1. Introduction
miRNA was initially discovered as a small temporal
RNA (stRNA) in C.elegans in 1993 (1). However, not much attention was paid to
this finding until seven years later when Let-7 (the second miRNA)
was identified (2). In the
following years, researchers became aware that miRNAs were a large
family of small non-coding RNAs that exist in species ranging from
plants to humans (3–8). Correspondingly, the functions of
miRNAs were found to not be limited to temporal regulation, but
were shown to be implicated in various biological processes,
including cell cycle (9,10), proliferation (11), apoptosis (12,13)
and development (6). In 2002, Calin
and colleagues (14) reported the
first direct evidence of miRNA playing a role in human cancer; they
found that miR-15 and miR-16 contribute to chronic lymphocytic
leukemia. Subsequently, more examples of miRNA correlated with
human cancers were noted. Iorio and colleagues (15) first demonstrated miRNA dysregulation
in human breast cancer by miRNA microarray; they found that
miR-10b, miR-125b, and miR-145 were down-regulated, while miR-21
and miR-155 were up-regulated, suggesting that these miRNAs may act
as potential tumor suppressor genes or oncogenes, respectively.
Following this finding, more functional studies had identified
specific miRNAs as pivotal regulators in different stages of breast
cancer development (initiation, progression and metastasis). In
this review we will summarize the current understanding regarding
the functions of miRNAs in breast cancer tumorigenesis.
Finally, based on these experimentally validated
specific breast cancer-associated miRNAs and their gene targets, we
summarize stage-specific miRNA functions of breast-derived cells
and tissues in different phases (Table
I). This approach reveals that some miRNAs, such as miR-21,
play a key role in all phases of breast cancer tumorigenesis. Other
miRNAs, such as miR-30, miR-17-5p, miR-9, are phase-specific. It
suggests that regulation of miRNAs themselves at specific stages
may be crucial for breast cancer tumorigenesis.
 | Table ImicroRNAs and their targets in
different phases. |
Table I
microRNAs and their targets in
different phases.
| Phase | microRNA | Expression and
role | Target | Refs. |
|---|
| Initiation | miR-200c | Down-regulated,
BT-IC self-renewal suppressor | Bmi-1 | (20) |
| Let-7 | Down-regulated,
BT-IC self-renewal suppressor | Ras | (21) |
| miR-30 | Down-regulated,
BT-IC self-renewal suppressor | Ubc9, integrin
β3 | (22,23) |
| Progression | miR-21 | Up-regulated,
anti-apoptotic factor | Unknown | (33) |
| miR-145 | Down-regulated,
inducing apoptosis | RTKN | (35) |
| miR-155 | Up-regulated,
anti-apoptotic factor | FOXO3a | (36) |
| miR-34a | Down-regulated,
inducing apoptosis | Bcl-2 | (39) |
| miR-17/20 | Down-regulated,
proliferation suppressor | Cyclin D1 | (41,42) |
| miR-27a | Up-regulated,
inducing proliferation | Myt-1, ZBTB10 | (43) |
| miR-17-5p | Down-regulated,
proliferation suppressor | AIBI | (44) |
|
miRs-106b/93/25 | Up-regulated,
inducing proliferation | pRb | (45) |
| miR-206 | Up-regulated,
inducing proliferation | ERα | (47) |
|
miR-18a/b/221/222 | Up-regulated,
inducing proliferation | ERα | (48,49) |
| miR-21 | Up-regulated,
inducing proliferation | PTEN | (50) |
| Metastasis | miR-205/200 | Down-regulated, EMT
suppressor | ZEB1, ZEB2 | (59) |
| miR-31 | Down-regulated, EMT
suppressor | RhoA | (60) |
| miR-155 | Up-regulated,
inducing EMT | E-cadherin | (61) |
| miR-21 | Up-regulated,
inducing EMT | TIMP1, TIMP3
PDCD4 | (66,67) |
|
miR-373/miR-520c | Up-regulated,
inducing EMT | CD44 | (53) |
|
miR-335/miR-126 | Up-regulated,
inducing EMT | TNC | (77) |
| miR-10b | Up-regulated,
inducing EMT | HOXD10 | (80) |
| miRNA-672 | Down-regulated, EMT
suppressor | PRDX6 | (83) |
| miR-126 | Down-regulated,
angiogenesis suppressor | VEGF | (87) |
| miR-9 | Up-regulated,
inducing EMT and angiogenesis | E-cadherin | (57) |
| miR-20b | Down-regulated,
angiogenesis suppressor | HIF-1α | (92) |
2. microRNAs as regulators in breast cancer
initiation
Currently, it is universally acknowledged that
cancers may arise from cancer stem cells, also termed as
tumor-initiating cells (T-IC), which are the primary cellular
components within a tumor that drives disease progression and are
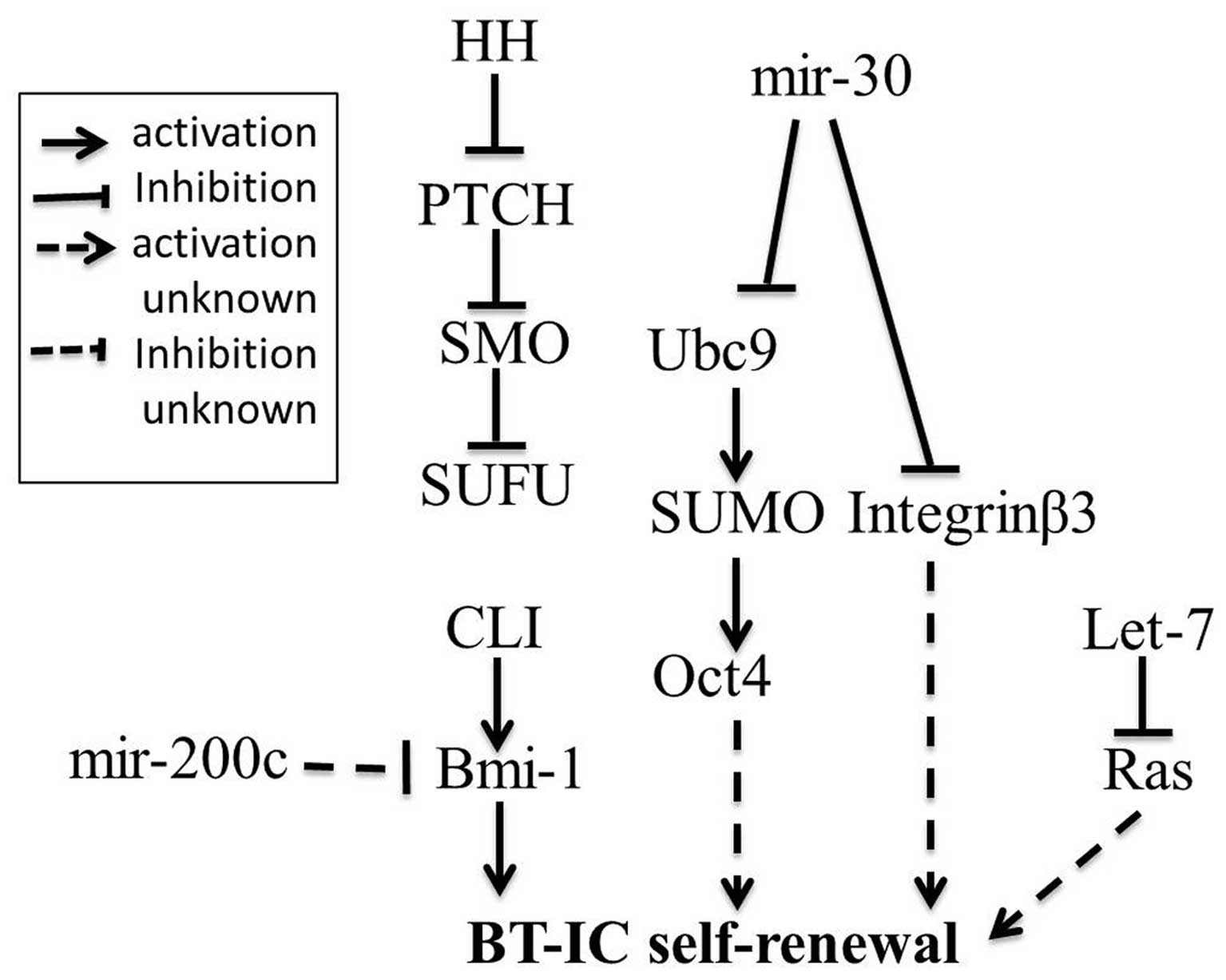
characterized by their stem-like ability to self-renew (16). Evidence of breast cancer-initiating
cells (BT-IC) was reported by Al-Hajj and Clarke (17). Such cells may be responsible for
breast cancer initiation. However, how the self-renewal of BT-ICs
is regulated remains obscure. A previous study showed that the
regulation of the self-renewal of breast cancer stem cells is
associated with the Hedgehog pathway. Bmi-1 is a downstream target
of Hedgehog pathway. Furthermore, Bmi-1 has been shown to be
required for self-renewal (18,19).
