Introduction
Pancreatic cancer is one of the most aggressive
malignancies in the world, with a poor prognosis and a high
mortality rate. The overall 5-year survival rate for pancreatic
cancer patients remains less than 5%. Although surgical approaches
have been developed, there has been no significant improvement in
survival rate over the past three decades (1), which is attributed to the high
incidence of metastasis at initial diagnosis (2). Further understanding of tumorigenesis
of pancreatic cancer may provide new clues for developing
prevention and treatment strategies.
Epidermal growth factor receptor (EGFR) plays a key
role in epithelial tumor formation. Recent studies indicated that
EGFR was detectable in over 95% of pancreatic cancer patients
(3), and that aberrant EGFR
activation can increase pancreatic cancer cell proliferation
(4). In recent years, EGFR
targeting therapy has become a popular mode of comprehensive tumor
treatment. However, selective targeting of EGFR was not as
effective as expected, because drug resistance would develop and
lead to unsatisfactory clinical effect (5–7).
Therefore, it remains necessary to explore additional therapeutic
combinations.
Hedgehog (Hh) signaling pathway plays an important
role in pancreatic carcinogenesis. It is closely associated with
pancreatic cancer occurrence and progression, and is involved in
cellular proliferation and invasion in vivo and in
vitro(8). Research showed that
EGFR and Hh signaling pathways were upregulated in many pancreatic
cancer cell lines (9,10). However, the relationship and the
synergetic mechanisms between these two pathways remain
unclear.
In this study, we introduced a lentiviral vector
containing shRNA that targets the EGFR gene into human pancreatic
cancer cells. The effects of EGFR RNAi alone or in conjunction with
Hh inhibition on proliferation and apoptosis were explored both
in vitro and in vivo, and the possible synergistic
mechanisms for Hh and EGFR signaling pathways were further
investigated.
Materials and methods
Cell line and culture conditions
The human pancreatic cancer cell lines, PANC-1,
ASPC-1 and Mia PaCa-2 were generously provided by Professor Beger
and Professor Kornmann of Ulm University, Germany. The cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco,
Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS, Sigma, St. Louis, MO, USA) and penicillin (100
U/ml).
Establishment of pancreatic cancer cell
clones expressing EGFR RNAi
The RNAi targeting human EGFR sequence (GeneBank
accession no. NM_201283) and the negative control sequence were
designed and constructed by GeneChem (Shanghai, China). PANC-1
cells were transfected with recombinant pFU-GW-RNAi lentivirus
vectors targeting the EGFR gene (PANC-1-si) or with negative
control vectors (PANC-1-nc). All constructs were confirmed using
sequence analysis. The constructs were stably transfected into
cells to generate knockdown clones. The cells were cultured for 48
h, and the transduction efficiency was assessed using FACS
analysis. The transfected cells were harvested and prepared for
subsequent studies.
Reverse transcriptase polymerase chain
reaction (RT-PCR)
Total RNA from wild-type pancreatic cancer cells and
clones EGFR knockdown cells was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). Aliquots (1 μg) of RNA were
DNase-treated and processed for first-strand cDNA synthesis using
the RT-PCR Kit (Toyobo, Osaka, Japan). cDNA was amplified using a
PCR thermal controller with initial denaturation at 94°C for 5 min,
followed by 30 cycles of: denaturation at 94°C for 30 sec,
annealing at 61°C for 30 sec, extension at 72°C for 60 sec, and a
final extension step at 72°C for 5 min. GAPDH was used for gene
expression normalization. Three independent experiments were
performed.
Western blot analysis
The total protein from wild-type pancreatic cancer
cells and the EGFR knockdown clones (PANC-1, PANC-1-nc and
PANC-1-si) were obtained using the Total Protein Extraction kit
(Keygen, Nanjing, China). Total protein per sample (70 μg) was
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and electroblotted onto nitrocellulose
membranes, and then probed with anti-phospho-Akt, anti-Akt,
anti-phospho-Erk1/2, anti-Erk1/2 (Cell Signaling Technology,
Beverly, MA, USA) and anti-EGFR (Abcam, Cambridge, UK) antibodies.
Three independent experiments were performed.
Colony formation assay
To assess the effect of EGFR knockdown on the colony
formation ability of PANC-1, PANC-1-nc and PANC-1-si cells, each
were respectively seeded onto 6-well plates at various low
densities. When clones appeared, they were fixed in methanol and
stained with Giemsa before they were manually counted. Three
independent experiments were performed.
Cell growth assays
The MTT assay was used to assess the effect of EGFR
knockdown on cell proliferation and cyclopamine sensitivity in
pancreatic cancer cells. Wild-type cancer cells (PANC-1) and two
transduced clones (PANC-1-nc and PANC-1-si) were cultured in
96-well plates at a density of 1.0×104 cells/well for 72
h prior to the proliferation assay. To assess cyclopamine
sensitivity, each cell group was seeded at a density of
1.0×104 cells/well in 96-well plates, and allowed to
attach for 24 h. They were then incubated in media containing the
indicated concentrations (0, 0.625, 1.25, 2.5, 5.0 and 10 μmol/l)
of cyclopamine for 48 h before initiation of the MTT assay. The
cell growth inhibition rate (GIR) was calculated as follows: GIR =
(1 − OD490 of treated cells/OD490 of
untreated cells) × 100%. Three independent experiments were
performed.
Flow cytometry analysis for
apoptosis
An Annexin V-PE Apoptosis Detection kit (BD
Biosciences) was used to measure apoptosis in PANC-1, PANC-1-nc and
PANC-1-si cells, with or without cyclopamine treatment. The cells
were seeded in 6-well plates and incubated until they were about
70% confluent. Then, the cyclopamine medium was replaced with fresh
media, and the cells were incubated for 48 h before analysis. The
labeled cells (1.0×104/sample) were analyzed using
FACScan flow cytometry (BD Biosciences) in conjunction with
CellQuest software. Three independent experiments were
performed.
Tumor xenografts in nude mice
All animal studies were reviewed and approved by the
Ethics Committee for Animal Studies at Peking University, China.
Briefly, 1.0×107 cells were injected subcutaneously into
the right axilla of 6-week-old female nude mice. The mice were
randomized into four treatment groups according to the cells
injected and paired with the following treatments: i) PANC-1-nc, or
PANC-1-si plus cyclopamine (50 mg/kg, dissolved in 0.1 ml PBS,
intraperitoneal injection, once every other day) (11) treatment, and ii) PANC-1-nc, or
PANC-1-si plus PBS treatment groups (0.1 ml intraperitoneal
injection, once every other day). The animals were monitored for
tumor formation every 2 days. Tumor size was measured in two
dimensions, and the volume was calculated using the equation V = L
× W2 × 0.5 (where V is the volume, L is the length and W
is the width). Four weeks later, all the mice were sacrificed, and
the tumors were excised and weighed.
