Introduction
Deguelin, one of the most critical rotenoids from
the flavonoid family, derived from the natural plants in the
Mundulea sericea family, has been shown to be effective as a
chemopreventive and therapeutic agent against different cancer
cells such as tumors of the colon, lung and breast (1–3). The
functions of human cancer cell lines through the induction of cell
cycle arrest and apoptosis can be down-regulated for specific cell
survival proteins, including Akt and mitogen-activated protein
kinase (MAPK) (4–6). Furthermore, deguelin inhibited the
transcriptional regulation of ornithine decarboxylase (7), NF-κB gene expression (8,9) and
hypoxia-inducible factor-1α (HIF-1α) (10).
DNA damage is associated with diseases such as
neuro-degeneration in age-related disease, cerebral ischemia and
brain trauma (11). Thus,
agent-induced DNA damage may lead to cell mutation and then cause
malignancy (12,13). To fully understand the actions of
anticancer drugs is critical and can offer more information
regarding the anticancer drug-induced side effects in patients.
Although substantial evidence has shown that
deguelin induced cell death of human cancer cell lines, there is no
information to address the effects of deguelin-provoked DNA damage
in human lung cancer cells. The purpose of the present study was to
investigate the effects of deguelin on DNA damage and DNA repair
associated gene expression (mRNA) in human lung cancer NCI-H460
cells. Our results revealed that deguelin induced DNA damage and
inhibited DNA associated gene expression in NCI-H460 cells in
vitro.
Materials and methods
Chemicals and reagents
Deguelin, dimethyl sulfoxide (DMSO), propidium
iodide (PI), Tris-HCl and Triton X-100 was obtained from
Sigma-Aldrich Corp. (St. Louis, MO, USA). RPMI-1640 medium, fetal
bovine serum (FBS), L-glutamine, penicillin-streptomycin and
trypsin-EDTA were obtained from Gibco Life Technologies (Grand
Island, NY, USA).
Cell culture
The human lung cell line (NCI-H460) was purchased
from the Food Industry Research and Development Institute (Hsinchu,
Taiwan) and maintained at 37°C with 5% CO2 and 95% air
in RPMI-1640 medium supplemented with 10% FBS, 2 mM L-glutamine,
100 units/ml penicillin and 100 μg/ml streptomycin. The medium was
changed every 2 days (14).
Deguelin was dissolved in DMSO and added directly to cell culture
medium at a final concentration of 0.5% DMSO. This concentration
had no effect on cell growth or other assays.
PI exclusion method and flow cytometric
assay
Approximately 2×105 cells/well of
NCI-H640 cells in 12-well plates were incubated with deguelin at
final concentrations of 0 (vehicle, 0.5% DMSO), 50, 250 and 500 nM
and for 24 h, or the cells were treated with 250 nM deguelin for 0,
24, 48 and 72 h. Cells from each treatment were stained with PI (5
μg/ml) and analyzed by flow cytometry (Becton-Dickinson, San Jose,
CA, USA) and cell viability was calculated as previously described
(15,16).
Comet assay
NCI-H460 cells at a density of 2×105
cells/well in 12-well plates were incubated with 0 (vehicle, 0.5%
DMSO), 50, 250 and 500 nM degulein and 5 μM hydrogen peroxide
(H2O2, positive control) for 48 h in
RPMI-1640 medium grown at 37°C in 5% CO2 and 95% air.
Cells were harvested for the examination of DNA damage using the
comet assay as previously described (17,18).
Comet tail length was calculated and quantified using the TriTek
CometScore™ software image analysis system (TriTek Corp.,
Sumerduck, VA, USA) as previously described (18).
DNA gel electrophoresis
NCI-H460 cells (1×106 cells/well) seeded
in 6-well plates were incubated with degulein at final
concentrations of 0 (vehicle, 0.5% DMSO), 50, 250 and 500 nM for 48
h. Cells from each treatment were individually isolated by using
DNA isolation kit (Genemark Technology Co., Ltd., Tainan, Taiwan)
(19). The DNA electrophoresis was
carried out in 1.5% agarose gel in Tris-borate EDTA (TBE) buffer
(Amresco, Solon, OH, USA) at 15 V for 2 h. DNA was stained with
ethidium bromide (EtBr, Sigma-Aldrich Corp.), then examined and
photographed by fluorescence microscope as previously described
(20,21).
Real-time PCR analysis
Approximately 1×106 cells/well of
NCI-H460 cells in 6-well plates were incubated with or without 0,
50 and 250 nM degulein for a 24-h treatment in RPMI-1640 medium
grown at 37°C in 5% CO2 and 95% air. The total RNA from
each treatment was extracted by using the Qiagen RNeasy Mini Kit
(Qiagen, Inc., Valencia, CA, USA) as previously described (14,22).