These findings clearly indicate that the Hedgehog pathway activates
breast cancer stem cell self-renewal by Bmi-1. A recent study has
implicated several miRNAs in the regulation of BT-IC self-renewal.
These miRNAs include miR-200c, Let-7, miR-30, but presently, little
is known about the mechanism by which it functions to regulate
BT-IC self-renewal. What is clear is that miR-200c strongly
suppressed the ability for self-renewal of breast cancer stem cells
(20). Studies also show that lack
of Let-7 is required for self-renewal in breast cancer stem cells;
moreover, Ras is determined as the direct target of Let-7 and its
silencing contributes to loss of BT-IC self-renewal (21). More recently, it is demonstrated
that up-regulated expression of miR-30 in breast cancer-initiating
cells inhibits their self-renewal capacity by reducing the
ubiquitin-conjugating enzyme 9 (Ubc9). Ubc9 has been shown to be
specific for small ubiquitin-related modifier (SUMO) activation.
SUMO may up-regulate Oct4 by stabilizing its structure (22,23).
Oct4 overexpression induced by Ubc9 contributes to the self-renewal
(24). Integrin β3 (ITGB3) is
another direct target of miR-30, which contributes to apoptosis
(22). A previous report has shown
that unligated ITGB3 recruits caspase-8 to the cell membrane and
activated caspase-8-mediates apoptosis in a death
receptor-independent manner (25),
while miR-30 induces apoptosis not in the death
receptor-independent manner but through an unclear pathway. In
addition, a recent study indicates that integrins play a role via
directly regulating the ability of BT-ICs to self-renew during the
initial steps of breast cancer tumorigenesis (26). These findings support two greatly
simplified models of regulation of BT-IC self-renewal (Fig. 1). The identification of miRNAs
functioning as regulators of BT-IC self-renewal partially expand
our understanding of the regulation of breast cancer
initiation.
Although the molecular mechanisms by which miRNAs
play a crucial role in tumor progression and metastasis have been
studied in great detail over the last decades, the role of miRNAs
in the early events of tumorigenesis has only recently been
demonstrated. As tumor formation is a multi-step process, the
initiating events may facilitate the development of effective
targeted therapeutic strategies for cancer.
3. Roles of microRNAs in breast cancer
progression
Cancer stem cells drive tumor progression and
heterogeneity by proliferating and generating some differentiated
cancer cells (27). These
differentiated cancer cells will gain the ability to anti-apoptosis
and full out of the control of the normal cell cycle during cancer
progression. Here, we will highlight those miRNAs identified as
regulators of anti-apoptosis and of the cell cycle in breast cancer
progression.
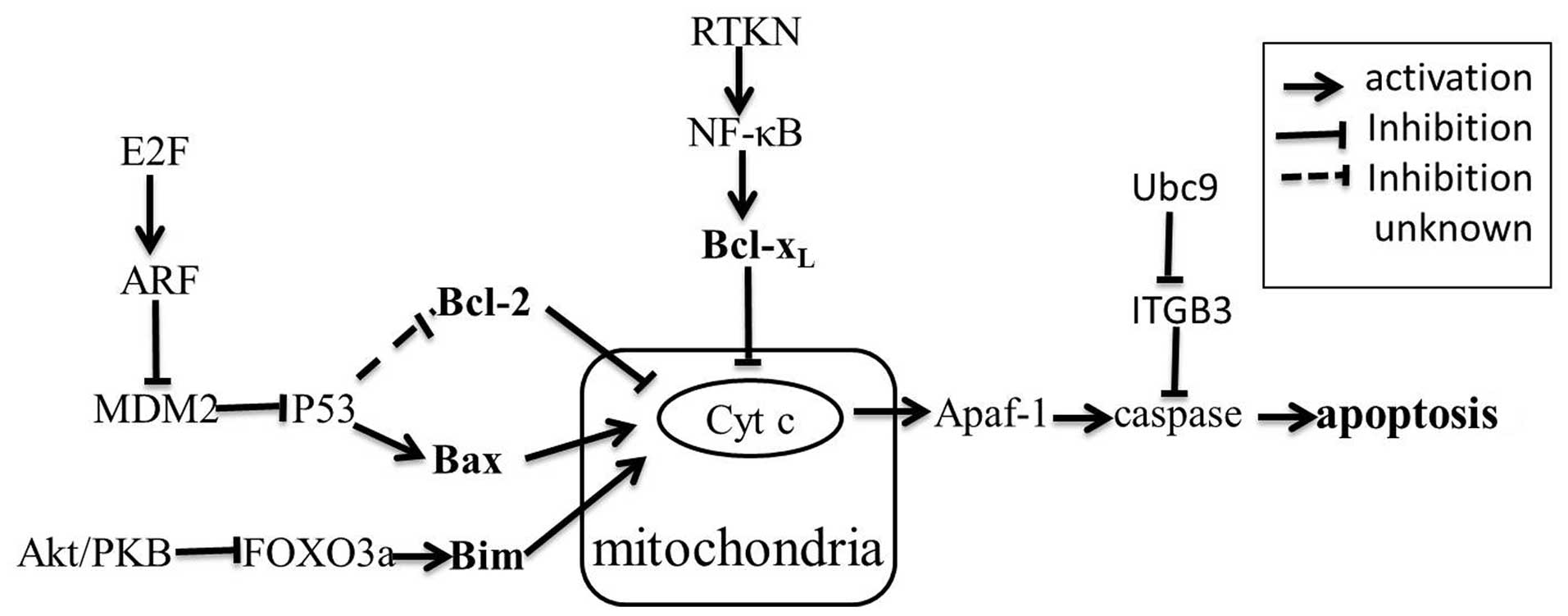
Anti-apoptosis
In normal breast tissue, apoptosis plays a key role
for performing the normal functions. The mechanism of apoptosis
still needs to be fully investigated. There is evidence that
mitochondria play an essential role in the apoptotic process
(Fig. 2). Several pathways
contribute to apoptosis, but the best characterized are the Akt/PKB
pathway, RTKN/NF-κB survival pathway and the p53-mediated apoptosis
pathway (28–30). Emerging research shows that miRNAs
are involved in these pathways. Bcl-2 family proteins can be
thought of as the central factors of the apoptotic pathway. The
Bcl-2 family is comprised of many proteins, which can be classified
into three functional groups. Group I members, including Bcl-2 and
Bcl-xL, possess anti-apoptotic activity; group II
members, including Bax and Bak, are characterized by pro-apoptotic
activity; group III members, such as Bim and Bad, also possess
pro-apoptotic activity (31). After
the identification of the down-regulation of miR-15/16 promoting
anti-apoptosis via up-regulating Bcl-2 expression in leukemias and
lymphomas, more miRNAs promoting anti-apoptosis by directly or
indirectly regulating Bcl-2 family proteins have been observed in
many types of cancer, including breast cancer (32).
