Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common cancer in the world (1). Postoperative quality of life for
patients with OSCC has improved in recent years (2). However, the 5-year survival rate has
not improved significantly. Furthermore, 30–40% of patients without
evidence of nodal disease at resection eventually die from
metastatic spread (3). The
identification of biomarkers for evaluating the progression of OSCC
is therefore urgent.
It has been suggested that β2-microglobulin (β2-M)
expression in tissues may be involved in OSCC progression and
metastasis (4). β2-M is a
non-glycosylated protein with a molecular mass of 11,800 Da and is
synthesized by all nucleated cells (5). It is present on the surface of all
nucleated cells except for red blood cells (6). β2-M forms the β chain of the major
histocompatibility complex (MHC) class I molecule [also known as
human leukocyte antigens (HLAs) in humans] and has a 7-stranded
β-pleated structure, which is believed to function in antigen
presentation to cytotoxic (CD8+) T lymphocytes (7). Upon recognition of foreign peptide
antigens on cell surfaces, T cells actively bind and lyse
antigen-presenting cancer cells. In β2-M-deficient mice, antibody
(Ab) responses are defective, and natural killer (NK) cells with
increased sensitivity attack cells lacking the MHC class I molecule
(8,9). In addition to the roles in immunity,
the level of β2-M is associated with proliferation, apoptosis and
metastasis in several cancer types (10,11),
and is a predictor of survival in patients with certain types of
cancer (12). β2-M was found to
promote the growth of human renal cell carcinoma through the
activation of the protein kinase A, cyclic AMP-responsive
element-binding protein, and vascular endothelial growth factor
axis (11). Overexpression of β2-M
in human prostate cancer cell lines leads to inhibition of tumor
growth in vivo and using the β2-M Ab to interrupt β2-M
signaling in human prostate cancer cell lines inhibits cancer cell
growth and induces cell apoptosis (13).
The aim of this study was to investigate β2-M
expression in normal oral mucosa and progressive OSCC and to assess
the clinical significance of β2-M expression. The results of our
study may contribute to a better understanding of the clinical
significance of alterations in β2-M expression and may lead to
further insights into the mechanisms to control progression and
metastatic spread of tumor cells in OSCC patients.
Materials and methods
Cell cultures
Normal human oral keratinocytes (NHOKs) and human
immortalized oral epithelial cells (HIOECs) (14,15)
were cultured in defined keratinocyte medium-SFM (cat. no. 10744;
Gibco, USA). CAL27 was purchased from ATCC (Manassas, VA). The
OSC-4 cells were from Kochi Medical School, Japan. The CAL27 cells
were cultured in DMEM (Invitrogen) with supplements (10% fetal
bovine serum, 1% glutamine and 1% penicillin-streptomycin). The
OSC-4 cells were cultured in RPMI-1640 (Invitrogen) with the same
supplements.
Western blotting
Protein extracts were prepared from 1×106
cells using standard procedures. Cell lysates containing 20 μg
protein were subjected to Western blot analysis. The primary Ab was
monoclonal mouse anti-β2-M (sc-13565, 1:1000; Santa Cruz
Biotechnology Inc.), and tubulin was detected as input control
using monoclonal mouse anti-tubulin (T9026, 1:50,000; Sigma), Blots
were developed with Immobilon Western Chemiluminescent HRP
Substrate (Millipore, USA).
Tissue specimens
Tissue specimens were obtained from the files of the
Department of Oral and Maxillofacial Surgery, Shanghai Ninth
People's Hospital, Shanghai Jiao Tong University School of
Medicine, China. All tissue samples had been fixed in 10% buffered
formalin and embedded in paraffin wax. For primary OSCC lesions
obtained from 50 untreated patients, who underwent surgery between
2008 and 2009, clinicopathological data, including gender, age,
tumor site, primary tumor stage (T), lymph node status (N) and
tumor-node-metastasis (M) were obtained from the patient clinical
records and pathological reports (Table
I). Clinical stage was determined according to the 2002
American Joint Committee on Cancer (AJCC) staging system.
Histopathological diagnosis and grading were confirmed using
haematoxylin and eosin-stained sections according to the criteria
mentioned in ‘Histological Typing of Tumors of the Upper
Respiratory Tract and Ear’, WHO, 2nd edition. All data were
re-examined independently by two of the authors. Metastatic OSCC
lesions from 25 patients were obtained prior to biotherapy or
chemotherapy between 2008 and 2009, and data including gender, age,
and metastatic type was collected. (Table II). Analyses of the tissue samples
are documented in Tables
III-V. Histologically normal
oral mucosa samples were obtained from 10 patients who underwent
dental extractions. The human studies were approved by the
institutional ethics committee.
 | Table IProfiles of the patients with primary
oral squamous cell carcinoma. |
Table I
Profiles of the patients with primary
oral squamous cell carcinoma.