Immunohistochemistry analysis of
xenograft tumors
Paraffin-embedded 4-μm-thick sections were prepared
and analyzed by H&E staining or immunohistochemical analysis
using a Cell and Tissue Staining kit according to the
manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA). To
assess the proliferative index of serial sections, Ki-67 staining
assay was carried out in representative non-necrotic areas of each
tumor using a rabbit anti-human Ki-67 antibody (Abcam). Tumor cell
apoptosis was evaluated using the TUNEL assay (Roche, Mannheim,
Germany) and was performed as described by the manufacturer.
Statistical analysis
Quantitative data were expressed as means ± SD.
Means were compared using one-way ANOVA and Student’s t-test.
Statistical analyses were performed using SPSS Version 13.0.
Differences were considered statistically significant if
P<0.05.
Results
Detection of endogenous EGFR in
pancreatic cancer cell lines and the effect of cyclopamine on its
expression
EGFR mRNA and protein expression were determined
using RT-PCR and western blot analyses, respectively, in three
pancreatic cancer cell lines (PANC-1, ASPC-1 and Mia PaCa-2). As
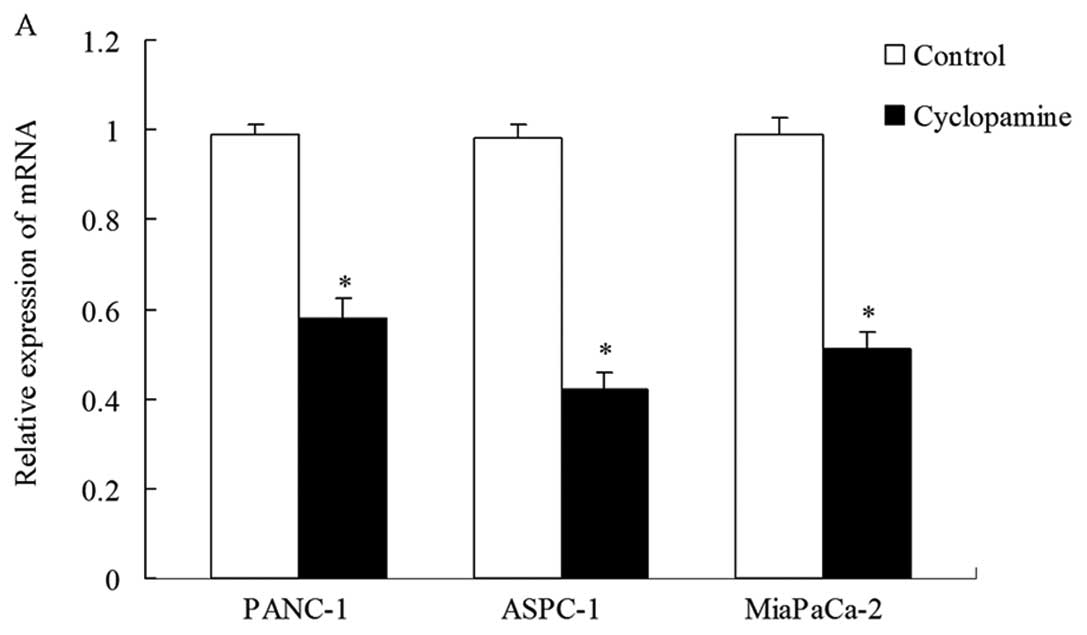
shown in Fig. 2A, the EGFR mRNA and
protein expression levels differed in the three cell lines. We then
studied the effect of cyclopamine, a naturally occurring Hh pathway
antagonist, on EGFR expression in these cell lines. As shown in
Fig. 2B, both protein and mRNA
expression levels decreased in all the three cell lines after 48 h
of treatment with 5 μmol/l cyclopamine. EGFR mRNA and protein
expression in PANC-1 cells was higher than in the other two cell
lines (*P<0.05). Therefore, we selected the PANC-1
cell line for follow-up assays in order to show EGFR inhibition
more clearly.
RNAi targeting EGFR in PANC-1 pancreatic
cancer cells
PANC-1 cells were transfected with either
recombinant pFU-GW-RNAi lentiviral vectors that target the EGFR
gene (PANC-1-si), or with negative control vectors (PANC-1-nc). The
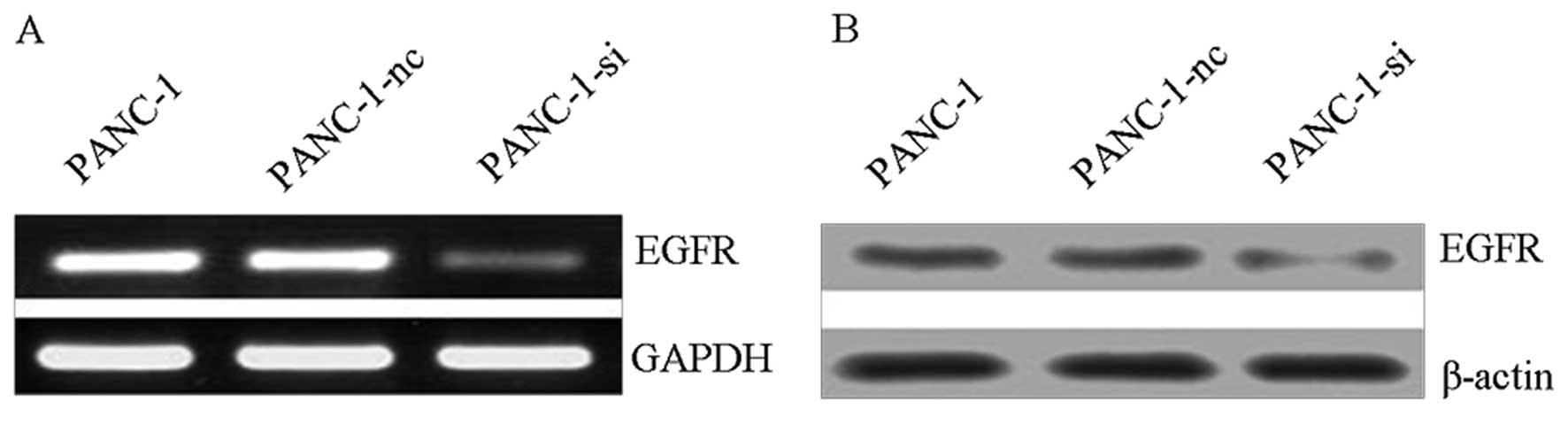
stably transfected cells were purified using FACS. RT-PCR and
western blot analyses probed for EGFR expression and revealed that
it was significantly downregulated in PANC-1-si transfected cells,
but not in PANC-1-nc transfected cells when both were compared with
untransfected cells (Fig. 3). The
results indicated that the EGFR-targeting RNAi could effectively
suppress EGFR gene expression in PANC-1 cells.