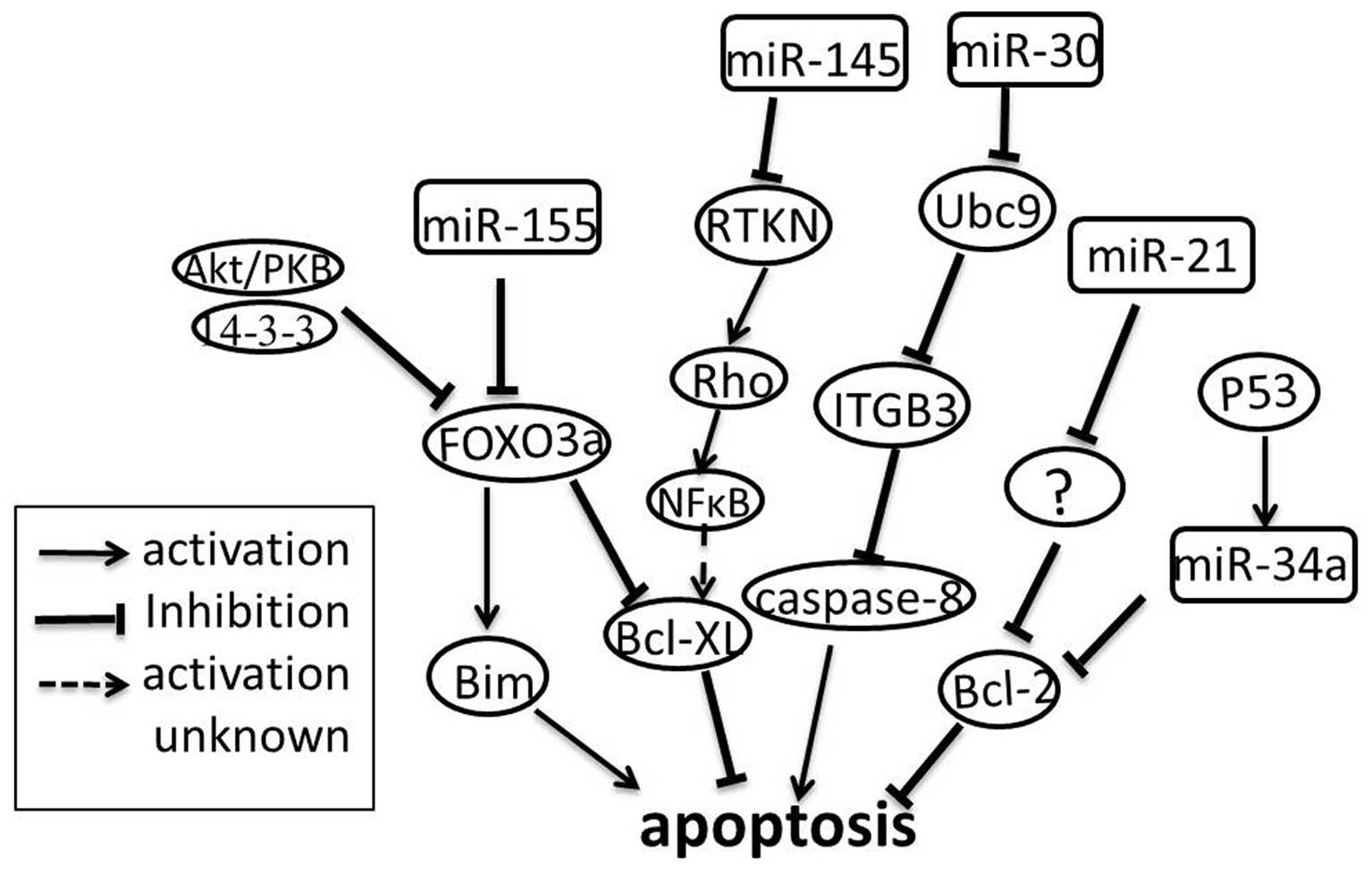
Studies by Si and colleagues (33) showed that treatment of MCF-7 breast
cancer cells with anti-miR-21 causes cell apoptosis. Moreover, they
detected a lower level of Bcl-2 expression both at the mRNA and at
the protein level in the anti-miR-21-transfected MCF-7 cells as
well as in tumors derived from the MCF-7 cells transfected with
anti-miR-21 (33). These
investigations reveal that miR-21 may act as an anti-apoptotic
factor by indirectly targeting Bcl-2, consistent with the previous
report for glioblastoma cells (34). In contrast, miR-145 was
down-regulated in MCF-7 cells, and overexpression of miR-145
suppressed MCF-7 cell growth and induced apoptosis (35). RTKN was confirmed as a direct target
of miR-145 in MCF-7 cells (35).
RTKN further enhanced the expression of Bcl-xL by
activating NF-κB (29). In normal
cells, miR-145 suppresses RTKN to maintain its low expression
level; while in cancer cells, miR-145 is down-regulated, leading to
increased cell survival. As mentioned above, miR-21 is up-regulated
and miR-145 is down-regulated in breast cancer cells, but each
contributes to anti-apoptosis via different pathways. How the two
miRNAs cooperate with each other to promote apoptosis resistance is
fully unknown, maybe the identification of the direct target of
miR-21 in regulating Bcl-2 expression will shed a new light on our
understanding of the complex regulatory mechanism. Similarly,
miR-155 is also up-regulated in breast cancer cells, and also
contributes to anti-apoptosis. Kong et al (36) demonstrated that miR-155 induces cell
survival by targeting FOXO3a in breast cancer. FOXO3a is a major
member of the forkhead transcription factor family characterized by
a distinctive forkhead DNA binding domain. They are localized in
the nucleus without growth factor stimulation, and function as
transcription factors to enhance apoptosis by promoting the
expression of Bim (pro-apoptotic member of the Bcl-2 family). When
phosphorylated by protein kinase B, FOXO3a is assembled into a
complex with the 14-3-3 protein. The complex is then exported from
the nucleus and loses its pro-apoptotic function (36,37).
miR-34a is shown to play a role in the p53-mediated apoptosis of
human lung cancer cells. Further study suggests that p53 can
directly regulate the gene encoding miR-34a. Bcl-2 is a target of
miR-34a and loss of miR-34a protect cells from apoptosis (Fig. 3) (30). miRNAs involved in p53-mediated
apoptosis are also validated in other cancer cell lines (38). However, little is known about such
miRNAs in breast cancer. A recent study examined whether miR-34a is
necessary to induce apoptotic cell death in a breast cancer cell
line. This process seems to be associated with p53, but the
mechanism remains largely unknown (39). Cleary, the events of miRNA-mediated
anti-apoptosis in breast cancer is still not yet fully realized and
many advances should be anticipated in this field in the
foreseeable future.