| No | Gender | Age (years) | Location stage | Pathological
grade | TNM | Clinical stage | Smoking Alcohol
consumption | β2-microglobulin |
|---|
| 1 | M | 54 | Palate | G1 | T3N1M0 | III | Yes | Homogeneous |
| 2 | F | 63 | Gingivae | G2 | T3N1M0 | III | No | Homogeneous |
| 3 | M | 48 | Tongue | G2 | T2N1M0 | III | Yes | Homogeneous |
| 4 | F | 54 | Tongue | G2 | T2N1M0 | III | No | Homogeneous |
| 5 | F | 62 | Floor of the
mouth | G1 | T4N1M0 | IV | Yes | Homogeneous |
| 6 | M | 35 | Tongue | G2 | T4N1M0 | IV | No | Homogeneous |
| 7 | F | 71 | Tongue | G3 | T3N1M0 | III | No | Homogeneous |
| 8 | M | 55 | Tongue | G2 | T4N1M0 | IV | No | Homogeneous |
| 9 | F | 64 | Gingivae | G1 | T3N0M0 | III | Yes | Heterogeneous |
| 10 | M | 65 | Tongue | G2 | T2N0M0 | II | No | Negative |
| 11 | M | 64 | Tongue | G2 | T3N0M0 | III | Yes | Heterogeneous |
| 12 | F | 54 | Tongue | G2 | T2N0M0 | II | No | Negative |
| 13 | F | 53 | Tongue | G1 | T1N0M0 | I | No | Negative |
| 14 | M | 57 | Tongue | G2 | T2N1M0 | III | Yes | Heterogeneous |
| 15 | M | 40 | Buccal | G2 | T3N1M0 | III | No | Homogeneous |
| 16 | M | 51 | Tongue | G2 | T4N2M0 | IV | No | Homogeneous |
| 17 | M | 44 | Tongue | G2 | T4N1M0 | IV | No | Homogeneous |
| 18 | F | 67 | Gingivae | G1 | T3N1M0 | III | Yes | Homogeneous |
| 19 | F | 73 | Floor of the
mouth | G2 | T4N1M0 | IV | Yes | Homogeneous |
| 20 | M | 63 | Tongue | G2 | T2N1M0 | III | No | Homogeneous |
| 21 | M | 58 | Tongue | G2 | T2N1M0 | III | Yes | Homogeneous |
| 22 | F | 65 | Gingivae | G1 | T3N1M0 | III | Yes | Homogeneous |
| 23 | M | 58 | Buccal | G2 | T4N1M0 | IV | No | Homogeneous |
| 24 | F | 53 | Palate | G2 | T2N1M0 | III | No | Heterogeneous |
| 25 | M | 54 | Floor of the
mouth | G3 | T4N0M0 | IV | No | Heterogeneous |
| 26 | M | 59 | Tongue | G1 | T1N1M0 | III | Yes | Heterogeneous |
| 27 | M | 60 | Tongue | G2 | T2N0M0 | II | No | Negative |
| 28 | F | 50 | Tongue | G1 | T4N0M0 | IV | No | Homogeneous |
| 29 | M | 51 | Buccal | G1 | T2N0M0 | II | No | Heterogeneous |
| 30 | M | 42 | Palate | G3 | T2N0M0 | II | Yes | Heterogeneous |
| 31 | M | 65 | Tongue | G2 | T2N0M0 | II | No | Negative |
| 32 | M | 76 | Tongue | G2 | T3N0M0 | III | Yes | Heterogeneous |
| 33 | F | 34 | Tongue | G2 | T2N0M0 | II | Yes | Negative |
| 34 | F | 44 | Tongue | G1 | T1N0M0 | I | Yes | Negative |
| 35 | F | 68 | Buccal | G2 | T2N1M0 | III | Yes | Heterogeneous |
| 36 | M | 60 | Buccal | G2 | T3N1M0 | III | No | Homogeneous |
| 37 | M | 68 | Tongue | G2 | T4N2M0 | IV | No | Homogeneous |
| 38 | M | 61 | Gingivae | G2 | T4N1M0 | IV | No | Homogeneous |
| 39 | F | 66 | Gingivae | G2 | T3N0M0 | III | Yes | Negative |
| 40 | F | 65 | Buccal | G2 | T4N1M0 | IV | Yes | Homogeneous |
| 41 | M | 65 | Tongue | G2 | T2N1M0 | III | No | Homogeneous |
| 42 | M | 58 | Tongue | G2 | T2N0M0 | II | Yes | Homogeneous |
| 43 | F | 72 | Gingivae | G2 | T3N1M0 | III | Yes | Homogeneous |
| 44 | M | 74 | Buccal | G3 | T4N1M0 | IV | Yes | Homogeneous |
| 45 | M | 60 | Palate | G2 | T2N0M0 | II | No | Heterogeneous |
| 46 | F | 73 | Tongue | G3 | T4N0M0 | IV | No | Heterogeneous |
| 47 | M | 49 | Tongue | G2 | T1N1M0 | III | Yes | Heterogeneous |
| 48 | M | 54 | Tongue | G2 | T2N0M0 | II | Yes | Negative |
| 49 | F | 67 | Buccal | G3 | T4N0M0 | IV | No | Heterogeneous |
| 50 | F | 52 | Tongue | G1 | T2N0M0 | II | No | Heterogeneous |
 | Table IIProfiles of the patients with
metastatic oral squamous cell carcinoma. |
Table II
Profiles of the patients with
metastatic oral squamous cell carcinoma.