Combined targeting of EGFR and Hh
signaling enhanced inhibition of proliferation and colony formation
in PANC-1 cells
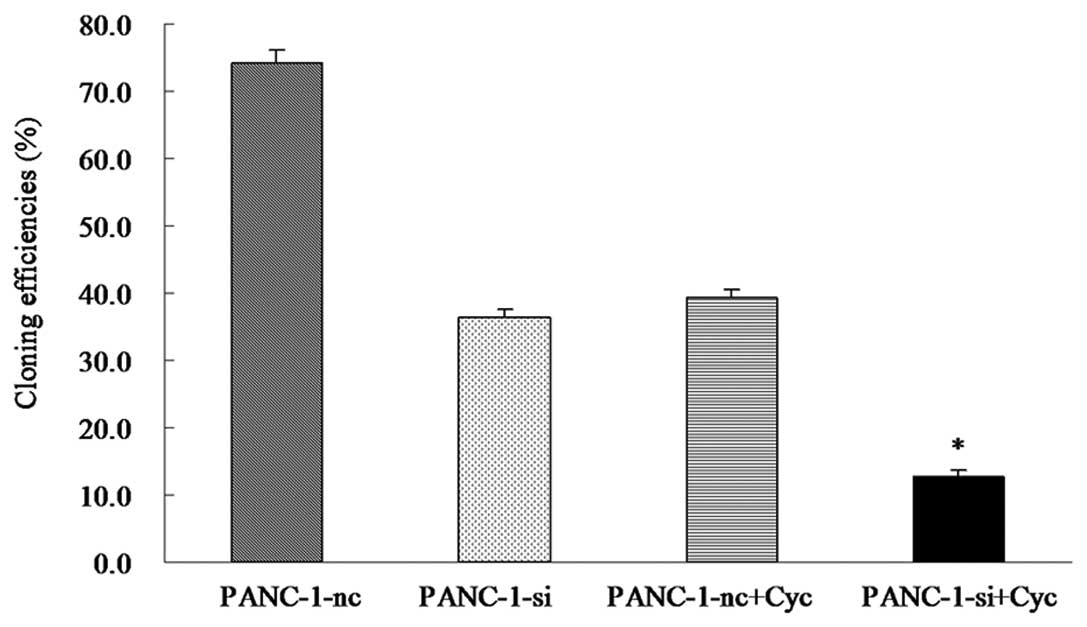
The effect of EGFR inhibition on cell growth was
measured using the MTT and colony formation assays. Colony
formation assay was used to assess cell survival, as shown in
Fig. 4. The results indicated that
the number of PANC-1-si+cyclopamine colonies was fewer than those
of PANC-1-nc cells and PANC-1-si cells (*P<0.05,
Fig. 4). There was no difference in
colony formation inhibition when PANC-1-nc+cyclopamine cells and
PANC-1-si cells were compared (P>0.05).
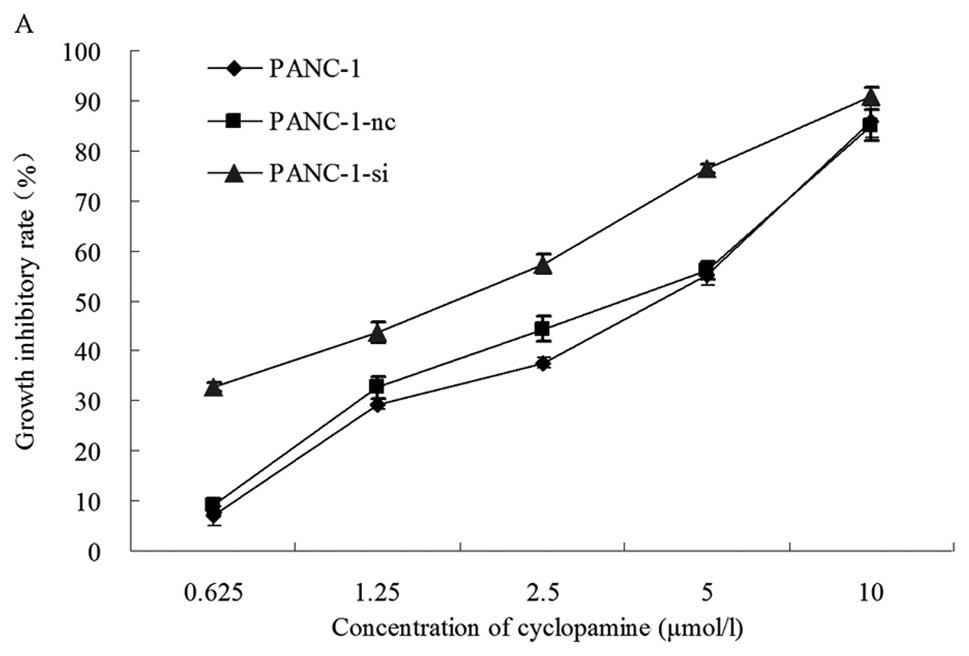
Cell proliferation was analyzed using the MTT assay.
Proliferation was inhibited notably for EGFR knockdown cells when
combined with Hh signaling inhibition by cyclopamine in the PANC-1
cell line. As shown in Fig. 5A,
cyclopamine inhibited cell growth in a dose-dependent manner. The
inhibitory rates after 72 h of treatment were 32.67% for PANC-1,
9.03% for PANC-1-nc and 6.99% for PANC-1-si cells. A low
concentration of cyclopamine (0.625 μmol/l) was sufficient to
significantly inhibit cell growth. PANC-1-si cells displayed the
highest sensitivity to cyclopamine, due to EGFR knockdown. However,
there was no difference in growth inhibition between the PANC-1 and
PANC-1-nc cell lines (P>0.05). This result showed that treatment
with cyclopamine resulted in a dose-dependent anti-proliferative
effect in PANC-1 cells. The half maximal inhibitory concentration
(IC50) for cyclopamine was determined from dose-response
curves. As shown in Fig. 5B, the
IC50 of cyclopamine was 2.978±0.336 μmol/l for PANC-1,
3.106±0.176 μmol/l for PANC-1-nc, and 1.698±0.057 μmol/l for
PANC-1-si cells. The IC50 for the EGFR knockdown cells
was significantly lower than those of wild-type and control
transfected cells (P=0.002 for PANC-1 cells, P=0.001 for PANC-1-nc
cells and P=0.002 for PANC-1-si cells). There was no difference in
the IC50 between the control (PANC-1) and the untreated
(PANC-1-nc) cells (P>0.05). These results showed that EGFR RNAi
combined with Hh signaling inhibition could significantly reduce
cell proliferation and colony formation in pancreatic cancer cells,
and could effectively enhance cyclopamine sensitivity in
vitro.
