components (20). The special
proclivity of the SACC cells to invade basement membrane-rich
tissues may be related to their high chemotactic response to the
ECM (3,6). Our study showed that blocking of
EMMPRIN could directly inhibit the attachment of SACC-LM cells to
the Matrigel-coated plates. These results indicate that EMMPRIN
induces the adhesion of the SACC-LM cells to the ECM.
Degradation of ECM are necessary steps in tumor
local invasion (21). MMPs, a major
family of enzymes that can degrade various components of the ECM,
are believed to play critical roles in tumor invasion (7). Among MMPs, MMP-2 (gelatinase-A) and
MMP-9 (gelatinase-B) are particularly upregulated in SACC and
contribute to the invasion of tumor cells by degrading the ECM
(22–24). Our previous study found that the
expression of MMP-2 and MMP-9 was correlated with EMMPRIN
expression in SACC (11). In the
present study, we found that blocking of EMMPRIN expression in
SACC-LM cells reduced the secretion of MMP-2 and MMP-9 when
cultured alone or co-cultured with fibroblasts, thus inhibiting the
invasion ability of SACC-LM cells through the reconstituted
basement membrane in vitro. Our results suggest that EMMPRIN
is involved in the invasion of SACC-LM cells by regulating the
secretion of MMP-2 and MMP-9 to participate in the degradation of
the ECM.
The search for MMP-inducing factors in tumor cells
led to the identification of EMMPRIN, whose name reflects its
activity. EMMPRIN has been found to facilitate tumor invasion and
metastasis by regulating the expression of MMPs (8–11).
EMMPRIN has also been found to promote tumor angiogenesis by
stimulating the expression of MMPs and the vascular endothelial
growth factor (VEGF) (25,26). Besides, EMMPRIN has been shown to
promote tumor growth in an anchorage-dependent manner by inducing
hyaluronan production (27), and to
stimulate cancer cell proliferation via the activation of ERK1/2
and p38 MAPK signaling pathways (28). In addition, the latest studies
showed that silencing of EMMPRIN gene expression via RNAi could
inhibit the invasion activity of cancer cells (29,30).
Our study suggested that blocking of EMMPRIN by its antibody could
effectively inhibit the in vitro invasion activity of
SACC-LM cells by inhibiting the expression of MMP-2 and MMP-9.
The interactions between tumor cells and the
surrounding stromal cells have important implications in the
invasion of many malignant tumors (31). Accumulating evidence suggests that
EMMPRIN facilitates tumor invasion by participating in tumor-stroma
interactions to stimulate the expression of MMPs in stromal cells
(10,18). In this study, we found that
co-culturing the SACC-LM cells and fibroblasts to mimic the
tumor-stroma interactions produced elevated levels of MMP-2 and
MMP-9 and gave rise to greatly increased in vitro invasion
activity of SACC-LM cells, as compared with cultures of SACC-LM
cells alone. We also found that blocking of EMMPRIN significantly
inhibited the MMP-2 and MMP-9 secretion, and invasion activity of
SACC-LM cells when cultured alone or co-cultured with fibroblasts
in vitro. These results suggest that EMMPRIN participates in
the tumor-stroma interactions by stimulating production of MMPs in
both the tumor and stromal cells and thus facilitates the invasion
of SACC-LM cells. Thus, further study of tumor-stroma interactions
mediated by EMMPRIN may shed more light on the mechanism of SACC
invasion.
In conclusion, this study showed that EMMPRIN played
an important role in the invasion of SACC-LM cells through
functionally mediating the expression of MMP-2 and MMP-9 both in
tumor and stromal cells, and its antibody could effectively inhibit
SACC-LM cells adhesion and invasion in vitro. Interruption
of tumor-stroma interactions by blocking tumor EMMPRIN could be a
target of anti-invasion therapy in SACC.
Acknowledgements
We thank Yuan Liu for his excellent technical
assistance and Lei Che for her secretarial assistance. This study
was supported by the Foundation of the Fourth Military Medical
University (no. 2010D14).
References
|
1
|
Li LJ, Li Y, Wen YM, Liu H and Zhao HW:
Clinical analysis of salivary gland tumor cases in West China in
past 50 years. Oral Oncol. 44:187–192. 2008.PubMed/NCBI
|
|
2
|
Fordice J, Kershaw C, El-Naggar A and
Goepfert H: Adenoid cystic carcinoma of the head and neck:
predictors of morbidity and mortality. Arch Otolaryngol Head Neck
Surg. 125:149–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dardick I: Color Attlas/Text of Salivary
Gland Tumor Pathology. Igaku-Shoin; New York: 1996
|
|
4
|
van der Wal JE, Snow GB and van der Waal
I: Intraoral adenoid cystic carcinoma. The presence of perineural
spread in relation to site, size, local extension, and metastatic
spread in 22 cases. Cancer. 66:2031–2033. 1990.PubMed/NCBI
|
|
5
|
Barrett AW and Speight PM: Perineural
invasion in adenoid cystic carcinoma of the salivary glands: a
valid prognostic indicator? Oral Oncol. 45:936–940. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shintani S, Alcalde RE, Matsumura T and
Terakado N: Extracellular matrices expression in invasion area of
adenoid cystic carcinoma of salivary glands. Cancer Lett. 116:9–14.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toole BP: Emmprin (CD147), a cell surface
regulator of matrix metalloproteinase production and function. Curr
Top Dev Biol. 54:371–389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
10
|
Tang Y, Kesavan P, Nakada MT and Yan L:
Tumor-stroma interaction: positive feedback regulation of
extracellular matrix metalloproteinase inducer (EMMPRIN) expression
and matrix metalloproteinase-dependent generation of soluble
EMMPRIN. Mol Cancer Res. 2:73–80. 2004.
|
|
11
|
Yang X, Dai J, Li T, et al: Expression of
EMMPRIN in adenoid cystic carcinoma of salivary glands: correlation
with tumor progression and patients’ prognosis. Oral Oncol.