| No | Gender | Age (years) | Location | Type |
β2-microglobulin |
|---|
| 1 | M | 58 | Tongue | Lymph node | Homogeneous |
| 2 | M | 64 | Buccal | Lymph node | Heterogeneous |
| 3 | F | 65 | Tongue | Lymph node | Homogeneous |
| 4 | F | 67 | Tongue | Lymph node | Homogeneous |
| 5 | F | 67 | Tongue | Lymph node | Homogeneous |
| 6 | M | 56 | Tongue | Lymph node | Homogeneous |
| 7 | M | 54 | Tongue | Lymph node | Homogeneous |
| 8 | F | 60 | Tongue | Lymph node | Homogeneous |
| 9 | M | 61 | Tongue | Lymph node | Homogeneous |
| 10 | F | 80 | Buccal | Lymph node | Homogeneous |
| 11 | F | 81 | Buccal | Lymph node | Homogeneous |
| 12 | F | 86 | Tongue | Lymph node | Homogeneous |
| 13 | M | 88 | Tongue | Lymph node | Homogeneous |
| 14 | F | 49 | Buccal | Lymph node | Heterogeneous |
| 15 | F | 49 | Buccal | Lymph node | Homogeneous |
| 16 | M | 43 | Tongue | Lymph node | Homogeneous |
| 17 | M | 67 | Tongue | Lymph node | Homogeneous |
| 18 | M | 78 | Tongue | Lymph node | Homogeneous |
| 19 | M | 86 | Buccal | Lymph node | Homogeneous |
| 20 | M | 38 | Tongue | Lymph node | Homogeneous |
| 21 | M | 67 | Buccal | Lymph node | Homogeneous |
| 22 | M | 88 | Tongue | Lymph node | Homogeneous |
| 23 | F | 55 | Tongue | Lymph node | Homogeneous |
| 24 | M | 49 | Tongue | Lymph node | Homogeneous |
| 25 | M | 67 | Tongue | Lymph node | Homogeneous |
 | Table IIIβ2-microglobulin antigen expression
in normal oral mucosa epithelial and oral squamous cell carcinoma
specimens. |
Table III
β2-microglobulin antigen expression
in normal oral mucosa epithelial and oral squamous cell carcinoma
specimens.
| Staining
pattern | Normal oral mucosa
epithelial specimens n (%) | Oral squamous cell
carcinoma specimens n (%) |
|---|
| Homogeneous | 0 (0) | 46 (61.3) |
| Heterogeneous | 10 (100) | 20 (26.7) |
| Negative | 0 (0) | 9 (12.0) |
| Total | 10 (100) | 75 (100.0) |
 | Table VProfile of β2-microglobulin antigen
expression in primary oral squamous cell carcinoma and
metastases. |
Table V
Profile of β2-microglobulin antigen
expression in primary oral squamous cell carcinoma and
metastases.
| Primary OSCC | Metastases |
|---|
|
|
|
|---|
| Staining
pattern | n (%) | n (%) |
|---|
| Homogeneous | 26 (52) | 20 (80) |
| Heterogeneous | 15 (30) | 5 (20) |
| Negative | 9 (18) | 0 (0) |
| Total | 50 (100) | 25 (100) |
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissue sections
were dewaxed with xylene and rehydrated by passage through
decreasing concentrations of ethanol (100–80%). Endogenous
peroxidase activity was blocked by a 20-min incubation at room
temperature with 3% H2O. The sections were heated using
a water bath at 100°C with 0.01 M citrate buffer solution (pH, 6.0)
for 20 min, and incubated with an optimal amount of
affinity-purified monoclonal mouse anti-human β2-M (sc-13565, 1:50;
Santa Cruz Biotechnology) overnight at 4°C. Sections were stained
with liquid DAB substrate-chromogen, and counterstained with
hematoxylin. Negative controls were carried out by omitting the
primary Ab. The percentage of stained tumor cells in each lesion
was enumerated independently by two investigators who had no
knowledge of the patient characteristics. Variations in the
percentage of stained cells as counted were within a 10% range. We
scored the staining results according the report of Kageshita et
al (16). Briefly, OSCC lesions
consisting of >75% immunostained OSCC cells within the entire
lesion were scored as homogeneously positive, those having 25–75%
immunostained OSCC cells were heterogeneously positive, and those
with <25% immunostained OSCC cells were negative.
Statistical analysis
Several clinicopathological factors were evaluated
in the primary OSCC lesions, including gender, age (≤61 years vs.
>61 years), T stage (T1, T2 vs. T3, T4), N status (negative vs.
positive) and clinical stage (stage I, II vs. stage III, IV).
Pearson Chi-square test, Continuity Correction test and Fisher's
exact test were used to evaluate the correlation between the
clinicopathological variables and the β2-M staining score using
SPSS software v13.0 (SPSS Inc., USA). Differences in the β2-M
staining score between primary OSCC samples and metastatic OSCC
samples were also analyzed using the Chi-square test. A P-value
<0.05 was considered to denote significant difference.