Briefly, RNA samples were reverse-transcribed for 30 min at 42°C
with High Capacity cDNA Reverse Transcription Kit according to the
standard protocol of the supplier (Applied Biosystems, Carlsbad,
CA, USA). For quantitative PCR from each sample that was performed
in the conditions: 2 min at 50°C, 10 min at 95°C, and 40 cycles of
15 sec at 95°C, 1 min at 60°C using 1 μl of the cDNA
reverse-transcribed as described above, 2X SYBR-Green PCR Master
Mix (Applied Biosystems) and 200 nM of forward and reverse primers
as shown in Table I. Finally, each
assay was run on an Applied Biosystems 7300 real-time PCR system in
triplicates and expression fold-change was derived using the
comparative CT method (15,18).
 | Table IPrimer sequences used for real-time
PCR. |
Table I
Primer sequences used for real-time
PCR.
| Primer name | Primer
sequence |
|---|
| BRCA1 | F:
CCAGGGAGTTGGTCTGAGTGA
R: ACTTCCGTAAGGCATCGTAACAC |
| DNA-PK | F:
CCAGCTCTCACGCTCTGATATG
R: CAAACGCATGCCCAAAGTC |
| MGMT | F:
CCTGGCTGAATGCCTATTTCC
R: TGTCTGGTGAACGACTCTTGCT |
| p53 | F:
GGGTTAGTTTACAATCAGCCACATT
R: GGGCCTTGAAGTTAGAGAAAATTCA |
| ATM | F:
TTTACCTAACTGTGAGCTGTCTCCAT
R: ACTTCCGTAAGGCATCGTAACAC |
| ATR | F:
GGGAATCACGACTCGCTGAA
R: CTAGTAGCATAGCTCGACCATGGA |
| GAPDH | F:
ACACCCACTCCTCCACCTTT
R: TAGCCAAATTCGTTGTCATACC |
Statistical analysis
The data are presented as the mean ± SD and
Student’s t-test was used to analyze differences between
deguelin-treated and untreated (control) groups. All the
statistical analyses were performed, and p<0.05 was considered
statistically significant.
Results
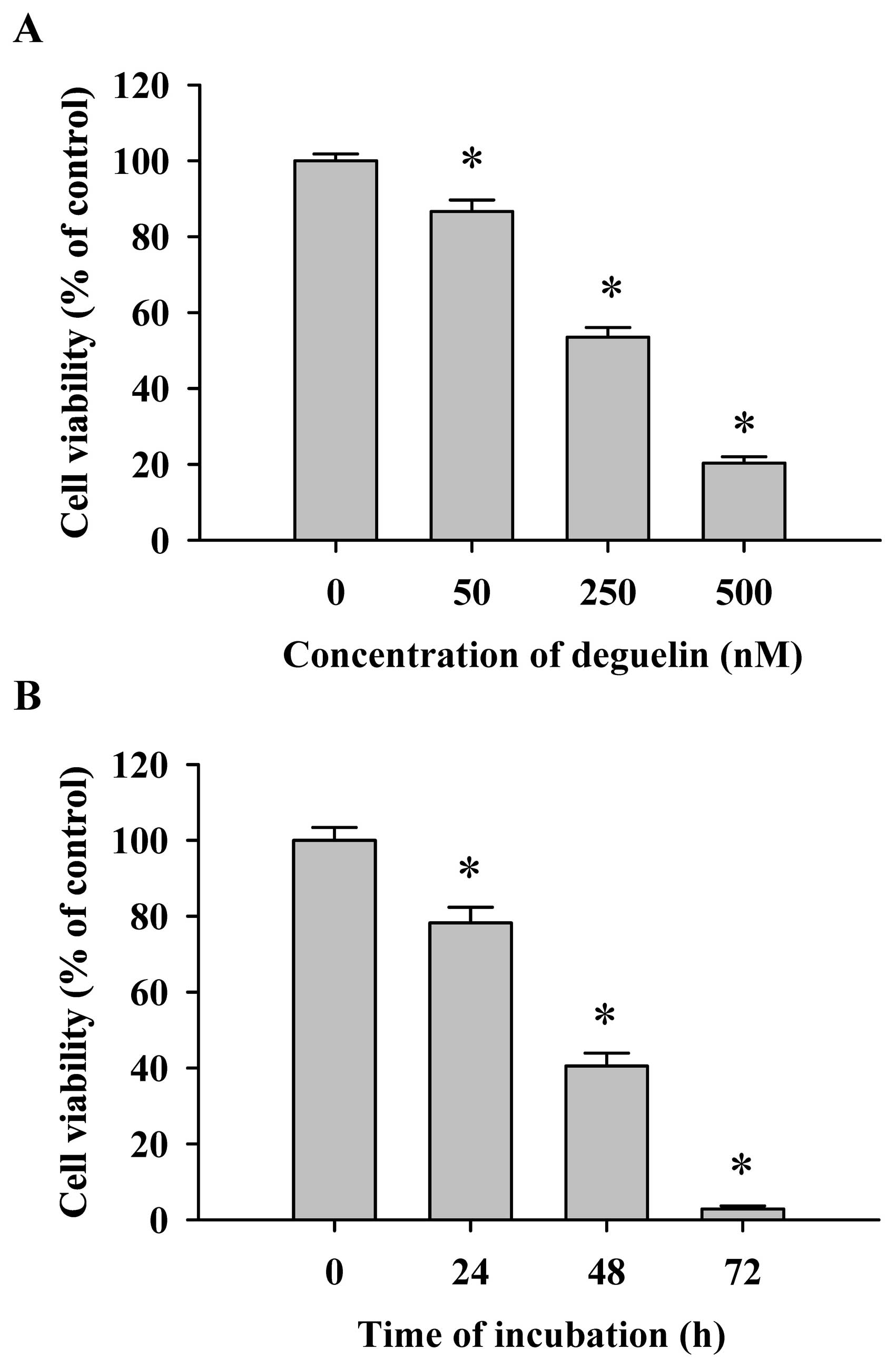
Flow cytometric assay for the effects of
deguelin on the percentage of viable NCI-H460 cells
Cells were treated with various concentrations (0,
50, 250 and 500 nM) of deguelin for 48 h or were treated with 250
nM of deguelin for 0, 24, 48 and 72 h. The cells from each
treatment were collected for the measurement of percentage of
viable NCI-H460 cells. The results shown in Fig. 1 indicate that deguelin decreased the
cell viability and these effects are dose- and time-dependent
(Fig. 1).
