Introduction
Oral squamous cell carcinoma (OSCC) is the most
common malignant tumor of epithelial origin in the head and neck
area, and it commonly metastasizes (1). Many studies have examined the
mechanism of metastasis (2).
However, the exact pathways involved are still unclear. The
transforming growth factor-β1 (TGF-β1) (3) and matrix metalloproteinase (MMP)
families (4,5) are involved in metastasis of
gastrointestinal (GI) tract tumors. TGF-β1 is expressed in normal
epithelium of the GI tract. In this setting, it is known to inhibit
proliferation through the caspase pathway (6).
During tumor progression, however, TGF-β1 can
stimulate cancer cell growth through a different mechanism and is
involved in metastasis (6). In
particular, TGF-β1 is involved in epithelial to mesenchymal
transition (EMT) (7). When cells of
epithelial origin acquire mesenchymal features, they start to
migrate into the connective tissue by breaking down the basement
membrane. In addition, TGF-β1 is highly expressed in areas of
inflammation and it activates the growth of fibroblasts (8). In a similar manner, TGF-β1 may
increase the growth of cancer cells that have undergone EMT.
The MMP family of enzymes is involved in tissue
remodeling under both physiologic and pathologic conditions
(9). MMPs are zinc-dependent
endopeptidases that can degrade extracellular matrix (ECM)
components (10). For example,
decreased expression of MMPs is related to impaired tooth eruption
(11). Overexpression of several
MMPs has been linked to increased invasive and metastatic potential
in a variety of cancers including OSCC (2,4,5). MMP-2
has been implicated in TGF-β1-induced invasion by pancreatic cancer
cells (4).
MMP-2 is highly expressed in OSCC with lymph node
metastasis, and OSCC cells in the metastatic lymph node highly
express TGF-β1 (12). Therefore,
TGF-β1 may be an attractive drug target for invasive OSCC. However,
TGF-β1 regulates biological process of normal cells. To administer
a TGF-β1 inhibitor systemically, an appropriate drug carrier is
essential to avoid unwanted effects of the agent on normal cells.
If the agent can be administered locally, systemic toxicity may be
reduced. Antisense oligonucleotides (ODNs) are a relatively
temporary way of inhibiting a target gene compared to small
inhibitory RNAs (siRNA). If a proper delivery system can be
identified for ODN, intracellular transportation also can be
achieved efficiently. Considering that downregulation of a key gene
is sometimes sufficient to cause apoptosis in cancer cells,
repeated local administration of antisense ODN may be
therapeutically useful for challenging OSCC.
The objective of this study was to evaluate the
therapeutic effect of antisense TGF-β1 ODN in SCC-9. First, we
assessed whether the ODN caused reduced protein expression by
reverse transcriptase-polymerase chain reaction (RT-PCR) and
western blot analysis. Second, cellular growth inhibition was
examined by real time-cell electronic sensing (RT-CES) analysis.
Third, the antitumor effect of antisense TGF-β1 ODN therapy was
evaluated in a tumor xenograft model. Finally, tumor cell
expression of proliferating cellular nuclear antigen (PCNA) and
MMP-2 in the xenograft model was evaluated by
immunohistochemistry.
Materials and methods
Cell culture and RT-CES
SCC-9 cells (human tongue squamous cell carcinoma)
(American Type Culture Collection; Manassas, VA, USA) were
maintained as monolayer cultures in Dulbecco’s modified Eagle’s
medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum containing L-glutamine, vitamins (Life
Technologies, Inc., Grand Island, NY, USA) and
penicillin-streptomycin (Flow Laboratories, Inc., Rockville, MD,
USA). The cells were incubated in a mixture of 5% CO2
and 95% air at 37°C. The cultures were maintained for no longer
than 12 weeks after recovery from frozen stocks.
Label-free detection of cellular growth was
conducted by RT-CES based on impedance measurements using the
xCELLigence® system (Roche Applied Science, Penzberg,
Germany) and special 96-well gold-plated culture dishes in which
SCC-9 cells were grown. The antisense TGF-β1 ODN was designed as
5′-CTGTTGTACAGGGCGAGC-3′. The sense TGF-β1 ODN was
5′-GCTCGCCCTGTACAACAG-3′. SCC-9 cells were transfected at 80%
confluence with sense or antisense TGF-β1 ODNs using Lipofectamine
2000 (Invitrogen) according to the manufacturer’s instructions.
Briefly, we added 20 μl of the ODN mixture at a concentration 10 pM
to each well 42 h after the initial culture under serum-free
conditions. In the control wells, cells were grown in the same
medium without ODNs. The cell index values, derived from the
measured impedance, were determined every hour for 4 days.
Extraction of total-RNA, cDNA synthesis
and RT-PCR
The culture medium was removed and total-RNA was
extracted using Tri-Reagent (Molecular Research Center, Inc.,
Cincinnati, OH, USA) according to the manufacturer’s protocol. The
concentration of total-RNA was measured using a spectrophotometer
(Ultrospec 2000 UV/visible spectrophotometer; Amersham, Pharmacia
Biotech, Piscataway, NJ, USA).