Results
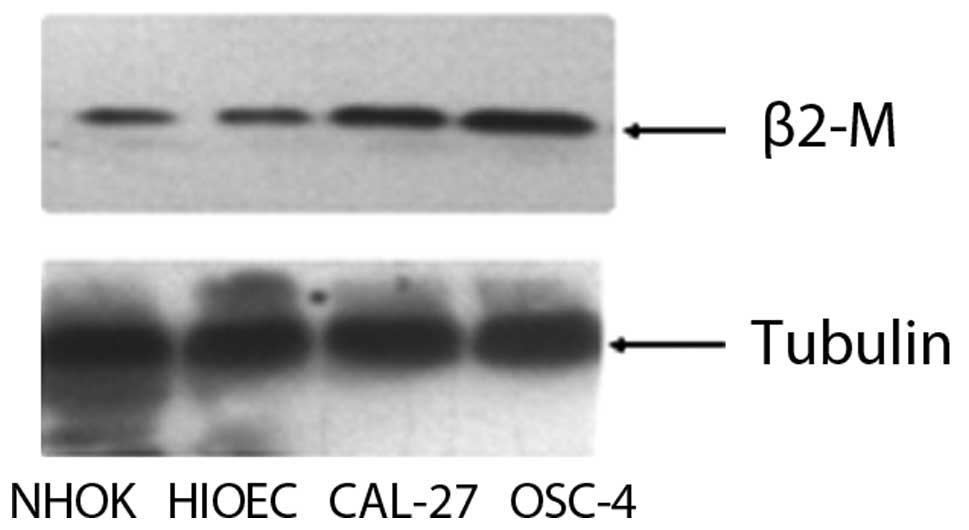
Expression of β2-M in primary cultured
NHOKs and HIOECs and the OSCC cell lines
We compared the expression levels of β2-M in NHOKs
and HIOECs and in the two OSCC cancer cell lines (OSC and CAL27) by
Western blotting. NHOKs were isolated and cultured as described
(14). HIOECs were established by
overexpression of HPV16 E6 and E7 protein (14). Western blot analysis revealed that
β2-M protein expression was increased in the OSC and CAL27 cells
compared to the NHOKs and HIOECs (Fig.
1).
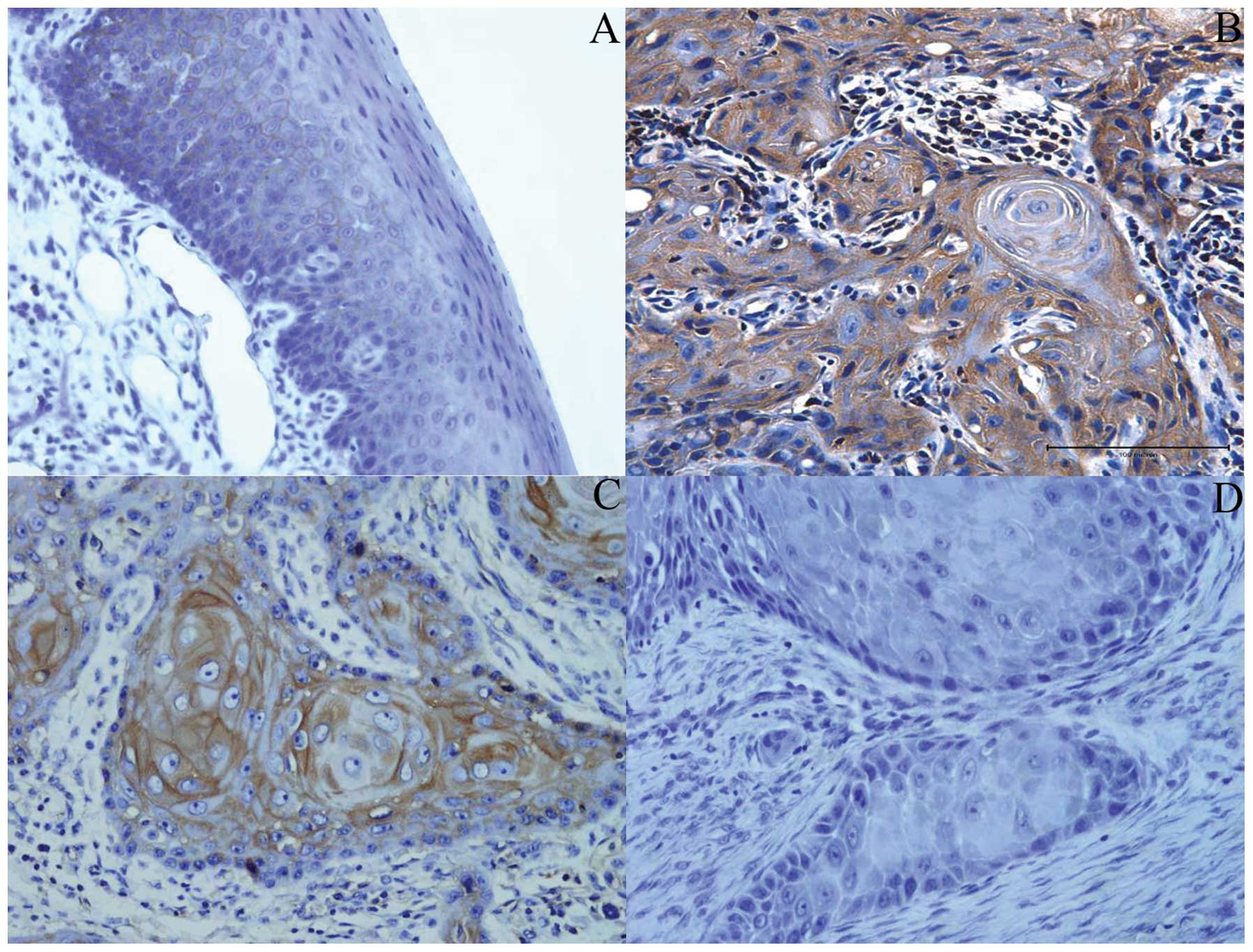
Expression of β2-M in normal oral mucosa
epithelial and OSCC tissue specimens
We performed immunohistochemical staining using
normal oral mucosa and OSCC tissue specimens. Ten human normal oral
mucosa samples and 75 human OSCC lesions (50 primary OSCC and 25
metastatic OSCC samples) were included. In the human normal oral
mucosa, a faint but consistent staining was observed, mainly in the
plasma membrane in oral mucosa epithelial cells. Stromal cells such
as fibroblasts and fibrocytes were not stained by the anti-β2-M Ab
(Fig. 2A). Most of the OSCC (88%)
tissue sections showed distinct homogeneous (Fig. 2B) or heterogeneous staining
(Fig. 2C), mainly in the cytoplasm
and cytoplasmic membrane of tumor epithelial cells. However, in a
few primary OSCC tissues, no staining or staining with weak
intensity for β2-M was noted in the cytoplasm and cytoplasmic
membrane of tumor epithelial cells (Fig. 2D). Compared with normal oral mucosa
specimens, the frequency of β2-M expression was significantly
increased in OSCC (P=0.031) (Table
III).