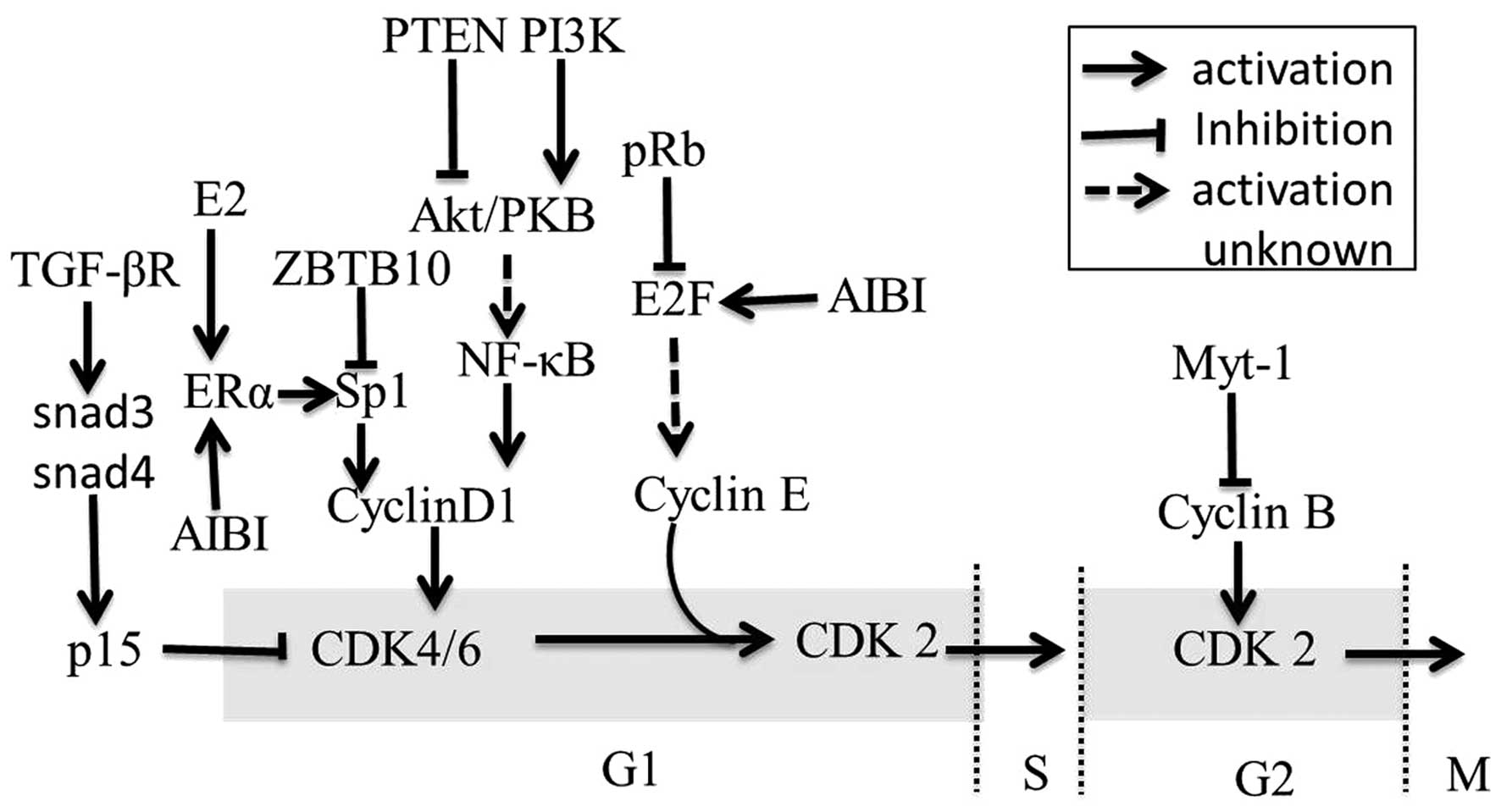
Cell cycle dysregulation
Cancer cells are characterized by deregulated cell
proliferation during cancer progression. Proliferation is
controlled by cell cycle in normal tissues (40). Numerous regulatory pathways
contribute to the cell cycle and their alterations are necessary
for cancer cells to overcome the control of the normal cell cycle
(Fig. 4). miRNAs may alter the cell
cycle by controlling regulators of these regulatory pathways. In
this section, the roles of miRNAs in breast cancer cell cycle
regulation will be discussed.
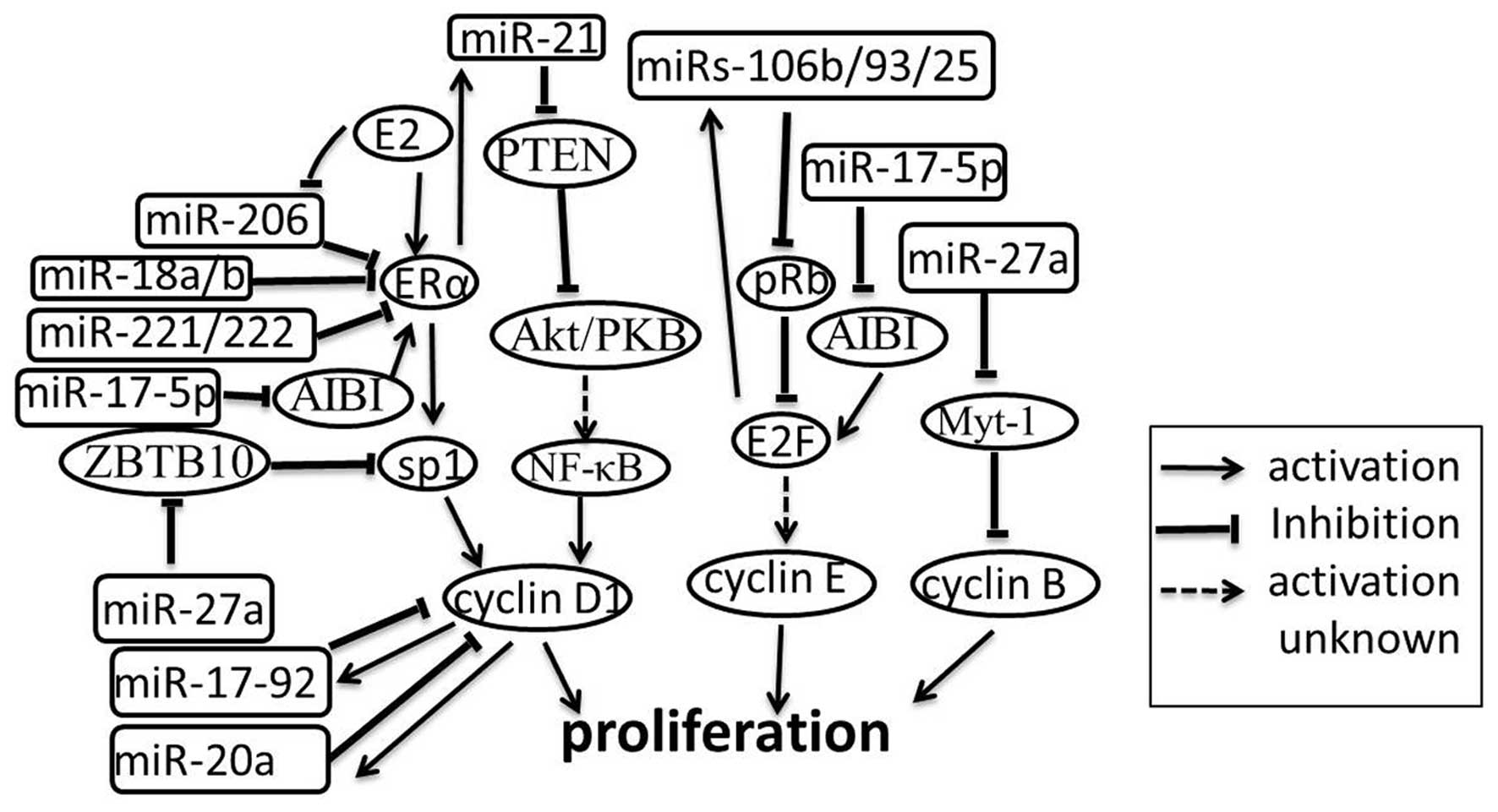
The cyclin/CDK (cyclin dependent kinase) pathway is
an important pathway in the regulation of the cell cycle. This
pathway can be regulated by several miRNAs in breast cancer and in
cell lines. For example, the miR-17-5p/miR-20a miRNA cluster is
shown to attenuate cyclin D1 through directly combining with the
3′-UTR binding site in MCF-7 cell line, thereby inhibiting S-phase
entry and halting cell proliferation. Correspondingly, the
miR-17/20 cluster is down-regulated and promotes cell proliferation
in breast cancer cells (41).