Synergetic effects of EGFR and Hh
signaling inhibition on apoptosis in pancreatic cancer cells
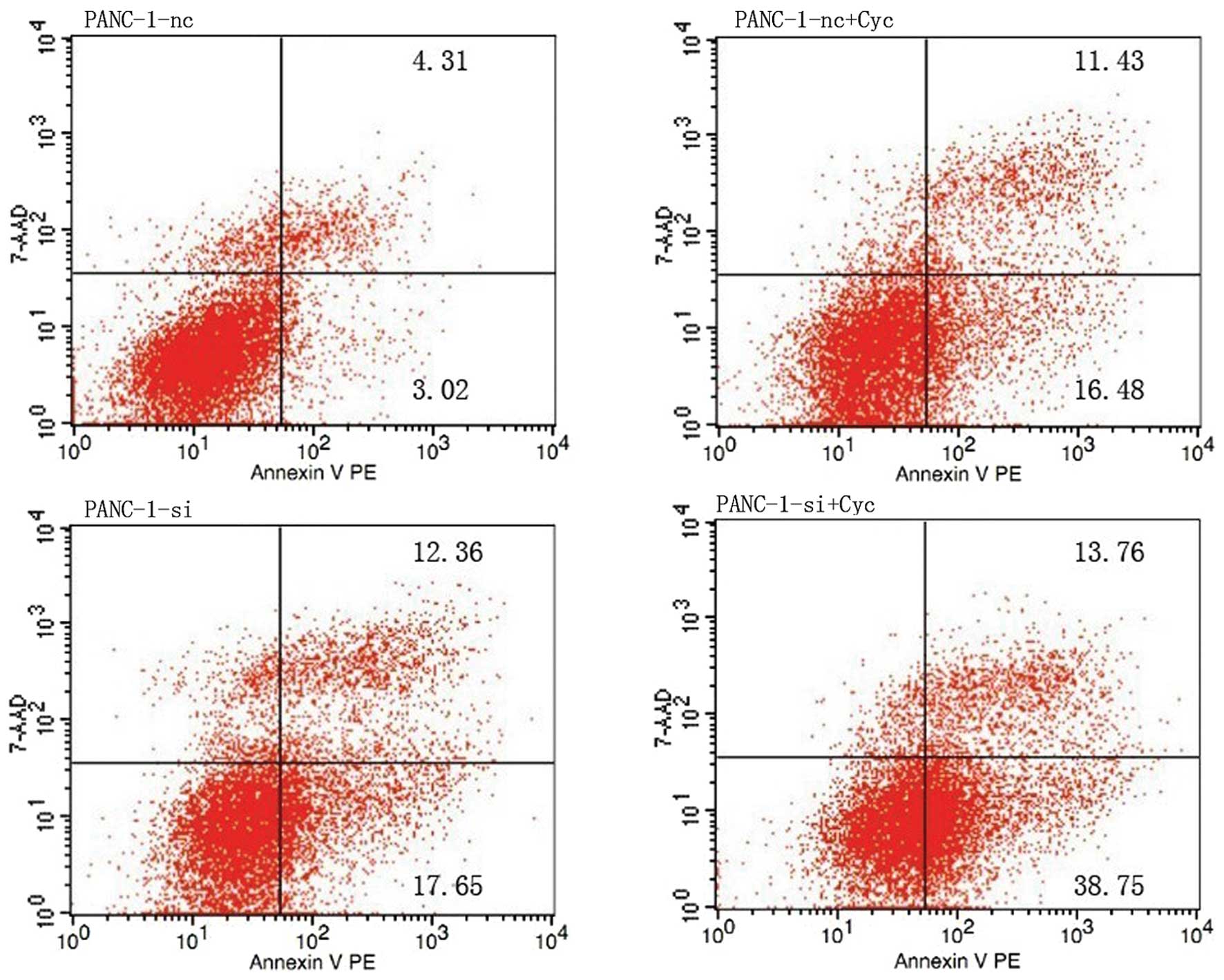
Apoptosis of cultured cells was analyzed using flow
cytometry. Four different conditions were tested: PANC-1-nc,
PANC-1-si, PANC-1-nc+Cyc and PANC-1-si+Cyc. As shown in Fig. 6, the prophase apoptosis percentage
of the control group (PANC-1-nc) was 3.117±0.121% (P=0.001); and
both the EGFR RNAi group (PANC-1-si) and the cyclopamine treatment
group (PANC-1-nc+Cyc) showed a moderately increased apoptosis,
compared with untreated PANC-1-nc cells (P<0.05). The prophase
apoptosis percentage of the combined treatment group
(PANC-1-si+Cyc) was as high as 38.97±1.237%, which was
significantly higher than the EGFR knockdown (PANC-1-si,
17.62±0.602%) and the control group (PANC-1-nc, 3.117±0.121%),
(P<0.05). The prophase apoptosis percentage of the combined
treatment group (PANC-1-si+Cyc) was significantly higher than that
of the PANC-1-si and PANC-1-nc+Cyc groups (P<0.05). The results
showed that EGFR downregulation might reduce the apoptosis
threshold in pancreatic cancer cells, and enhanced apoptosis when
combined with cyclopamine treatment.
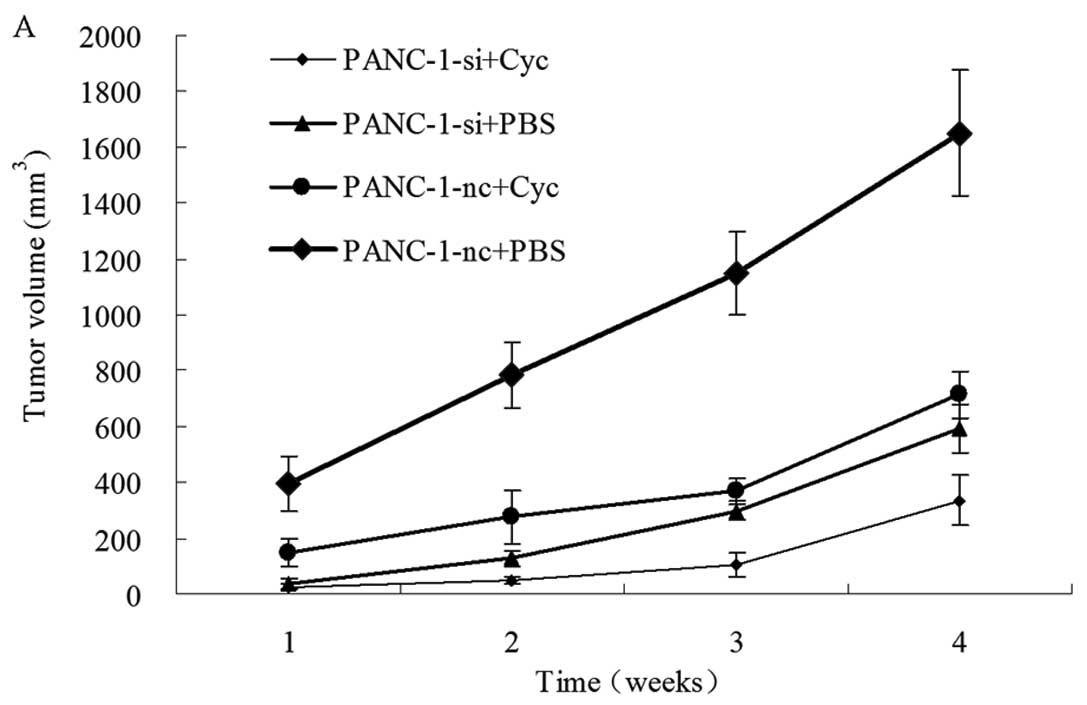
Effects of EGFR RNAi and cyclopamine on
PANC-1 xenograft growth
To investigate whether the in vitro antitumor
effects of EGFR inhibition and its synergistic effects with
cyclopamine could be reproduced in vivo, we established
xenograft models using PANC-1-nc and PANC-1-si cells. All of the
PANC-1-nc injected mice, and most of the PANC-1-si injected mice
developed measurable tumors one week after injection. The mice then
received treatments of either cyclopamine or PBS once every 48 h.