Association of β2-M expression with
various clinicopathological features in primary OSCC tissues
Of the 50 primary OSCC samples, 26 (52%) exhibited a
homogeneous distribution of β2-M staining, and 15 (30%) exhibited a
heterogeneous distribution within the OSCC cells, while 9 (18%)
were negative for β2-M staining (Table
IV). Of the 23 patients classified as T1, T2 in 50 primary OSCC
cases, 9 (39.1%) showed heterogenous staining and 8 (34.8%) showed
negative staining, while only 6 (26.1%) exhibited homogeneous
staining. In contrast, of the 27 patients classified as T3, T4, 20
(74.1%) presented with homogeneous staining, whereas only 6 (22.2%)
showed heterogenous staining and 1 (3.7%) showed negative staining.
Compared with primary OSCC of T1, T2 stage, the intensity of β2-M
expression was significantly increased in the primary OSCC
specimens of T3, T4. Up-regulation of β2-M expression was also
associated with lymph node invasion of OSCC. β2-M expression was
significantly increased in N-positive patients compared to
N-negative patients (82.8 vs. 9.5%, P=0.000). Regarding clinical
stage, in highly malignant stages (III, IV) 67.6% of samples showed
homogeneous staining whereas in low malignant stages (I, II), only
1% of samples showed homogeneous staining. These data suggest that
the staining scores for β2-M were significantly associated with
large tumor size (T3, T4 vs. T1, T2, P=0.001), positive nodal
status (N-positive vs. N-negative, P=0.000), and advanced clinic
stage (III, IV vs. I, II, P=0.000) (Table IV). In contrast, there were no
correlations between β2-M expression and gender, age, smoking and
alcohol consumption, and pathologic grade.
 | Table IVAssociation of β2-microglobulin
antigen expression with clinicopathological characteristics in
primary OSCC lesions. |
Table IV
Association of β2-microglobulin
antigen expression with clinicopathological characteristics in
primary OSCC lesions.
| | β2-microglobulin
staining pattern | |
|---|
| |
| |
|---|
| N | Homogeneous n
(%) | Heterogeneous n
(%) | Negative n (%) | P-value |
|---|
| Gender |
| Female | 19 | 10 (52.6) | 4 (21.1) | 5 (26.3) | 0.368 |
| Male | 31 | 16 (51.6) | 11 (35.5) | 4 (12.9) | |
| Age (years) |
| ≤61 | 29 | 14 (48.3) | 9 (31.0) | 6 (20.7) | 0.748 |
| >61 | 21 | 12 (57.1) | 6 (28.6) | 3 (14.3) | |
| Smoking and alcohol
consumption |
| No | 28 | 16 (57.1) | 7 (25.0) | 5 (17.9) | 0.652 |
| Yes | 22 | 10 (45.5) | 8 (36.4) | 4 (18.2) | |