Further studies reveal a novel regulatory mechanism in which cyclin
D1 induces an miRNA signature including miR-17/20 through the
binding of the miR-17/20 promoter region (42). In addition to the miR-17/20 cluster,
miR-27a is also associated with the cyclin/CDK pathway. ZBTB10 and
Myt-1 are identified as direct targets of miR-27a. ZBTB10 (a
putative Sp repressor) can inhibit the proliferation of breast
cancer cells by suppressing cyclin D1 indirectly and Myt-1 can
block cell cycle progression at the G2/M phase through suppression
of cyclin B (43). E2Fs are
critical regulators of the cell cycle; they can activate the
expression of the miRs-106b/93/25 cluster. E2F is a downstream
target of pRb and miRs-106b/93/25 can silence pRb. Furthermore,
miR-17-5p is down-regulated in breast cancer cell lines, which has
been shown to limit the oncogene AIB1, which enhances the
transcriptional activity of the estrogen receptor (ER) and E2F1,
leading to proliferation suppression (44). Thus, a negative feedback loop is
generated (Fig. 5) (45).
Another cell cycle regulatory pathway in breast
cancer is the E2/ERα/Sp1 pathway (46). Upon activation of the receptor
estrogen receptor α (ERα), the pathway ERα/Sp1 enhances
proliferation via activating cyclin D1, which eventually leads to
the G1/S-phase transition. The regulation between the ER and miRNAs
has been extensively investigated.
miR-206 is up-regulated in ERα-negative breast
tumors and cell lines and inhibits ERα translation by binding to
the 3′UTR of ERα mRNA (47). In
addition to miR-206, ERα mRNA is also a direct target of miR-18a,
miR-18b, miR-193b, miR-302c and miR-221/222 in breast cancer cells.
Similar to miR-206, miR-18a, miR-18b and miR-221/222 are also
up-regulated in ERα-negative cell lines, suggesting an important
role of these miRNAs in the development of ERα-negative breast
cancers (48,49). The latter study also suggested that
miR-206 expression was strongly inhibited by ERα agonists (E2 and
PPT), but not by an ERβ agonist (DPN) and progesterone in MCF-7
cells. This finding suggests the existence of a feedback loop
between ER and miRNAs (47). In
contrast, E2 increased the expression of miR-21 and Let-7 family
members in ERα positive breast cancers (50). A previous publication reported that
E2 can down-regulate miR-21 expression and thus increases the
protein expression of miR-21 target genes programmed cell death 4
(PDCD4), PTEN and Bcl-2 in MCF-7 breast cancer cells (51). Whatever the reasons for this
discrepancy, an attractive speculation is that multiple signaling
pathways exist in the regulation of breast cancer cell growth
linked to ERs and miRNAs. E2-induced activated ERα directly binds
to the miR-21 promoter sequence and increases the levels of miR-21
(50), synchronously recruiting
other transcriptional cofactors that bind to target DNA elements
thus affecting cell growth in ERα-positive breast tumors. In
ERα-negative breast tumors, the E2/ERα signaling pathway is blocked
and miR-21 will be decreased. These events may lead to the
activition of other ER isoforms which induce the expression of some
miRNAs, such as miR-206, miR-18a, miR-18b, miR-221 and miR-222,
leading to further inhibition of ERα expression (49)and the activation of other pathways
controlling cell growth and proliferation. For the complex
regulation of cell proliferation, miRNAs and coregulators are up-
or down-regulated by a variety of interacting mechanisms, the
investigation of which has only begun. Additional studies need to
be undertaken for a more in-depth understanding.
4. microRNAs in breast cancer
metastasis
After initiation and progression, cancer cells will
proceed to the final step: invasion and metastasis (52,53).
To initiate the process, tumor cells must first penetrate the
epithelial basement membrane and then invade the interstitial
stroma. Traversal of basement membranes may require the
epithelial-mesenchymal transition (EMT). Distant metastases require
tumor-induced angiogenesis that allows for expansion of the primary
tumor and permits easy access to the vascular compartment due to
defects in the basement membrane of newly formed vessels (54). Many factors, including miRNAs, have
been identified as regulators in these processes in different human
tumor types. This section will focus on the functions of these
factors in breast cancer (Fig.
6).
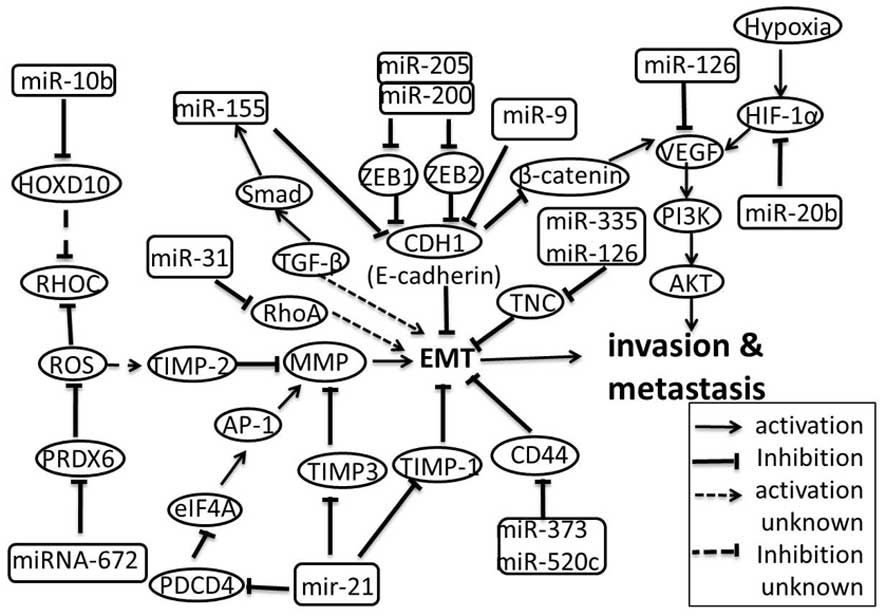
EMT is characterized by loss of cell adhesion.
E-cadherin plays an inhibitory role for cell adhesion molecules
(CAM) in metastasis and mediates cell-cell binding. Loss of
E-cadherin is a marker that EMT is involved in the progression of
carcinoma in situ to invasive breast cancer (55,56).
miR-9 directly targets CDH1, which is the E-cadherin coding gene,
leading to increased cell motility and invasiveness of SUM149 human
breast cancer cells (57). Zinc
finger E-box binding homeobox 1 (ZEB1) and ZEB2 are shown to be
crucial EMT activators in breast cancer by inhibiting E-cadherin
expression (58). The miR-200
family and miR-205 directly target ZEB1 and ZEB2, suggesting that
down-regulation of these miRNAs is an essential early step in
metastasis (59). In addition, a
recent study indicates that miR-31 prevents metastasis at multiple
steps by inhibiting the expression of prometastatic genes. RhoA is
one of such genes, which may enhance EMT in human breast cancer
(60). In contrast, miR-155 is
overexpressed in breast cancer by the TGF-β/Smad4 pathway and
mediates TGF-β-induced EMT by directly targeting RhoA in NMuMG
cells. Further study suggests that miR-155 also mediates EMT by
indirectly down-regulating E-cadherin (61). These findings reveal that RhoA
functions as the target of both tumor suppressor miRNAs (miR-31)
and oncogenic miRNAs (miR-155). Both the up- and down-regulation of
RhoA contribute to the EMT in different cell lines, suggesting that
RhoA regulates EMT in a multiphasic manner.