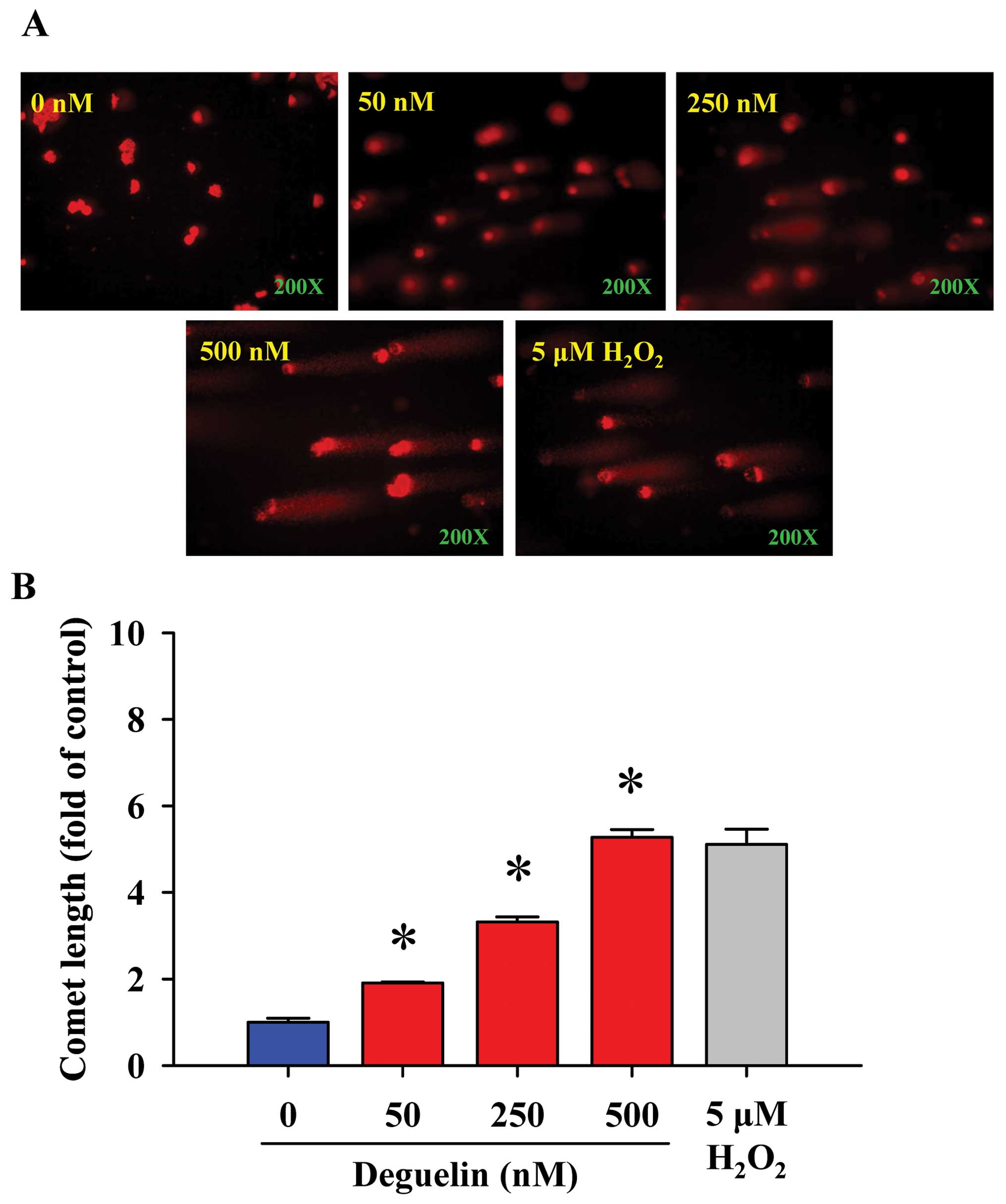
Comet assay for the effects of
deguelin-triggered DNA damage in NCI-H460 cells
We investigated that deguelin-induced DNA damage of
NCI-H460 cells in vitro. The comet assay was selected for
determining DNA damage and the results are shown in Fig. 2, indicating that deguelin provoked
DNA damage in NCI-H460 cells in a dose-dependent manner. The higher
concentration of deguelin led to a longer DNA migration smear
(comet tail). It is well documented that H2O2
is a highly reactive oxygen species and it has been used as
positive control for numerous studies (23,24).
The results from present studies indicated that 5 μM
H2O2-induced comet tail occurred and was used
as a positive control.