| Tumor size | | | | | |
| ≤4 cm (T1+T2) | 23 | 6 (26.1) | 9 (39.1) | 8 (34.8) | 0.001 |
| >4 cm
(T3+T4) | 27 | 20 (74.1) | 6 (22.2) | 1 (3.7) | |
| Lymph nodes |
| Negative (0) | 21 | 2 (9.5) | 10 (47.6) | 9 (42.9) | 0.000 |
| Positive
(1–2) | 29 | 24 (82.8) | 5 (17.2) | 0 (0.0) | |
| Clinical stage |
| Early (I, II) | 13 | 1 (7.7) | 4 (30.8) | 8 (61.5) | 0.000 |
| Advanced (III,
IV) | 37 | 25 (67.6) | 11 (29.7) | 1 (2.7) | |
| Pathological
grade |
| G1 | 11 | 5 (45.5) | 4 (36.4) | 2 (18.2) | |
| G2 | 33 | 19 (57.6) | 7 (21.2) | 7 (21.2) | 0.237 |
| G3 | 3 | 2 (33.3) | 4 (66.7) | 0 (0.0) | |
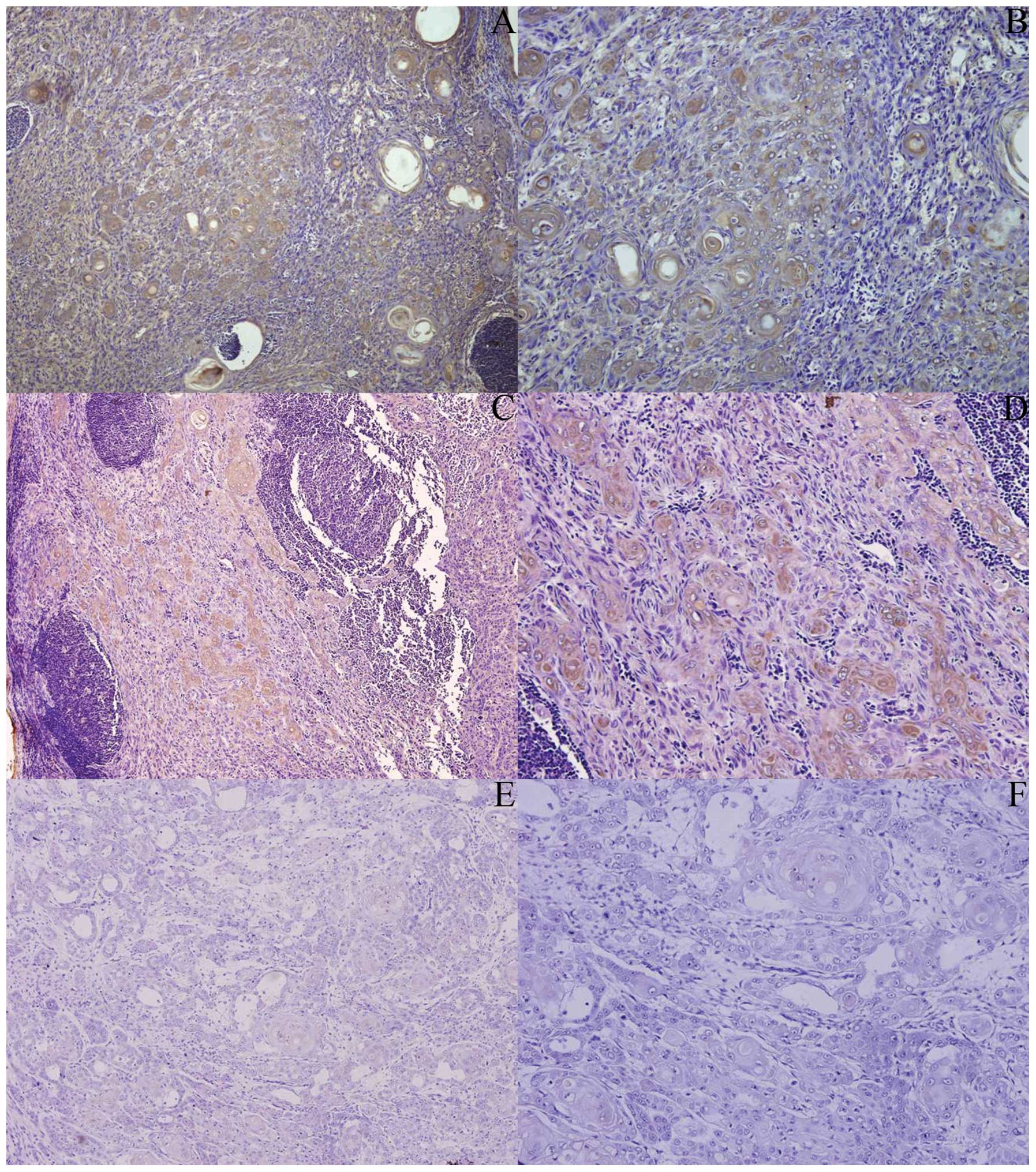
Up-regulation of β2-M expression in OSCC
tissues correlates with tumor metastasis
Intensity of β2-M staining in OSCC metastatic
lesions was significantly stronger than that in the primary OSCC
lesions (Fig. 3). Ninenty-two
percent of metastatic lesions exhibited a homogeneous distribution
of β2-M expression while 52% of primary OSCC lesions exhibited
homogeneous staining for β2-M (P=0.027) (Table V).
Discussion
In the present study, β2-M expression in OSCC
lesions was evaluated and correlated with tumor progression and
metastasis in OSCC patients. The results showed that β2-M
expression was up-regulated in OSCC cell lines and OSCC lesions,
and was associated with OSCC progression, invasion and metastasis.
Consistent with our results, it was previously found that the
suppression of β2-M expression using small interfering RNA (siRNA)
was sufficient to decrease cell migration and invasion in
vitro (4). The results of our
and other research studies (4),
indicate that OSCC lesions should be included in the spectrum of
tumors with increased levels of β2-M expression.
Recent studies have used a wide range of
experimental approaches to assess the mitogenic role of β2-M in
malignancies. (17–19). These studies have provided strong
evidence to show that β2-M acts similarly to a prototypical
oncogenic factor capable of stimulating growth and progression of
various types of cancers, including breast cancer (17), prostate cancer (18), lung cancer (19), gastrointestinal (20), nasopharyngeal cancers (21), multiple myeloma (22), and particularly, lymphocytic
malignancies (23), such as
non-Hodgkin's lymphoma and multiple myeloma. Similar studies have
also reported that β2-M is a growth-promoting factor contributing
to the growth and progression of renal cell carcinoma (24,25).