For invasion to take place, cyclic attachment to
matrix components must be released. Metalloproteinases (MMP) play
an important role in this event. MMP can degrade the ECM, which is
the extracellular part of tissue and mediates cell attachment
(62). The tissue inhibitor of
metalloproteinases (TIMPs) inhibits the activity of MMP (63) and contains a consensus miR-21
binding site. Previous study reported that miR-21 directly targets
TIMP3 in glioma cells and leads to increases of their migratory and
invasive abilities (64,65). A recent study showed for the first
time that miR-21 negatively regulates TIMP3 expression in breast
cancer via the binding of the 3′UTR of TIMP3 mRNA and promotes
breast cancer invasion in multiple cell lines in vitro
(66). In addition, miR-21 also
affects invasion and metastasis by directly suppressing expression
of tropomyosin 1 (TPM1), PDCD4 and maspin (67). As an actin-binding protein, TPM1 is
capable of stabilizing microfilaments and controlling cell motility
(68). The actin microfilaments are
components of the cytoskeleton, and mediate a variety of essential
biological functions in all eukaryotic cells, including providing
the driving force for cells (69).
TPM1 mRNA expression has been shown to be reduced in the metastatic
breast cancer MDA-MB-231 and MDA-MB-435 cell lines and in
metastatic colon cancer SW620 cell line (70). These facts suggest that suppression
of TPM1 expression by miR-21 is a general way for metastatic tumor
cells to disrupt the ECM and contribute to metastasis. Similarly,
PDCD4 expression is blocked by miR-21 in breast cancer and colon
cancer, suggesting that this interplay may be a general
carcinogenic pathway, rather than a tissue-specific mechanism
(71,72). The mechanism by which PDCD4
regulates cell invasion remains unclear, however, present evidence
supports that PDCD4 inhibits AP-1 by binding to the eukaryotic
translation initiation factor 4A (eIF4A). Subsequently, AP-1 and
other cis-acting elements together interact with the AP-1 site at
the MMP promoter (73,74). Earlier evidence suggests that
miR-373 and miR-520c can stimulate migration and invasion of MCF-7
and MDA-MB-435 cells, at least in part through direct suppression
of CD44 (53), which functions as a
cell surface receptor for several ECM components and mediates
cell-cell or cell-substrate interactions through recognition of
elements of the ECM (75,76). In addition, miR-335 and miR-126 are
also reported to be associated with the ability of breast cancer
cells to metastasize to the lung and bone by directly suppressing
the ECM component tenascin c (TNC) (77). Taken together, these observations
reveal that miRNA can induce cell migration and invasion by
directly degrading the ECM or disrupting recognition between the
cell and the ECM.
RhoC is an extensive researched prometastatic gene
(78,79), which is reported a member of the Ras
superfamily of small GTPases, playing a role in modulating assembly
of actin-myosin contractile filaments and focal adhesion complex.
Ma and colleagues (80) initially
observed that miR-10b is highly expressed in metastatic breast
cancer cells and they further found that the miR-10b is induced by
Twist and it inhibits translation of the mRNA encoding HOXD10 (a
homeobox transcription factor that promotes or maintains a
differentiated phenotype in epithelial cells). RhoC increases with
the decrease of HOXD10 leading to tumor cell invasion and
metastasis (80,81). A later study indicated that RhoC is
dispensable for tumor initiation but essential for metastasis
(78). As a protein with both
glutathione peroxidase and phospholipase A2 activities,
peroxiredoxin (PRDX) 6 was previously described playing a crucial
role in reactive oxygen species (ROS) resistance. Lehtonen et
al (82) first demonstrated
that PRDX may be associated with human lung carcinoma. Chang and
colleagues (83) found that PRDX6
is up-regulated in highly invasive and potentially metastatic
MDA-MB-231 HM breast cancer cells compared with their parental
cells. Furthermore, they demonstrated that overexpression of PRDX6
in breast cancer cells promoted their invasive and metastatic
potential in vitro and in vivo (82,84).
RhoC was up-regulated and TIMP-2 was down-regulated, in the cells
with up-regulation of PRDX6 (83).
When PRDX6 was knockdown by miRNA-672, RhoC was down-regulated and
TIMP-2 was up-regulated (83).
These findings indicate that miRNA-672 indirectly regulates breast
cancer cell invasiveness and metastasis via down-regulating PRDX6
expression. However, how PRDX6 regulates RhoC and TIMP-2 as well as
whether PRDX6 is an instigator of metastasis or merely a
correlative product during progression of breast cancer are still
beyond present understanding (85).
As the available knowledge has shown that PRDX6 functions as an
anti-oxidative protein to protect cells from damage by ROS,
therefore it is reasonable to believe that ROS may play a key role
in the regulation of RhoC and TIMP-2 by PRDX6. Further study into
the mechanism of these relationships may add to the current state
of knowledge of these signaling pathways, and will improve our
understanding of metastasis-related interaction between miRNAs and
cancer protein-coding genes.
To obtain sufficient nutrients and oxygen for
metastasis of solid tumors, the formation of new blood vessels
(angiogenesis) is necessary (86).
It is now well established that tumor-induced angiogenesis is
driven by the overexpression of angiogenic factors such as vascular
endothelial growth factor (VEGF), which is the most potent inducer
of angiogenesis. Of their wide range of biological actions, the
role of miRNAs in tumor angiogenesis has received the greatest
attention. Recent studies have shown that VEGF-A may be
well-regulated by miR-126 in normal tissues and miR-126 was
restrictly expressed in human breast cancer where the VEGF/PI3K/AKT
signaling pathway was vigorously activated. In addition, miR-126
directly targeted VEGF and its expression was decreased in human
breast cancer, revealing that miR-126 plays a role in angiogenesis
(87–90). Yet, another pathway regulating VEGF
expression was presented by Ma et al (57) who described that the up-regulation
of VEGF-A mRNA by miR-9 depends on its ability to down-regulate
E-cadherin expression and to activate β-catenin-mediated
transcription. E-cadherin has been identified as the direct target
of miR-9 and VEGF-A has been described as a transcriptional target
gene of β-catenin. The data illustrates a novel mechanism by which
miR-9 promotes angiogenesis through stimulation of VEGF-A
expression in breast cancer. A recent study proposed that the VEGF
expression in breast cancer cells is mediated by HIF-1 in a
miR-20b-dependent manner. Hypoxia is one of the features within the
tumor microenvironment. Hypoxia inducible factor1 (HIF-1) is a
heterodimeric transcription factor consisting of HIF-1α and HIF-1β
subunits (91,92). Under oxygenated conditions, HIF-1α
is rapidly degraded, while in hypoxic conditions, this factor is
stabilized and contributes to angiogenesis by directly activating
the VEGF gene (93). Taking into
account the above discussed evidence on the involvement of miRNAs
in breast cancer-induced angiogenesis it would be of interest to
address the functional relationship among these miRNAs. These
regulators adjust the same target-VEGF by different pathways under
specific conditions and trigger angiogenesis. One interesting
question to be addressed is whether these miRNAs share the same
specific expression pattern or not. Therefore, more novel miRNAs
which participate in the process of VEGF-mediated angiogenesis in
breast cancer should be identified to understand these expression
patterns.