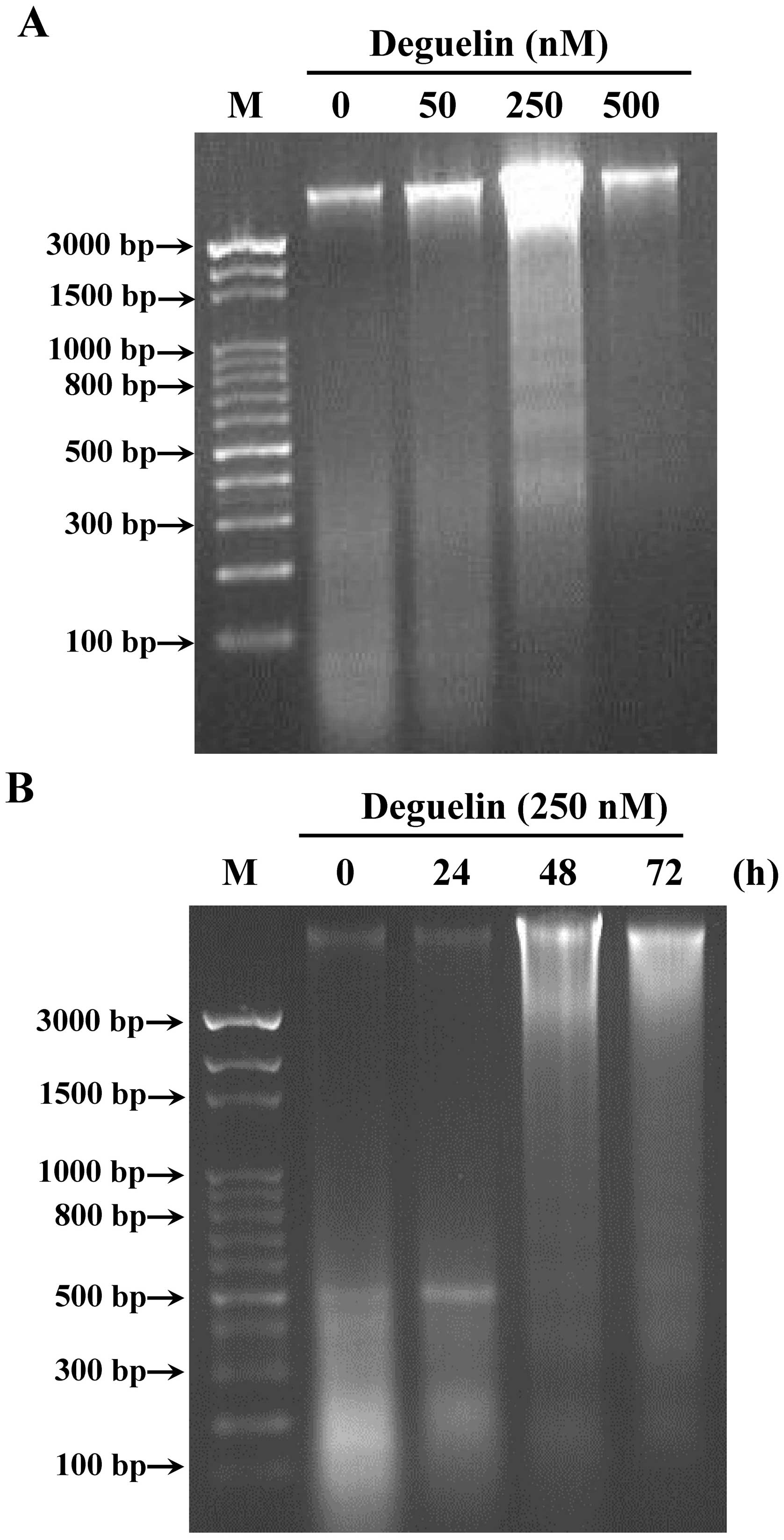
DNA gel electrophoresis for the effects
of deguelin-induced DNA damage and fragmentation in NCI-H460
cells
In comet assay, we found that deguelin induced DNA
damage in NCI-H460 cells. Thus, DNA gel electrophoresis was used to
investigate whether or not deguelin causes DNA fragmentation in
NCI-H460 cells. Thus, DNA was isolated from NCI-H460 cells after
treatment with deguelin for 48 h and then DNA fragments were
determined by DNA gel electrophoresis. The results showed that
deguelin induced DNA damage and fragments in NCBI-H460 cells in a
dose- and time-dependent manner (Fig.
3). The highest dose of deguelin (500 nM) incubation of
NCI-H460 cells led to more DNA damage and fragments than that of
low dose (50 nM) deguelin incubation.
Real-time PCR for examining the effects
of deguelin on DNA damage and repair gene expression in NCI-H460
cells
Based on the above results, deguelin induced DNA
damage and fragments in NCI-H460 cells. We further investigated the
effects of deguelin on gene expression of DNA damage and repair in
NCI-H460 cells. We also used DNA agarose gel electrophoresis for
examining the products (Fig. 3).