The xenograft tumor size was measured once a week. As shown in
Fig. 7, the tumors from PANC-1-si
injected mice were significantly smaller than tumors from the
control cells (PANC-1-si+PBS vs. PANC-1-nc+PBS, P<0.05).
Cyclopamine treatment could inhibit xenograft tumor growth compared
with PBS (PANC-1-nc+Cyc vs. PANC-1-nc+PBS, P<0.05). Furthermore,
the combined treatment using EGFR RNAi and cyclopamine
(PANC-1-si+Cyc) resulted in additional tumor growth inhibition
(Fig. 7A). Twenty-eight days after
tumor inoculation, the average tumor volume of the combined
treatment was significantly decreased when compared with those from
PANC-1-nc+PBS PANC-1-si+PBS and PANC-1-nc+Cyc xenografts
(P<0.05, Fig. 7B).
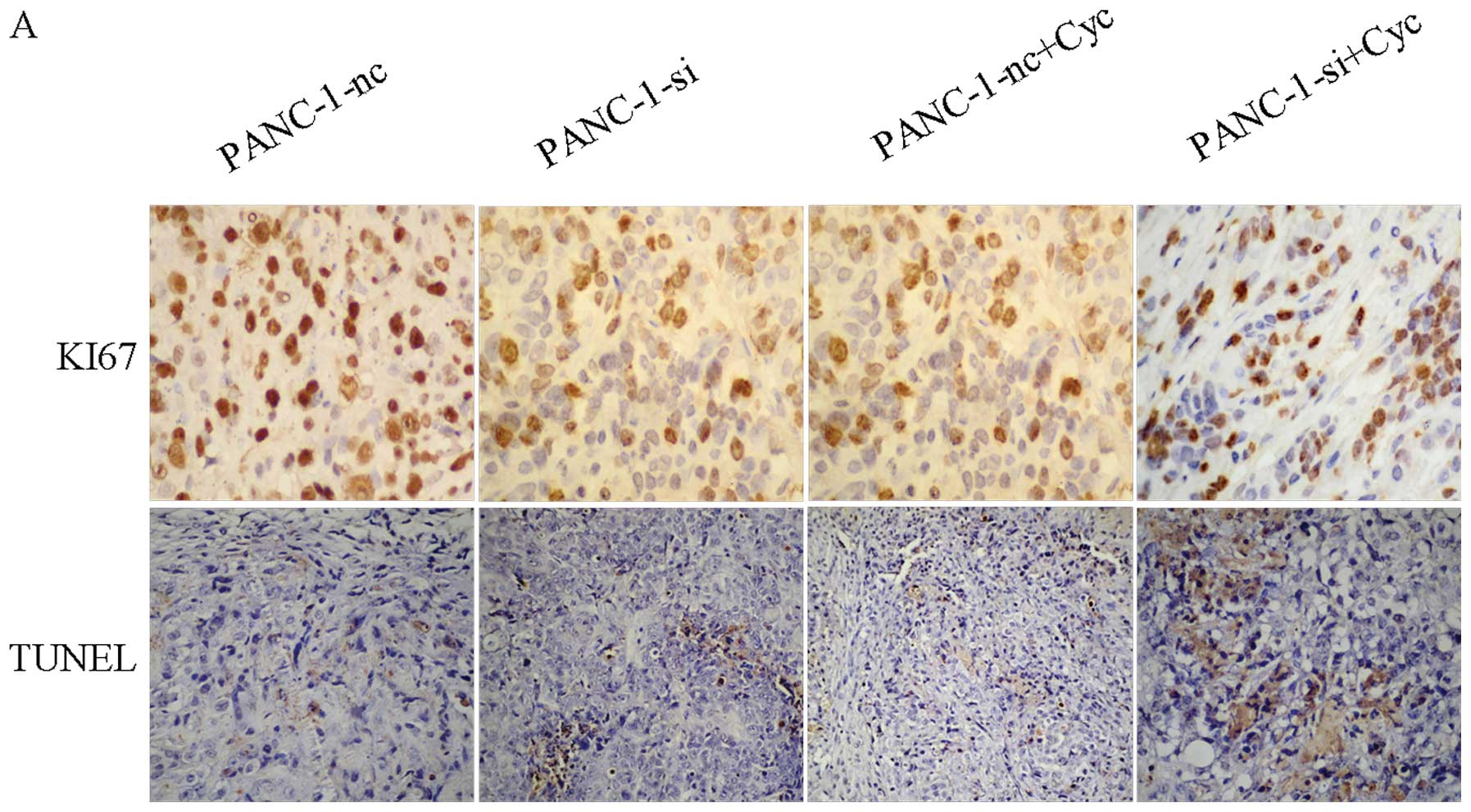
Proliferation and apoptosis in xenograft
tumors
We performed the Ki-67 staining assay and the TUNEL
assay to evaluate proliferation in xenografts. As shown in Fig. 8, analysis of the proliferative index
for the xenograft tumors revealed that both suppression of EGFR and
combined treatment with cyclopamine significantly inhibited Ki-67
immunoreactivity, compared with the control groups. Combined
treatment (PANC-1-si+Cyc xenografts) showed the lowest Ki-67
immunoreactivity. Tumor cell apoptosis was also assessed using the
TUNEL assay (Fig. 8A). As shown in
Table I, the apoptotic index for
PANC-1-si+Cyc cells was significantly higher than PANC-1-nc cells
and PANC-1-si cells (*P<0.05). The results indicated
that inhibition of both EGFR and Hh signaling pathways may have a
synergistic effect on proliferation and apoptosis.