5. Conclusion
Breast cancer develops because of complex multistep
processes. Generally speaking, there are three phases (initiation,
progression and metastasis) in the complex multistep process. These
phases are composed of a sequence of events, including self-renewal
apoptosis, cell cycle and mobility. miRNAs are an evolutionarily
conserved class of small, approximately 22-nucleotide non-coding
RNAs that decrease gene expression post-transcriptionally in a
sequence-specific manner, which participates in these events and
some members extensively contribute to breast tumorigenesis. Over
the past years, the utilizations of high-throughput technologies,
such as microarray, a large number of ectopic miRNAs have been
observed in breast cancer but the critical roles of most of these
miRNAs remain largely unknown. Among these miRNAs, one miRNA can
potentially regulate the expression of hundreds of genes, and on
the other hand, a single transcript can be targeted by multiple
miRNAs. However, knowledge of how an miRNA simultaneously
down-regulates multiple proteins in the same pathway and an
understanding of the miRNA target genes and their biologic
functions is limited. There is no doubt that to further understand
breast cancer pathogenesis, identifying the genome-wide targets of
these miRNAs is essential. In addition, identifying the factors
determining tissue-and cell-specific expression of miRNAs is also
pivotal. As described above, miRNAs may act cooperatively through
multiple target sites in one gene (94), or one miRNA may regulate a group of
functionally related genes. Interestingly, unwanted cross-reaction
does not appear in these two regulatory patterns. In addition, the
mechanism does not seem to be specific of miRNA itself but the
specific expression of miRNA. If only partially complementary
sequences exist, targets will be repressed, regardless of the
target gene specificities. In fact, miRNAs are expressed in
specific cells and tissues at specific developmental stages and
conditions. These facts clearly show that the system determining
the specific expression of miRNAs plays a key role in regulating
gene expression. In recent years, numerous miRNAs and their targets
have been confirmed in breast cancer and have been recognized as
new therapeutic targets. However, these new therapeutic targets may
not work, for the destruction of interplay between one miRNA and
its target will be restored by another miRNA with similar function.
Here, we postulate that the miRNA specificity determining system is
composed of the effective targets, and miRNAs function as signal
molecules together with other regulatory elements mediating breast
cancer tumorigenesis in a stage-specific manner. Nevertheless, at
the current stage little is known about these systems and various
aspects of them need to be clarified in a future study. Taken
together, the miRNA specificity determining system may serve as
more effective potential target for breast cancer therapy in
comparison with miRNA.
References
|
1
|
Lee R, Feinbaum R and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
2
|
Reinhart BJ, Slack FJ, Basson M, et al:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vetter G, Saumet A, Moes M, et al: miR-661
expression in SNAI1-induced epithelial to mesenchymal transition
contributes to breast cancer cell invasion by targeting Nectin-1
and StarD10 messengers. Oncogene. 29:4436–4448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: MicroRNA pathways in flies and
worms: growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palatnik JF, Allen E, Wu X, et al: Control
of leaf morphogenesis by microRNAs. Nature. 425:257–263. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatfield SD, Shcherbata HR, Fischer KA,
Nakahara K, Carthew RW and Ruohola-Baker H: Stem cell division is
regulated by the microRNA pathway. Nature. 435:974–978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O’Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005.PubMed/NCBI
|
|
11
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong AW and Nemunaitis J: Modulation of
miRNA activity in human cancer: a new paradigm for cancer gene
therapy? Cancer Gene Ther. 15:341–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calin G, Dumitru C, Shimizu M, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iorio MV, Ferracin M, Liu CG, et al:
MicroRNA gene expression deregulation in human breast cancer.
Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Hajj M: Cancer stem cells and oncology
therapeutics. Curr Opin Oncol. 19:61–64. 2007.PubMed/NCBI
|
|
17
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Dontu G, Mantle ID, et al: Hedgehog
signaling and Bmi-1 regulate self-renewal of normal and malignant
human mammary stem cells. Cancer Res. 66:6063–6071. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dimri GP, Martinez JL, Jacobs JJL, et al:
The Bmi-1 oncogene induces telomerase activity and immortalizes
human mammary epithelial cells. Cancer Res. 62:4736–4745.
2002.PubMed/NCBI
|
|
20
|
Shimono Y, Zabala M, Cho RW, et al:
Downregulation of miRNA-200c links breast cancer stem cells with
normal stem cells. Cell. 138:592–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu F, Yao H, Zhu P, et al: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu F, Deng H, Yao H, Liu Q, Su F and Song
E: Mir-30 reduction maintains self-renewal and inhibits apoptosis
in breast tumor-initiating cells. Oncogene. 29:4194–4204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müller S, Hoege C, Pyrowolakis G and
Jentsch S: SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell
Biol. 2:202–213. 2001.
|
|
24
|
Park SW, Hu X, Gupta P, Lin YP, Ha SG and
Wei LN: SUMOylation of Tr2 orphan receptor involves Pml and
fine-tunes Oct4 expression in stem cells. Nat Struct Mol Biol.
14:68–75. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stupack DG, Puente XS, Boutsaboualoy S,
Storgard CM and Cheresh DA: Apoptosis of adherent cells by
recruitment of caspase-8 to unligated integrins. J Cell Biol.
155:459–470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pontier SM and Muller WJ: Integrins in
mammary-stem-cell biology and breast-cancer progression - a role in
cancer stem cells? J Cell Sci. 122:207–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu CA, Wang MJ, Chi CW, Wu CW and Chen
JY: Rho/Rhotekin-mediated NF-kappaB activation confers resistance
to apoptosis. Oncogene. 23:8731–8742. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raver-Shapira N, Marciano E, Meiri E, et
al: Transcriptional activation of miR-34a contributes to
p53-mediated apoptosis. Mol Cell. 26:731–743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar
|
|
32
|
Cimmino A, Calin G, Fabbri M, et al:
miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2006.
|
|
34
|
Chan J, Krichevsky A and Kosik K:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang S, Bian C, Yang Z, et al: miR-145
inhibits breast cancer cell growth through RTKN. Int J Oncol.
34:1461–1466. 2009.PubMed/NCBI
|
|
36
|
Kong W, He L, Coppola M, et al:
MicroRNA-155 regulates cell survival, growth, and chemosensitivity
by targeting FOXO3a in breast cancer. J Biol Chem. 285:17869–17879.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sunters A, Fernández de Mattos S, Stahl M,
et al: FoxO3a transcriptional regulation of Bim controls apoptosis
in paclitaxel-treated breast cancer cell lines. J Biol Chem.
278:49795–49805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Le MT, Teh C, Shyh-Chang N, et al:
MicroRNA-125b is a novel negative regulator of p53. Genes Dev.
23:862–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kato M, Paranjape T, Ullrich R, et al: The
mir-34 microRNA is required for the DNA damage response in vivo in
C. elegans and in vitro in human breast cancer cells.
Oncogene. 28:2419–2424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fu M: Minireview: cyclin D1: normal and
abnormal functions. Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu Z, Wang C, Wang M, et al: A cyclin
D1/microRNA 17/20 regulatory feedback loop in control of breast
cancer cell proliferation. J Cell Biol. 182:509–517. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the G2-M
checkpoint in MDA-MB-231 breast cancer cells. Cancer Res.
67:11001–11011. 2007. View Article : Google Scholar
|
|
44
|
Hossain A, Kuo MT and Saunders GF:
Mir-17-5p regulates breast cancer cell proliferation by inhibiting
translation of AIB1 mRNA. Mol Cell Biol. 26:8191–8201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brosh R, Shalgi R, Liran A, et al:
p53-Repressed miRNAs are involved with E2F in a feed-forward loop
promoting proliferation. Mol Syst Biol. 4:2292008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Castro-Rivera E, Samudio I and Safe S:
Estrogen regulation of cyclin D1 gene expression in ZR-75 breast
cancer cells involves multiple enhancer elements. J Biol Chem.
276:30853–30861. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Adams BD, Furneaux H and White BA: The
micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen
receptor-(ER) and represses ER messenger RNA and protein expression
in breast cancer cell lines. Mol Endocrinol. 21:1132–1147. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leivonen SK, Makela R, Ostling P, et al:
Protein lysate microarray analysis to identify microRNAs regulating
estrogen receptor signaling in breast cancer cell lines. Oncogene.