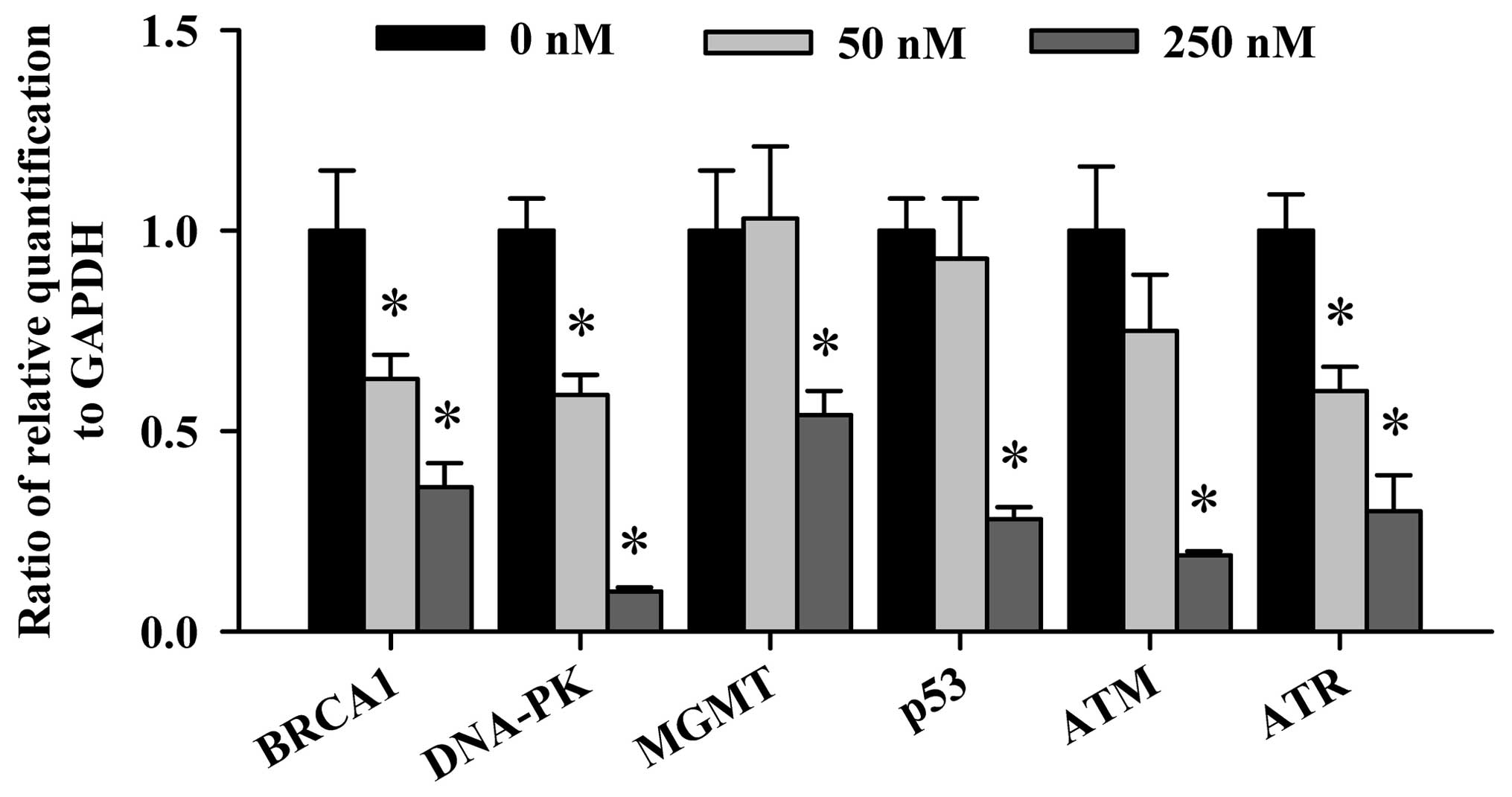
The real-time PCR results are shown in Fig. 5 and indicate that all the examined
gene expressions associated with DNA damage and repair such as the
BRCA1, DNA-PK, MGMT, p53, ATM
and ATR mRNA were decreased (Fig. 4) in NCI-H460 cells after a 24-h
treatment of deguelin. Especially, the gene levels of BRCA1,
DNA-PK, ATM and ATR expression were inhibited
dose-dependently in NCI-H460 cells. However, the gene levels of
MGMT and p53 mRNA expression were decreased in
NCI-H460 cells only at high dose of deguelin exposure.
Discussion
Several reports have demonstrated that deguelin can
induce cytotoxic effects and induce apoptosis in many human cancer
cell lines (1,4,26–28).
However, there is no report addressing deguelin-induced DNA damage
in human lung cancer cells. In the present study, a dose-dependent
increase in DNA damage (Fig. 2) was
observed in human lung cancer NCI-H460 cells associated with a loss
of cell viability in a dose- and time-dependent manner (Fig. 1). These findings indicated: i) DNA
damage from comet assay (single cell gel electrophoresis) occured
in the tail moment of the comets from NCI-H460 cells, the longer
the comet tail the higher the DNA damage (Fig. 2) in a dose-dependent manner; ii) DNA
fragments from DNA gel electrophoresis indicated that high dose of
deguelin treatment led to high fragmentation in NCI-H460 cells
(Fig. 3).
Comet assay is a highly sensitive technique for DNA
damage examination and thus it has been used for screening the
effects of agent on DNA damage in cells (28–30).