 | Table IApoptotic index. |
Table I
Apoptotic index.
| Cells | Control (NS) | Treatment group
(cyclopamine) | P-value |
|---|
| PANC-1-nc | 3.85±0.52 | 12.76±2.29 | 0.003 |
| PANC-1-si | 14.73±1.00 | 20.87±4.38a | 0.002 |
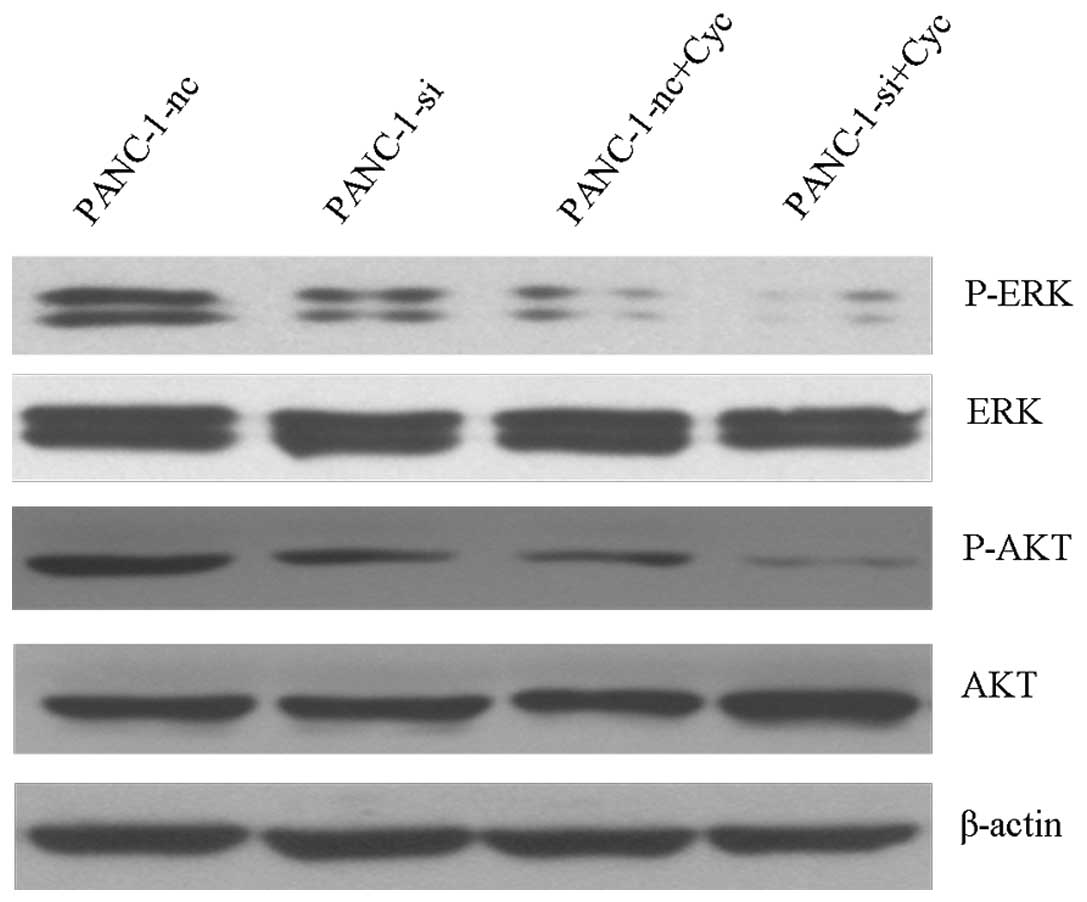
Synergistic effects of EGFR and Hh
inhibition on PI3K/Akt and Raf/MEK/ERK activation
To investigate possible mechanisms for the
synergistic apoptosis effect, we analyzed the PI3K/Akt and
Raf/MEK/ERK activation using western blot analysis. As shown in
Fig. 9, EGFR knockdown combined
with cyclopamine-mediated Hh inhibition produced a pronounced
decrease in both AKT and ERK phosphorylation, when compared with
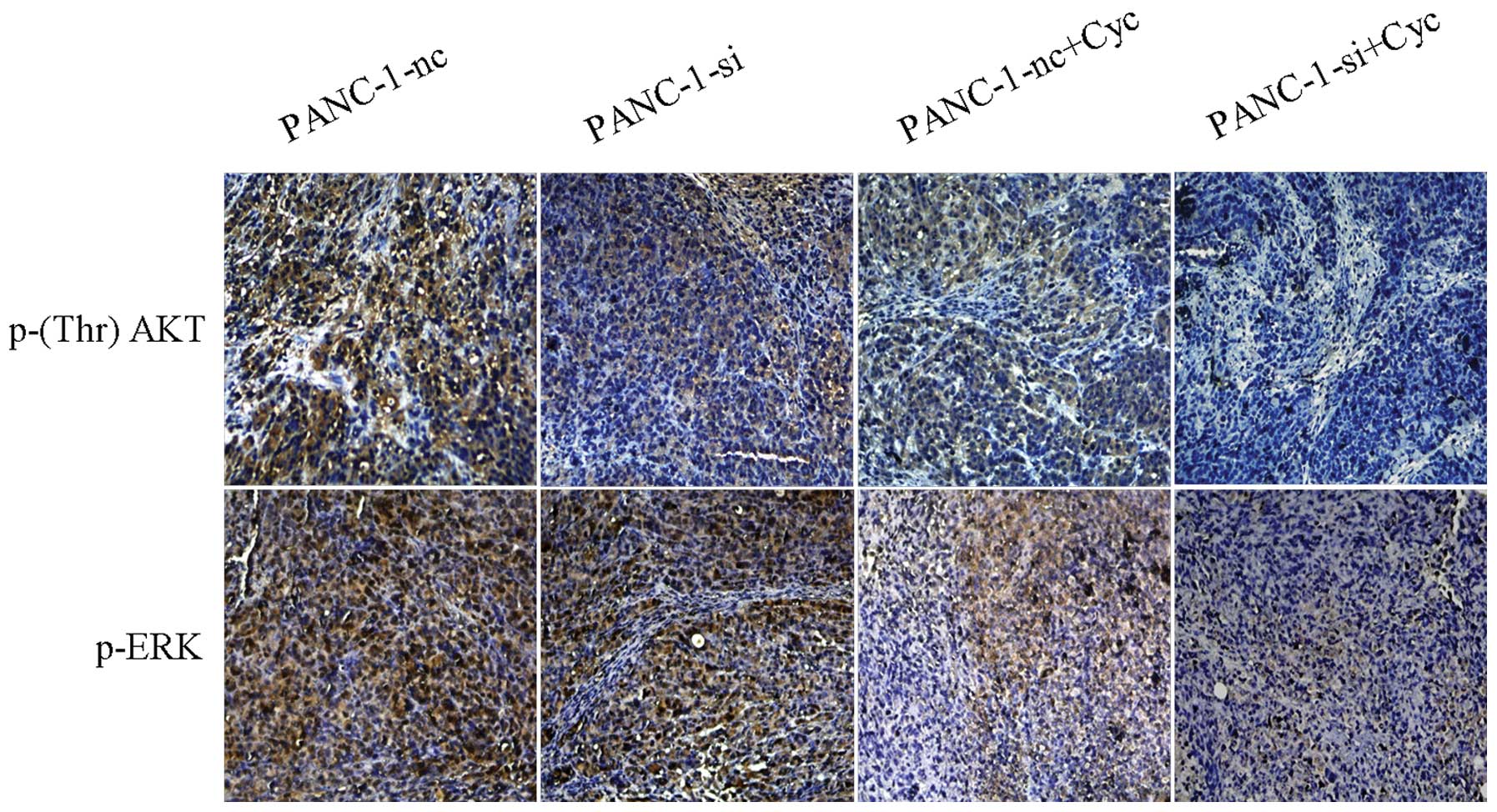
PANC-1-si or PANC-1-nc+Cyc cells. Moreover, phosphorylated AKT/ERK
expression in PANC-1-si+Cyc tumor groups displayed a significant
reduction compared with the control group (Fig. 10).