However, previous studies have shown that β2-M/MHC
class I can serve as important signal-transducing molecules in
regulating tumor immunity and progression (26). Increased susceptibility to tumor
formation was noted in β2-M gene-knockout mice, which suggests
potential regulation of cancer growth by β2-M (26). Loss of β2-M expression is clinically
important as it has been described in various patient-derived tumor
cells, such as in melanomas (27)
and cervical carcinoma (28). The
possible explanation is that β2-M is expressed at a constant level
on the cell surface. When expression of the β2-M molecule is below
a normal level, defects in the β2-M/MHC class I signaling pathway
may result in tumor immune escape. When expression of the β2-M
molecule is higher than normal, β2-M promotes tumorigenesis and
metastasis as an oncogene.
In some cancers, immunohistochemical evidence
suggests that absence of functional (β2-M-associated) HLA class I
molecules may be due to a mutational loss of β2-M (29); and in other cancers, a decreased
level or the loss of β2-M in tumor cells was found due to the loss
of the β2-M locus, or promoter methylation (30,31).
Under these conditions, loss of β2-M prevents the synthesis of
wild-type β2-M protein, which may lead to alterations in MHC class
I surface expression. In our study, was not observed loss of β2-M
in the OSCC lesions. In contrast, levels of β2-M expression were
up-regulated in progressive OSCC lesions. The balance of β2-M
expression at the cell surface was disturbal, which contributed to
human cancer growth. Therefore, β2-M has a wider function than just
a housekeeping gene or the role on stabilization and presentation
of MHC class I molecular in cells.
Recently, marked antitumor activity has been
observed by down-regulation of β2-M levels using either
sequence-specific siRNA or antibodies in cases of both solid tumors
and blood malignancies (13). In
prostate cancer and renal cancer, growth inhibition of tumors was
observed when patients were treated with anti-β2-M polyclonal or
monoclonal antibodies (32), and in
myeloma and other hematological malignancies, tumor cell apopotosis
was observed using monoclonal β2-M Ab and sequence-specific siRNA
to β2-M (33). Thus, β2-M, as an
oncogenic factor in various cancer types, appears to be an
excellent new target for interrupting human cancer growth. In our
study, the association of β2-M expression with progression and
metastasis of OSCC lesions was statistically significant. Whether
we can inhibit OSCC progression, invasion or migration by using a
similar anti-β2-M polyclonal or monoclonal antibody needs further
study.
Acknowledgements
This study was supported by grants from the Doctoral
Innovation Foundations of Shanghai Jiao Tong University School (no.
BXJ 0922) and National Nature Science Foundation of China (no.
30630065 and 30973344).
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
MHC
|
major histocompatibility complex
|
|
HLAs
|
human leukocyte antigens
|
|
NK
|
natural killer
|
|
HIOECs
|
human immortalized oral epithelial
cells
|
References
|
1
|
Imai T, Toyota M, Suzuki H, et al:
Epigenetic inactivation of RASSF2 in oral squamous cell carcinoma.
Cancer Sci. 99:958–966. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scully C and Bagan JV: Recent advances in
Oral Oncology 2007: imaging, treatment and treatment outcomes. Oral
Oncol. 44:211–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai ZG, Shi XJ, Gao Y, Wei MJ, Wang CY and
Yu GY: Beta-catenin expression pattern in primary oral squamous
cell carcinoma. Chin Med J (Engl). 121:1866–1870. 2008.PubMed/NCBI
|
|
4
|
Chen CH, Su CY, Chien CY, et al:
Overexpression of beta2-microglobulin is associated with poor
survival in patients with oral cavity squamous cell carcinoma and
contributes to oral cancer cell migration and invasion. Br J
Cancer. 99:1453–1461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi C, Zhu Y, Su Y, Chung LW and Cheng T:
Beta2-microglobulin: emerging as a promising cancer therapeutic
target. Drug Discov Today. 14:25–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winchester JF, Salsberg JA and Levin NW:
Beta-2 microglobulin in ESRD: an in-depth review. Adv Ren Replace
Ther. 10:279–309. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Margalit A, Sheikhet HM, Carmi Y, et al:
Induction of antitumor immunity by CTL epitopes genetically linked
to membrane-anchored beta2-microglobulin. J Immunol. 176:217–224.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christianson GJ, Brooks W, Vekasi S, et
al: Beta 2-microglobulin-deficient mice are protected from
hypergammaglobulinemia and have defective antibody responses
because of increased IgG catabolism. J Immunol. 159:4781–4792.
1997.PubMed/NCBI
|
|
9
|
Hoglund P, Glas R, Menard C, et al:
Beta2-microglobulin-deficient NK cells show increased sensitivity
to MHC class I-mediated inhibition, but self tolerance does not
depend upon target cell expression of H-2Kb and Db heavy chains.