Furthermore, a measurement for trend-break formation during the
process of excision repair of DNA could be used (31,32).
In our earlier studies, we have shown that deguelin induced
apoptosis in human cancer cell lines (data not shown), but we also
found that deguelin induced apoptosis based on DNA fragmentation
occur in NCI-H460 cells after exposure to deguelin from DNA agarose
gel electrophoresis assay (Fig. 3).
Our earlier studies also showed that deguelin-induced apoptosis may
be through the production of reactive oxygen species (ROS) in
NCI-H460 cells (data not shown); thus, we suggest that deguelin
induced DNA damage may be via the production of ROS. Further
studies are needed to establish the role of the interaction of
deguelin with DNA in cancer cells.
Numerous evidence has shown that in cells, agents
can induce DNA damage which can be reduced by DNA repair system
through eliminating DNA lesions (33–35).
In the present study, our results from the comet assay (Fig. 2) and DNA gel electrophoresis
indicated that deguelin-induced DNA damage (Fig. 3) in NCI-H460 cells. Furthermore,
results were obtained from real-time PCR (Fig. 4) which indicated that DNA repair
gene expression including BRCA1, DNA-PK, MGMT,
p53, ATM and ATR were inhibited in
deguelin-treated NCI-H460 cells. Importantly, the gene levels of
BRCA1, DNA-PK, ATM, ATR and
DNA-PK expressions were reduced dose-dependently.
Cells after stimulation by agents cause DNA damage
and the DNA damage checkpoints are signal transduction pathways
which are involved in the cell cycle and cellular responses to DNA
damage in order to maintain genomic integrity (36–38).
Especially, the ATM and ATR are two master checkpoint kinases
activated by double-stranded DNA breaks (DSBs) (39). In UV-damaged DNA and incompletely
replicated DNA, the ATR kinase is responsible for initiating the
DNA damage checkpoint (40). BRCA1
(tumor suppressor) plays critical roles in DNA repair, cell cycle
checkpoint control and maintenance of genomic stability in human
breast and ovarian cancer (41).
Moreover, DNA-PK plays a critical role in DNA damage repair
(42). MGMT reduces cytotoxicity of
therapeutic or environmental alkylating agents (43).
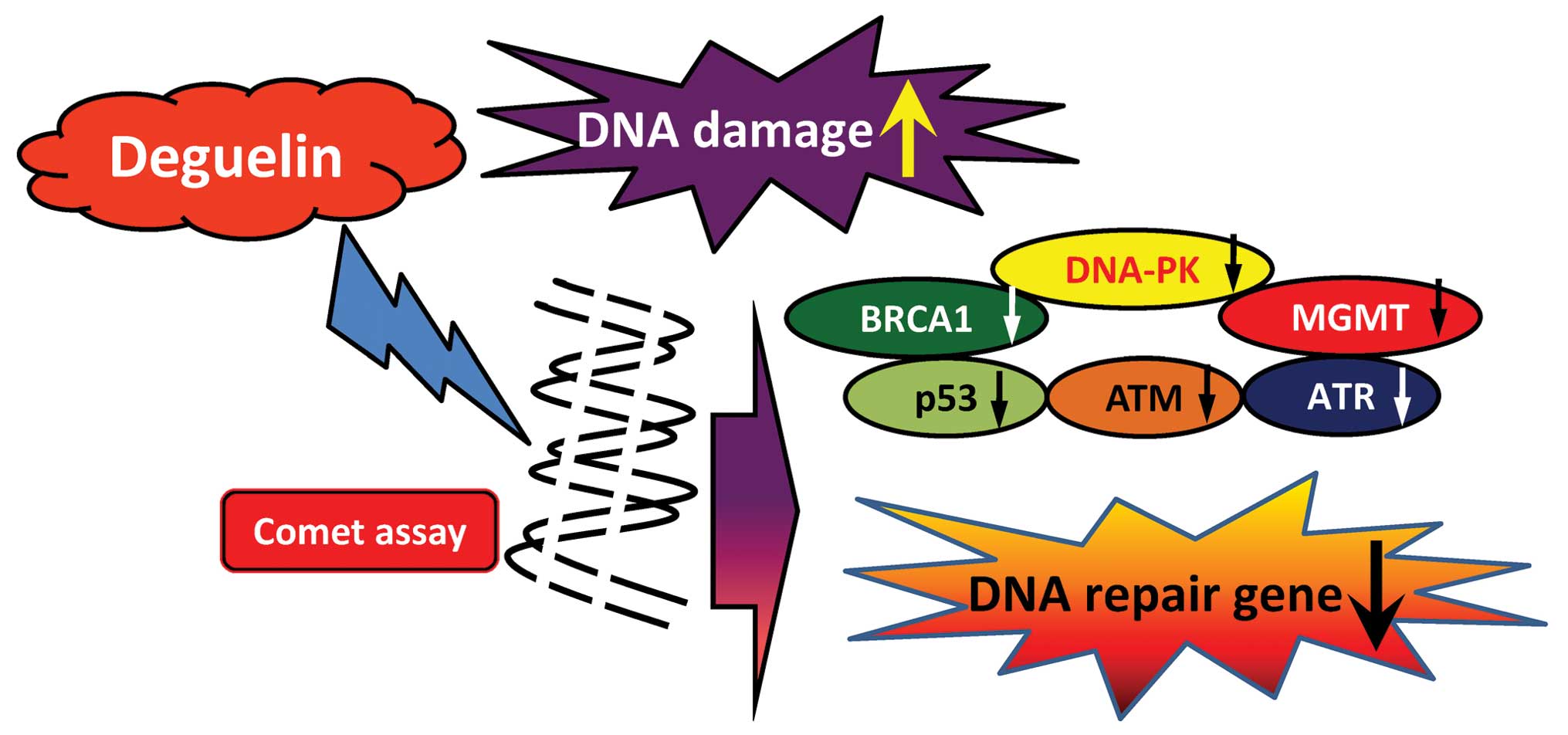
In conclusion, the possible flow charts for
deguelin-affected DNA damage in human lung cancer NCI-H460 cells
are summarized in Fig. 5 which
indicates that deguelin induced DNA damage followed by the
inhibition of DNA repair associated gene expressions (mRNA)
including BRCA1, DNA-PK, MGMT, p53, ATM and ATR, resulting in
maintenance of DNA damage (Fig.