Discussion
The epidermal growth factor receptor (EGFR) belongs
to a family of receptor tyrosine kinases, and plays a key role in
cell proliferation, survival, migration and differentiation in
epithelial tumor formation (12).
EGFR is localized mainly to cell membranes and is activated by the
EGF ligand (13). A recent study
revealed that the overexpression or deregulation of EGFR induced
rapid growth, strong invasion ability, and poor prognosis for many
kinds of solid tumors, including pancreatic cancer (14).
In recent years, therapy targeting EGFR has been a
popular method for comprehensive tumor treatment, which includes
EGFR monoclonal antibodies and small molecular tyrosine kinase
inhibitors. For example, erlotinib, an EGFR-tyrosine kinase
inhibitor, has been approved by the FDA as a comprehensive
treatment for advanced pancreatic cancer. However, monotherapy that
targets EGFR has not been as effective as expected, and the
clinical effect remains unsatisfactory. Patients with advanced
pancreatic cancer have a poor prognosis and there have been no
improvements in survival since the introduction of gemcitabine as a
therapeutic option. A randomized phase III trial showed that
improvement in patient survival using erlotinib or gemcitabine
singly was modest (6.24 vs. 5.91 months), while the 1-year survival
rate using erlotinib combined with gemcitabine was 23 vs. 17% when
combining placebo with gemcitabine, a statistically significant
difference (6). Therefore, there is
an urgent need to explore the crosstalk mechanism between EGFR and
other signaling pathways to find a more efficient combinatory
antitumor strategy.
The Hh signaling pathway contributes to pancreatic
cancer occurrence and progression, and participates in maintaining
the biological characteristics of pancreatic cancer stem cells
(15). Upregulated Hh pathway
activity has been observed in human pancreatic cancer (16,17).
Inhibition of Hh signaling by cyclopamine, a smoothened antagonist,
inhibited pancreatic cancer growth in vitro and in
vivo, suggesting that this signaling pathway has an early and
critical role in pancreatic cancer formation (18). Specifically blocking Hh signaling
can inhibit cell proliferation and invasion in vivo and
in vitro, and may become one of the best hopes for targeted
pancreatic cancer therapy. Some scholars have demonstrated a
synergy between Hh and EGFR pathways in pancreatic cancer cell
lines (19). Researchers have found
that sonic Hedgehog (SHH) and EGFR positivity were significantly
higher in 49 pancreatic cancer specimens compared with 49 matched
normal specimens, as detected by immunohistochemistry (79.6 vs.
14.3%, 73.5 vs. 16.3%, P<0.05), and SHH expression was
positively correlated to EGFR expression (r1=0.232,
P<0.05) (9). Another study
showed that there was an additive effect between anti-Hh and
anti-EGFR pathways that enhanced sensitivity to antitumor drugs in
esophageal and prostate cancer cell lines (20). Hu et al(21) found that Hh and EGFR were
overexpressed in pancreatic cancer cell lines and the antitumor
effects of Iressa, a tyrosine kinase inhibitor, were enhanced by
downregulation of EGFR gene expression while blocking Hh signaling
with cyclopamine.
In this study, we confirmed that EGFR was expressed
in pancreatic cancer cell lines, and its expression was suppressed
to some extent by cyclopamine. Our results indicate that EGFR
knockdown could efficiently inhibit cell proliferation, increase
apoptosis, and enhance cyclopamine sensitivity in human pancreatic
cancer cells. EGFR downregulation significantly lowered the
apoptotic threshold and enhanced sensitivity to cyclopamine.
Cyclopamine appeared to selectively induce apoptosis in tumor cells
without adverse effects to normal tissues in vivo(22), which suggests cyclopamine is a
promising drug for preventing pancreatic cancer progression.
Compared with singly blocking EGFR expression, the addition of
cyclopamine to EGFR knockdown further inhibited cell proliferation
and tumor growth in both cell lines and xenografts. The
combinational treatment inhibited pancreatic cancer cell
proliferation and colony formation, and effectively enhanced
cyclopamine sensitivity in vitro. These results point to a
synergistic effect between the Hh and EGFR signaling pathways in
the regulation of cell proliferation and apoptosis. However, the
mechanism between the two pathways remains unclear.
Two important downstream signaling pathways are
controlled by EGFR. The first is the RAS-RAF-MEK-MAPK pathway,
which influences gene transcription and cell proliferation. The
second is the PI3K-Akt pathway, which regulates apoptosis
resistance and survival signals (23,24).
They might also be involved in pancreatic cancer cell resistance to
EGFR-targeted therapy (25,26). In the Ras-Raf-MEK-ERK/MAPK pathway,
ERK plays a dual role as a membrane protein and as a transcription
factor. Activated MAPKs phosphorylate and regulate specific
intranuclear transcription factors (27), thus inducing cell differentiation
and proliferation that contribute to tumorigenesis and migration
(28). AKT is a protooncogene,
coding for a serine/threonine protein kinase (29), which is activated by phospholipid
binding and activation loop phosphorylation at Thr308 by PDK1
(30,31), and by phosphorylation within the
C-terminus at Ser473 (32). AKT
promotes cell survival by phosphorylating and inactivating
apoptosis-inducing targets, including Bad (33), forkhead transcription factors
(34) and caspase-9 (35). Since ERK and AKT are closely linked
to cell survival and growth, we analyzed ERK and AKT
phosphorylation using western blot analysis. The result showed that
combining RNAi-silenced EGFR with Hh signal inhibition produced a
marked decrease in both AKT and ERK phosphorylation in vivo
and in vitro. The synergistic effect of dual EGFR and Hh
signaling inhibition on proliferation and apoptosis, as presented
in this study, suggests that combined treatment is likely to be a
more efficient antitumor strategy than inhibiting either signal
alone.