46:755–760. 2010.PubMed/NCBI
|
|
12
|
Li SL: Establishment of a human cancer
cell line from adenoid cystic carcinoma of the minor salivary
gland. Zhonghua Kou Qiang Yi Xue Za Zhi. 25:29–31. 1990.(In
Chinese).
|
|
13
|
Dong L, Wang YX, Li SL, et al: TGF-beta1
promotes migration and invasion of salivary adenoid cystic
carcinoma. J Dent Res. 90:804–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quemener C, Gabison EE, Naïmi B, et al:
Extracellular matrix metalloproteinase inducer up-regulates the
urokinase-type plasminogen activator aystem promoting tumor cell
invasion. Cancer Res. 67:9–15. 2007. View Article : Google Scholar
|
|
15
|
Chen W, Zhang HL, Jiang YG, Li JH and Sun
MY: Inhibition of CD146 gene expression via RNA interference
reduces in vitro perineural invasion on ACC-M cell. J Oral Pathol
Med. 38:198–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng HC, Takahashi H, Murai Y, et al:
Upregulated EMMPRIN/CD147 might contribute to growth and
angiogenesis of gastric carcinoma: a good marker for local invasion
and prognosis. Br J Cancer. 95:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueda K, Yamada K, Urashima M, et al:
Association of extracellular matrix metalloproteinase inducer in
endometrial carcinoma with patient outcomes and clinicopathogenesis
using monoclonal antibody 12C3. Oncol Rep. 17:731–735. 2007.
|
|
18
|
Xu J, Xu HY, Zhang Q, et al: HAb18G/CD147
functions in invasion and metastasis of hepatocellular carcinoma.
Mol Cancer Res. 5:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stockinger H, Ebel T, Hansmann C, et al:
EC/16/760 neurothelin/basigin/M6/EMMPRIN Workshop Panel Report.
Leucocyte Typing VI: White Cell Differentiation Antigens. Kishimoto
T, Kikutani H, von den Borne AEGKr, et al: Garland Publishing; New
York: pp. 760–763. 1997
|
|
20
|
Kleinman HK, McGarvey ML, Hassell JR, et
al: Basement membrane complexes with biological activity.
Biochemistry. 25:312–318. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liotta LA and Kohn EC: The
microenvironment of the tumor-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shirasuna K, Saka M, Hayashido Y, Yoshioka
H, Sugiura T and Matsuya T: Extracellular matrix production and
degradation by adenoid cystic carcinoma cells: participation of
plasminogen activator and its inhibitor in matrix degradation.
Cancer Res. 53:147–152. 1993.
|
|
23
|
Vihinen P and Kähäri VM: Matrix
metalloproteinases in cancer: prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Freitas VM, Vilas-Boas VF, Pimenta DC, et
al: SIKVAV, a laminin alpha1-derived peptide, interacts with
integrins and increases protease activity of a human salivary gland
adenoid cystic carcinoma cell line through the ERK 1/2 signaling
pathway. Am J Pathol. 171:124–138. 2007. View Article : Google Scholar
|
|
25
|
Tang Y, Nakada MT, Kesavan P, et al:
Extracellular matrix metalloproteinase inducer stimulates tumor
angiogenesis by elevating vascular endothelial cell growth factor
and matrix metalloproteinases. Cancer Res. 65:3193–3199. 2005.
|
|
26
|
Tang Y, Nakada MT, Rafferty P, et al:
Regulation of vascular endothelial growth factor expression by
EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res.
4:371–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marieb EA, Zoltan-Jones A, Li R, et al:
Emmprin promotes anchorage-independent growth in human mammary
carcinoma cells by stimulating hyaluronan production. Cancer Res.
64:1229–1232. 2004. View Article : Google Scholar
|
|
28
|
Li M, Zhai Q, Bharadwaj U, et al:
Cyclophilin A is overexpressed in human pancreatic cancer cells and
stimulates cell proliferation through CD147. Cancer. 106:2284–2294.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Lin J, Kanekura T, et al: A small
interfering CD147-targeting RNA inhibited the proliferation,
invasiveness, and metastatic activity of malignant melanoma. Cancer
Res. 66:11323–11330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu C, Pan Y, He B, et al: Inhibition of
CD147 gene expression via RNA interference reduces tumor cell
invasion, tumorigenicity and increases chemosensitivity to
cisplatin in laryngeal carcinoma Hep2 cells. Oncol Rep. 25:425–432.
2011.PubMed/NCBI
|
|
31
|
Bhowmick NA and Moses HL: Tumor-stroma
interactions. Curr Opin Genet Dev. 15:97–101. 2005. View Article : Google Scholar : PubMed/NCBI
|