5).
Acknowledgements
This study was supported by grant 99-CCH-IRP-08 from
the Changhua Christian Hospital, Changhua, Taiwan.
References
|
1
|
Murillo G, Salti GI, Kosmeder JW II,
Pezzuto JM and Mehta RG: Deguelin inhibits the growth of colon
cancer cells through the induction of apoptosis and cell cycle
arrest. Eur J Cancer. 38:2446–2454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee HY, Oh SH, Woo JK, et al:
Chemopreventive effects of deguelin, a novel Akt inhibitor, on
tobacco-induced lung tumorigenesis. J Natl Cancer Inst.
97:1695–1699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murillo-Godinez G: The flugge’s drops. Rev
Med Inst Mex Seguro Soc. 47:2902009.
|
|
4
|
Lee JH, Lee DH, Lee HS, Choi JS, Kim KW
and Hong SS: Deguelin inhibits human hepatocellular carcinoma by
antiangiogenesis and apoptosis. Oncol Rep. 20:129–134.
2008.PubMed/NCBI
|
|
5
|
Chun KH, Kosmeder JW II, Sun S, et al:
Effects of deguelin on the phosphatidylinositol 3-kinase/Akt
pathway and apoptosis in premalignant human bronchial epithelial
cells. J Natl Cancer Inst. 95:291–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HY: Molecular mechanisms of
deguelin-induced apoptosis in transformed human bronchial
epithelial cells. Biochem Pharmacol. 68:1119–1124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerhauser C, Mar W, Lee SK, et al:
Rotenoids mediate potent cancer chemopreventive activity through
transcriptional regulation of ornithine decarboxylase. Nat Med.
1:260–266. 1995. View Article : Google Scholar
|
|
8
|
Nair AS, Shishodia S, Ahn KS, Kunnumakkara
AB, Sethi G and Aggarwal BB: Deguelin, an AKT inhibitor, suppresses
IkappaBalpha kinase activation leading to suppression of
NF-kappaB-regulated gene expression, potentiation of apoptosis, and
inhibition of cellular invasion. J Immunol. 177:5612–5622. 2006.
View Article : Google Scholar
|
|
9
|
Dell’Eva R, Ambrosini C, Minghelli S,
Noonan DM, Albini A and Ferrari N: The Akt inhibitor deguelin, is
an angiopreventive agent also acting on the NF-kappaB pathway.
Carcinogenesis. 28:404–413. 2007.PubMed/NCBI
|
|
10
|
Oh SH, Woo JK, Jin Q, et al:
Identification of novel antiangiogenic anticancer activities of
deguelin targeting hypoxia-inducible factor-1 alpha. Int J Cancer.
122:5–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maier CM and Chan PH: Role of superoxide
dismutases in oxidative damage and neurodegenerative disorders.
Neuroscientist. 8:323–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paz-Elizur T, Sevilya Z, Leitner-Dagan Y,
Elinger D, Roisman LC and Livneh Z: DNA repair of oxidative DNA
damage in human carcinogenesis: potential application for cancer
risk assessment and prevention. Cancer Lett. 266:60–72. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lavelle C, Salles B and Wiesmuller L: DNA
repair, damage signaling and carcinogenesis. DNA Repair (Amst).
7:670–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji BC, Hsu WH, Yang JS, et al: Gallic acid
induces apoptosis via caspase-3 and mitochondrion-dependent
pathways in vitro and suppresses lung xenograft tumor growth in
vivo. J Agric Food Chem. 57:7596–7604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu KC, Huang AC, Wu PP, et al: Gallic
acid suppresses the migration and invasion of PC-3 human prostate
cancer cells via inhibition of matrix metalloproteinase-2 and -9
signaling pathways. Oncol Rep. 26:177–184. 2011.PubMed/NCBI
|
|
17
|
Yu FS, Yang JS, Yu CS, et al: Safrole
induces apoptosis in human oral cancer HSC-3 cells. J Dent Res.
90:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
19
|
Kuo CL, Wu SY, Ip SW, et al: Apoptotic
death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma
cells is mediated through the ROS, mitochondrial depolarization and
caspase-3-dependent signaling responses. Int J Oncol. 39:319–328.