Eur J Immunol. 28:370–378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang WC, Wu D, Xie Z, et al:
Beta2-microglobulin is a signaling and growth-promoting factor for
human prostate cancer bone metastasis. Cancer Res. 66:9108–9116.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nomura T, Huang WC, Zhau HE, et al:
Beta2-microglobulin promotes the growth of human renal cell
carcinoma through the activation of the protein kinase A, cyclic
AMP-responsive element-binding protein, and vascular endothelial
growth factor axis. Clin Cancer Res. 12:7294–7305. 2006. View Article : Google Scholar
|
|
12
|
Tsimberidou AM, Kantarjian HM, Wen S, et
al: The prognostic significance of serum beta2 microglobulin levels
in acute myeloid leukemia and prognostic scores predicting
survival: analysis of 1,180 patients. Clin Cancer Res. 14:721–730.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang WC, Havel JJ, Zhau HE, et al:
Beta2-microglobulin signaling blockade inhibited androgen receptor
axis and caused apoptosis in human prostate cancer cells. Clin
Cancer Res. 14:5341–5347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sdek P, Zhang ZY, Cao J, Pan HY, Chen WT
and Zheng JW: Alteration of cell-cycle regulatory proteins in human
oral epithelial cells immortalized by HPV16 E6 and E7. Int J Oral
Maxillofac Surg. 35:653–657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Yang X, Zhong LP, et al:
Decreased expression of Annexin A1 correlates with pathologic
differentiation grade in oral squamous cell carcinoma. J Oral
Pathol Med. 38:362–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kageshita T, Hirai S, Ono T, Hicklin DJ
and Ferrone S: Down-regulation of HLA class I antigen-processing
molecules in malignant melanoma: association with disease
progression. Am J Pathol. 154:745–754. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klein B, Levin I, Kfir B, Mishaeli M,
Shapira J and Klein T: The significance of soluble interleukin-2,
soluble interleukin-2 receptors, soluble ICAM-1 and beta
2-microglobulin in breast cancer patients. Tumour Biol. 16:290–296.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Freeman MR: Beta2 microglobulin: a
surprising therapeutic target for prostate cancer and renal cell
carcinoma. J Urol. 178:10–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nissen MH, Bjerrum OJ, Plesner T, Wilken M
and Rorth M: Modification of beta-2-microglobulin in sera from
patients with small cell lung cancer: evidence for involvement of a
serine protease. Clin Exp Immunol. 67:425–432. 1987.PubMed/NCBI
|
|
20
|
Kaplan B, Martin BM, Livoff A, Yeremenko
D, Livneh A and Cohen HIL: Gastrointestinal beta2microglobulin
amyloidosis in hemodialysis patients: biochemical analysis of
amyloid proteins in small formalin-fixed paraffin-embedded tissue
specimens. Mod Pathol. 18:1610–1617. 2005.
|
|
21
|
Lee JK, Tsai SC, Hsieh JF, Ho YJ, Sun SS
and Kao CH: Beta-2-microglobulin (beta 2M) as a tumor marker in
nasopharyngeal carcinoma. Anticancer Res. 20:4765–4768.
2000.PubMed/NCBI
|
|
22
|
Kim JE, Yoo C, Lee DH, Kim SW, Lee JS and
Suh C: Serum albumin level is a significant prognostic factor
reflecting disease severity in symptomatic multiple myeloma. Ann
Hematol. 89:391–397. 2009. View Article : Google Scholar
|
|
23
|
Delgado J, Pratt G, Phillips N, et al:
Beta2-microglobulin is a better predictor of treatment-free
survival in patients with chronic lymphocytic leukaemia if adjusted
according to glomerular filtration rate. Br J Haematol.
145:801–805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Federico M, Guglielmi C, Luminari S, et
al: Prognostic relevance of serum beta2 microglobulin in patients
with follicular lymphoma treated with anthracycline-containing
regimens. A GISL study. Haematologica. 92:1482–1488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Albitar M, Vose JM, Johnson MM, et al:
Clinical relevance of soluble HLA-I and beta2-microglobulin levels
in non-Hodgkin's lymphoma and Hodgkin's disease. Leuk Res.
31:139–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sousa AO, Mazzaccaro RJ, Russell RG, et
al: Relative contributions of distinct MHC class I-dependent cell
populations in protection to tuberculosis infection in mice. Proc
Natl Acad Sci USA. 97:4204–4208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hicklin DJ, Wang Z, Arienti F, Rivoltini
L, Parmiani G and Ferrone S: Beta2-Microglobulin mutations, HLA
class I antigen loss, and tumor progression in melanoma. J Clin
Invest. 101:2720–2729. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mehta AM, Jordanova ES, Kenter GG, Ferrone
S and Fleuren GJ: Association of antigen processing machinery and
HLA class I defects with clinicopathological outcome in cervical
carcinoma. Cancer Immunol Immunother. 57:197–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
del Campo AB, Aptsiauri N, Mendez R, et
al: Efficient recovery of HLA class I expression in human tumor
cells after beta2-microglobulin gene transfer using adenoviral
vector: implications for cancer immunotherapy. Scand J Immunol.
70:125–135. 2009.PubMed/NCBI
|
|
30
|
Koene GJ, Arts-Hilkes YH, van der Ven KJ,
et al: High level of chromosome 15 aneuploidy in head and neck
squamous cell carcinoma lesions identified by FISH analysis:
limited value of beta2-microglobulin LOH analysis. Tissue Antigens.
64:452–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feenstra M, Veltkamp M, van Kuik J, et al:
HLA class I expression and chromosomal deletions at 6p and 15q in
head and neck squamous cell carcinomas. Tissue Antigens.
54:235–245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang J, Qian J, Wezeman M, et al:
Targeting beta2-microglobulin for induction of tumor apoptosis in
human hematological malignancies. Cancer Cell. 10:295–307. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Zhang X, Wang J, et al:
Anti-beta2-microglobulin monoclonal antibodies induce apoptosis in
myeloma cells by recruiting MHC class I to and excluding growth and
survival cytokine receptors from lipid rafts. Blood. 110:3028–3035.
2007. View Article : Google Scholar
|