28:3926–3936. 2009. View Article : Google Scholar
|
|
49
|
Zhao JJ, Lin J, Yang H, et al:
MicroRNA-221/222 negatively regulates estrogen receptor alpha and
is associated with tamoxifen resistance in breast cancer. J Biol
Chem. 283:31079–31086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bhat-Nakshatri P, Wang G, Collins NR, et
al: Estradiol-regulated microRNAs control estradiol response in
breast cancer cells. Nucleic Acids Res. 37:4850–4861. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wickramasinghe NS, Manavalan TT, Dougherty
SM, Riggs KA, Li Y and Klinge CM: Estradiol downregulates miR-21
expression and increases miR-21 target gene expression in MCF-7
breast cancer cells. Nucleic Acids Res. 37:2584–2595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gupta GP and Massague J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang Q, Gumireddy K, Schrier M, et al:
The microRNAs miR-373 and miR-520c promote tumour invasion and
metastasis. Nat Cell Biol. 10:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vincent-Salomon A and Thiery JP:
Epithelial-mesenchymal transition in breast cancer development.
Breast Cancer Res. 5:101–106. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tryndyak VP, Beland FA and Pogribny IP:
E-cadherin transcriptional down-regulation by epigenetic and
microRNA-200 family alterations is related to mesenchymal and
drug-resistant phenotypes in human breast cancer cells. Int J
Cancer. 126:2575–2583. 2010.PubMed/NCBI
|
|
57
|
Ma L, Young J, Prabhala H, et al: miR-9, a
MYC/MYCN-activated microRNA, regulates E-cadherin and cancer
metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
58
|
Blagosklonny MV, Dykxhoorn DM, Wu Y, et
al: miR-200 enhances mouse breast cancer cell colonization to form
distant metastases. PLoS One. 4:e71812009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gregory PA, Bert AG, Paterson EL, et al:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Valastyan S, Reinhardt F, Benaich N, et
al: A pleiotropically acting microRNA, miR-31, inhibits breast
cancer metastasis. Cell. 137:1032–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kong W, Yang H, He L, et al: MicroRNA-155
is regulated by the transforming growth factor beta/Smad pathway
and contributes to epithelial cell plasticity by targeting RhoA.
Mol Cell Biol. 28:6773–6784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Baker AH, George SJ, Zaltsman AB, Murphy G
and Newby AC: Inhibition of invasion and induction of apoptotic
cell death of cancer cell lines by overexpression of TIMP-3. Br J
Cancer. 79:1347–1355. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bode W, Reinemer P, Huber R, Kleine T,
Schnierer S and Tschesche H: The X-ray crystal structure of the
catalytic domain of human neutrophil collagenase inhibited by a
substrate analogue reveals the essentials for catalysis and
specificity. EMBO J. 13:1263–1269. 1994.PubMed/NCBI
|
|
64
|
Gabriely G, Wurdinger T, Kesari S, et al:
MicroRNA 21 promotes glioma invasion by targeting matrix
metalloproteinase regulators. Mol Cell Biol. 28:5369–5380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Selaru FM, Olaru AV, Kan T, et al:
MicroRNA-21 is overexpressed in human cholangiocarcinoma and
regulates programmed cell death 4 and tissue inhibitor of
metalloproteinase 3. Hepatology. 49:1595–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song B, Wang C, Liu J, et al: MicroRNA-21
regulates breast cancer invasion partly by targeting tissue
inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res.
29:292010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Perry SV: Vertebrate tropomyosin:
distribution, properties and function. J Muscle Res Cell Motil.
22:5–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar
|
|
70
|
Varga AE, Stourman NV, Zheng Q, et al:
Silencing of the Tropomyosin-1 gene by DNA methylation alters tumor
suppressor function of TGF-beta. Oncogene. 24:5043–5052. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lu Z, Liu M, Stribinskis V, et al:
MicroRNA-21 promotes cell transformation by targeting the
programmed cell death 4 gene. Oncogene. 27:4373–4379. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Asangani IA, Rasheed SAK, Nikolova DA, et
al: MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor
suppressor Pdcd4 and stimulates invasion, intravasation and
metastasis in colorectal cancer. Oncogene. 27:2128–2136. 2007.
View Article : Google Scholar
|
|
73
|
Yang HS, Matthews CP, Clair T, et al:
Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated
protein kinase kinase kinase kinase 1 expression to suppress colon
carcinoma cell invasion. Mol Cell Biol. 26:1297–1306. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Benbow U and Brinckerhoff CE: The AP-1
site and MMP gene regulation: what is all the fuss about? Matrix
Biol. 15:519–526. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Murai T, Maruyama Y, Mio K, Nishiyama H,
Suga M and Sato C: Low cholesterol triggers membrane
microdomain-dependent CD44 shedding and suppresses tumor cell
migration. J Biol Chem. 286:1999–2007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lesley J, Hyman R and Kincade PW: CD44 and
its interaction with extracellular matrix. Adv Immunol. 54:271–335.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tavazoie S, Alarcón C, Oskarsson T, et al:
Endogenous human microRNAs that suppress breast cancer metastasis.
Nature. 451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hakem A: RhoC is dispensable for
embryogenesis and tumor initiation but essential for metastasis.
Genes Dev. 19:1974–1979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Clark E, Golub T, Lander E and Hynes R:
Genomic analysis of metastasis reveals an essential role for RhoC.
Nature. 406:532–535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Myers C, Charboneau A, Cheung I, Hanks D
and Boudreau N: Sustained expression of homeobox D10 inhibits
angiogenesis. A J Pathol. 161:2099–2109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lehtonen ST, Svensk A-M, Soini Y, et al:
Peroxiredoxins, a novel protein family in lung cancer. Int J
Cancer. 111:514–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chang XZ, Li DQ, Hou YF, et al:
Identification of the functional role of peroxiredoxin 6 in the
progression of breast cancer. Breast Cancer Res. 9:R762007.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kümin A, Huber C, Rülicke T, Wolf E and
Werner S: Peroxiredoxin 6 is a potent cytoprotective enzyme in the
epidermis. Am J Pathol. 169:1194–1205. 2006.PubMed/NCBI
|
|
85
|
Chang XZ, Li DQ, Hou YF, et al:
Identification of the functional role of peroxiredoxin 6 in the
progression of breast cancer. Breast Cancer Res. 9:R762007.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Suarez Y and Sessa WC: MicroRNAs as novel
regulators of angiogenesis. Circ Res. 104:442–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhu N, Zhang D, Xie H, et al:
Endothelial-specific intron-derived miR-126 is down-regulated in
human breast cancer and targets both VEGFA and PIK3R2. Mol Cell
Biochem. 351:157–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gerber HP, McMurtrey A, Kowalski J, et al:
Vascular endothelial growth factor regulates endothelial cell
survival through the phosphatidylinositol 3′-Kinase/Akt signal
transduction pathway. J Biol Chem. 273:30336–30343. 1998.
|
|
89
|
Iva N and Karl-Heinz P: EGFL7 meets
miRNA-126: an angiogenesis alliance. J Angiogenes Res. 2:92010.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fish JE, Santoro MM, Morton SU, et al:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Boudreau N and Myers C: Breast
cancer-induced angiogenesis: multiple mechanisms and the role of
the microenvironment. Breast Cancer Res. 5:140–146. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cascio S, D’Andrea A, Ferla R, et al:
miR-20b modulates VEGF expression by targeting HIF-1α and STAT3 in
MCF-7 breast cancer cells. J Cell Physiol. 224:242–249.
2010.PubMed/NCBI
|
|
93
|
Bos R, Zhong H, Hanrahan CF, et al: Levels
of hypoxia-inducible factor-1α during breast carcinogenesis. J Natl
Cancer Inst. 93:3092001.
|
|
94
|
Krek A, Grun D, Poy MN, et al:
Combinatorial microRNA target predictions. Nature Genet.
37:495–500. 2005. View
Article : Google Scholar
|