2011.
|
|
20
|
Chen HY, Lu HF, Yang JS, et al: The novel
quinolone CHM-1 induces DNA damage and inhibits DNA repair gene
expressions in a human osterogenic sarcoma cell line. Anticancer
Res. 30:4187–4192. 2010.PubMed/NCBI
|
|
21
|
Yang JS, Chen GW, Hsia TC, et al: Diallyl
disulfide induces apoptosis in human colon cancer cell line (COLO
205) through the induction of reactive oxygen species, endoplasmic
reticulum stress, caspases casade and mitochondrial-dependent
pathways. Food Chem Toxicol. 47:171–179. 2009. View Article : Google Scholar
|
|
22
|
Ho YT, Yang JS, Li TC, et al: Berberine
suppresses in vitro migration and invasion of human SCC-4 tongue
squamous cancer cells through the inhibitions of FAK, IKK,
NF-kappaB, u-PA and MMP-2 and -9. Cancer Lett. 279:155–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riviere J, Ravanat JL and Wagner JR:
Ascorbate and H2O2 induced oxidative DNA
damage in jurkat cells. Free Radic Biol Med. 40:2071–2079.
2006.
|
|
24
|
Visvardis EE, Tassiou AM and Piperakis SM:
Study of DNA damage induction and repair capacity of fresh and
cryopreserved lymphocytes exposed to H2O2 and gamma-irradiation
with the alkaline comet assay. Mutat Res. 383:71–80. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee H, Lee JH, Jung KH and Hong SS:
Deguelin promotes apoptosis and inhibits angiogenesis of gastric
cancer. Oncol Rep. 24:957–963. 2010.PubMed/NCBI
|
|
26
|
Peng XH, Karna P, O’Regan RM, et al:
Down-regulation of inhibitor of apoptosis proteins by deguelin
selectively induces apoptosis in breast cancer cells. Mol
Pharmacol. 71:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hail N Jr and Lotan R: Apoptosis induction
by the natural product cancer chemopreventive agent deguelin is
mediated through the inhibition of mitochondrial bioenergetics.
Apoptosis. 9:437–447. 2004. View Article : Google Scholar
|
|
28
|
Pool-Zobel BL, Lotzmann N, Knoll M, et al:
Detection of genotoxic effects in human gastric and nasal mucosa
cells isolated from biopsy samples. Environ Mol Mutagen. 24:23–45.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donatus IA, Sardjoko and Vermeulen NP:
Cytotoxic and cytoprotective activities of curcumin. Effects on
paracetamol-induced cytotoxicity, lipid peroxidation and
glutathione depletion in rat hepatocytes. Biochem Pharmacol.
39:1869–1875. 1990.PubMed/NCBI
|
|
30
|
Ashby J, Tinwell H, Lefevre PA and Browne
MA: The single cell gel electrophoresis assay for induced DNA
damage (comet assay): measurement of tail length and moment.
Mutagenesis. 10:85–90. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tice RR, Andrews PW and Singh NP: The
single cell gel assay: a sensitive technique for evaluating
intercellular differences in DNA damage and repair. Basic Life Sci.
53:291–301. 1990.PubMed/NCBI
|
|
32
|
Olive PL, Banath JP and Durand RE:
Detection of etoposide resistance by measuring DNA damage in
individual chinese hamster cells. J Natl Cancer Inst. 82:779–783.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang MR, Li YC, Yang Y and Wu JR: c-Myc
degradation induced by DNA damage results in apoptosis of cho
cells. Oncogene. 22:3252–3259. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marnett LJ: Oxyradicals and DNA damage.
Carcinogenesis. 21:361–370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Epe B: Role of endogenous oxidative DNA
damage in carcinogenesis: what can we learn from repair-deficient
mice? Biol Chem. 383:467–475. 2002.PubMed/NCBI
|
|
36
|
Cimprich KA and Cortez D: ATR: an
essential regulator of genome integrity. Nat Rev Mol Cell Biol.
9:616–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lou Z, Minter-Dykhouse K, Wu X and Chen J:
MDC1 is coupled to activated CHK2 in mammalian DNA damage response
pathways. Nature. 421:957–961. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y and Kulesz-Martin M: p53 protein at
the hub of cellular DNA damage response pathways through
sequence-specific and non-sequence-specific DNA binding.
Carcinogenesis. 22:851–860. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shiotani B and Zou L: Single-stranded DNA
orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell.
33:547–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi JH, Sancar A and Lindsey-Boltz LA:
The human ATR-mediated DNA damage checkpoint in a reconstituted
system. Methods. 48:3–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Venkitaraman AR: Cancer susceptibility and
the functions of BRCA1 and BRCA2. Cell. 108:171–182. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mi J, Dziegielewski J, Bolesta E,
Brautigan DL and Larner JM: Activation of DNA-PK by ionizing
radiation is mediated by protein phosphatase 6. PLoS One.
4:e43952009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jesien-Lewandowicz E, Jesionek-Kupnicka D,
Zawlik I, et al: High incidence of MGMT promoter methylation in
primary glioblastomas without correlation with TP53 gene mutations.
Cancer Genet Cytogenet. 188:77–82. 2009. View Article : Google Scholar : PubMed/NCBI
|