In conclusion, our present study showed that the Hh
signaling pathway is involved in regulating EGFR expression, and
point to a synergistic effect of Hh and EGFR. Hh pathway inhibition
enhanced the effect of selective EGFR targeting on cell
proliferation, and increased apoptosis in human pancreatic cancer
cells in vivo and in vitro. Silencing EGFR in
combination with cyclopamine may be a potential therapeutic
strategy that significantly reduces tumor size and induces
apoptosis. The synergistic mechanism of Hh and EGFR signaling
pathways partly contributed to ERK and AKT phosphorylation.
Although results have been encouraging, additional studies are
warranted.
Acknowledgements
This research was supported by grants from the
National Natural Science Foundation of China (no. 30972897 to
Y.-M.Y). Thanks to Professor Ze-Bin Mao of the Department of
Biochemistry and Molecular Biology in Peking University Health
Science Center for his assistance and technical support.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Kornmann M, Beger HG and Link KH:
Chemosensitivity testing and test-directed chemotherapy in human
pancreatic cancer. Recent Results Cancer Res. 161:180–195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papageorgio C and Perry MC: Epidermal
growth factor receptor-targeted therapy for pancreatic cancer.
Cancer Invest. 25:647–657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yarden Y: The EGFR family and its ligands
in human cancer. signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37(Suppl 4): S3–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelley RK and Ko AH: Erlotinib in the
treatment of advanced pancreatic cancer. Biologics. 2:83–95.
2008.
|
|
6
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
7
|
Rajput A, Koterba AP, Kreisberg JI, Foster
JM, Willson JK and Brattain MG: A novel mechanism of resistance to
epidermal growth factor receptor antagonism in vivo. Cancer Res.
67:665–673. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feldmann G, Dhara S, Fendrich V, et al:
Blockade of hedgehog signaling inhibits pancreatic cancer invasion
and metastases: a new paradigm for combination therapy in solid
cancers. Cancer Res. 67:2187–2196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu WG, Wang CY, Liu T, Xiong JX and Yang
ZY: Expression of sonic hedgehog, EGFR and PCNA proteins in
pancreatic cancer and their correlations to cell proliferation. Ai
Zheng. 26:947–951. 2007.(In Chinese).
|
|
10
|
Ciardiello F and Tortora G: EGFR
antagonists in cancer treatment. N Engl J Med. 358:1160–1174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berman DM, Karhadkar SS, Hallahan AR, et
al: Medulloblastoma growth inhibition by hedgehog pathway blockade.
Science. 297:1559–1561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jorissen RN, Walker F, Pouliot N, Garrett
TP, Ward CW and Burgess AW: Epidermal growth factor receptor:
mechanisms of activation and signalling. Exp Cell Res. 284:31–53.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi F, Telesco SE, Liu Y, Radhakrishnan R
and Lemmon MA: ErbB3/HER3 intracellular domain is competent to bind
ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA.
107:7692–7697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Larsen AK, Ouaret D, El Ouadrani K and
Petitprez A: Targeting EGFR and VEGF(R) pathway cross-talk in tumor
survival and angiogenesis. Pharmacol Ther. 131:80–90. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CJ, Dosch J and Simeone DM: Pancreatic
cancer stem cells. J Clin Oncol. 26:2806–2812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berman DM, Karhadkar SS, Maitra A, et al:
Widespread requirement for Hedgehog ligand stimulation in growth of
digestive tract tumours. Nature. 425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thayer SP, di Magliano MP, Heiser PW, et
al: Hedgehog is an early and late mediator of pancreatic cancer
tumorigenesis. Nature. 425:851–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen JK, Taipale J, Cooper MK and Beachy
PA: Inhibition of Hedgehog signaling by direct binding of
cyclopamine to Smoothened. Genes Dev. 16:2743–2748. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buck E, Eyzaguirre A, Haley JD, Gibson NW,
Cagnoni P and Iwata KK: Inactivation of Akt by the epidermal growth
factor receptor inhibitor erlotinib is mediated by HER-3 in
pancreatic and colorectal tumor cell lines and contributes to
erlotinib sensitivity. Mol Cancer Ther. 5:2051–2059. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dragovich T and Campen C:
Anti-EGFR-targeted therapy for esophageal and gastric cancers: an
evolving concept. J Oncol. 2009:8041082009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu WG, Liu T, Xiong JX and Wang CY:
Blockade of sonic hedgehog signal pathway enhances
antiproliferative effect of EGFR inhibitor in pancreatic cancer
cells. Acta Pharmacol Sin. 28:1224–1230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qualtrough D, Buda A, Gaffield W, Williams
AC and Paraskeva C: Hedgehog signalling in colorectal tumour cells:
induction of apoptosis with cyclopamine treatment. Int J Cancer.
110:831–837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klapper LN, Kirschbaum MH, Sela M and
Yarden Y: Biochemical and clinical implications of the ErbB/HER
signaling network of growth factor receptors. Adv Cancer Res.
77:25–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tortora G, Bianco R, Daniele G, et al:
Overcoming resistance to molecularly targeted anticancer therapies:
rational drug combinations based on EGFR and MAPK inhibition for
solid tumours and haematologic malignancies. Drug Resist Updat.
10:81–100. 2007. View Article : Google Scholar
|
|
25
|
Faller BA and Burtness B: Treatment of
pancreatic cancer with epidermal growth factor receptor-targeted
therapy. Biologics. 3:419–428. 2009.PubMed/NCBI
|
|
26
|
Jimeno A, Rubio-Viqueira B, Amador ML, et
al: Epidermal growth factor receptor dynamics influences response
to epidermal growth factor receptor targeted agents. Cancer Res.
65:3003–3010. 2005.PubMed/NCBI
|
|
27
|
Hill CS and Treisman R: Transcriptional
regulation by extracellular signals: mechanisms and specificity.
Cell. 80:199–211. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bononi A, Agnoletto C, De Marchi E, et al:
Protein kinases and phosphatases in the control of cell fate.
Enzyme Res. 2011:3290982011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Franke TF, Yang SI, Chan TO, et al: The
protein kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scheid MP, Marignani PA and Woodgett JR:
Multiple phosphoinositide 3-kinase-dependent steps in activation of
protein kinase B. Mol Cell Biol. 22:6247–6260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chappell WH, Steelman LS, Long JM, et al:
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and
importance to inhibiting these pathways in human health.
Oncotarget. 2:135–164. 2011.PubMed/NCBI
|
|
33
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cardone MH, Roy N, Stennicke HR, et al:
Regulation of cell death protease caspase-9 by phosphorylation.
Science. 282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|