The cDNA was synthesized from total-RNA using a
reverse transcriptase kit (Invitrogen). The reactions were primed
with 3 μl of oligo(dt) and 1 μl of 10 mM dNTP mixture. DEPC-treated
tertiary distilled water was added to reach a total volume of 40
μl. The reaction was performed at 65°C for 5 min and the
temperature was slowly decreased to room temperature (RT). Then, 2
μl of 10X first-strand buffer, 4 μl of 25 mM MgCl2, 2 μl
of 0.1 M DTT, 1 μl of RNased Block ribonucleotide inhibitor (40
U/μl) and 1 μl RTase were added. The total volume was 50 μl, and
the reaction was performed at 42°C for 1 h.
PCR was performed with 2.5 μl of cDNA. 20-mer
primers were selected from the human TGF-β1 coding region sequence
(forward, GCCAGAGTGGTTATCTTTTG; backward, GCTG AAGCAATAGTTGGTGT).
The primer sequences for glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were: forward, AGGACTCATGACCACAGTCCA and backward,
TGTTGCTGTAGCCAAATTCGTT. The reaction mixture consisted of primers,
10X reaction buffer, 25 mM MgCl2, 1 mM dNTP and TaqDNA
polymerase (Promega, Madison, WI, USA). The total volume was 50 μl.
The PCR conditions were: after denaturing at 95°C for 10 min, the
temperature was cycled at 95°C for 30 sec, 58°C for 30 sec, 60°C
for 30 sec. Each specimen underwent 33 cycles. The PCR products
were run on a 1.5% agarose gel and stained with ethidium bromide
solution.
Western blot analysis
Whole cells were lysed in ProteoJET™ Mammalian Cell
Lysis Reagent (Thermo Scientific) containing protease inhibitor
cocktail (Sigma-Aldrich). Proteins were separated on 10%
SDS-polyacrylamide gels and transferred to PVDF membranes. Blots
were blocked with 5% skim milk powder in Tris-buffered DMEM (20 mM
Tris-HCl, 137 mM NaCl, pH 7.6) containing 0.1% Tween-20 (TBS-T
buffer) for 1 h at RT. Western blot analyses were performed with
TGF-β1 (sc-146) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) and anti-β-actin (Sigma-Aldrich) antibodies. Primary
antibodies were added to the TBS-T buffer at a 1:1,000 dilution and
incubated for 90 min at RT prior to incubation with HRP-conjugated
secondary antibodies (1:5,000 dilution; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. Proteins were detected using
ChemiDoc™ XRS+ (Bio-Rad, Hercules, CA, USA).
Xenograft study
Seven-week-old male athymic nude mice were purchased
from Orient Bioco (Seoul, Korea). The mice were used in accordance
with the Animal Care and Use Guidelines of the College of Dentistry
at Gangneung-Wonju National University (GWNU 2011–26). SCC-9 cells
were harvested from subconfluent cultures and a viable cell
suspension was used for injections. To induce anesthesia, the mice
were injected intramuscularly with 1.5 mg/kg of zolazepam
(Zoletil®; Virbac Laboratories, Carros, France) and 3.5
mg/kg of xylazine hydrochloride (Rumpun®; Bayer Korea,
Seoul, Korea). The injection solution consisted of 5×105
cells resuspended in 50 μl of Ca2+- and
Mg2+-free Hanks’ balanced salt solution (HBSS), and this
was injected into the submandibular gland in the flank using a
27-gauge hypodermic needle with a 1-ml syringe as described
previously (13). After the
injections, mice were randomly assigned to 1 of 3 groups (sense
TGF-β1 ODN, antisense TGF-β1 ODN and DMEM). Treatment was started
after a tumor mass was definitively identified. Tumor size was
calculated using the following formula: mass volume = a (long
distance) × b (short distance)2/2 (14).
Sense and antisense TGF-β1 ODNs were synthesized by
Bioneer (Chungwon, Korea). The drug was administered daily into the
main mass. The dosages were: i) 100 μl of DMEM, ii) 100 μl of 100
pM sense TGF-β1 ODN or iii) 100 μl of 100 pM antisense TGF-β1 ODN.
The vehicle for the sense and antisense ODN was Lipofectamine 2000
(Invitrogen). Changes in mass size and body weight were observed
daily. To compare mass size and body weight, the initial values at
the start of treatment were set to 1. The relative mass size and
body weight at each observation point were calculated. The
differences in mass size and body weight between groups were
compared by independent sample t-tests. The death of a mouse was
recorded and the data were used for the survival analysis. Survival
was compared by the Kaplan-Meier method and the difference between
the groups was evaluated by the log rank test. The significance
level was set at P<0.05.
Immunohistochemical staining
The following primary antibodies for
immunohistochemical analysis were purchased: mouse monoclonal
antibody to PCNA (sc-25280) (Santa Cruz Biotechnology, Inc.) and
mouse monoclonal antibody to MMP-2 (sc-13595) (Santa Cruz
Biotechnology, Inc.). The dilution rates were 1:50 for PCNA and
1:20 for MMP-2. The largest tumor from each mouse was used for
immunohistochemical staining and 4-μm-thick sections were cut.
Universal LSAB+ kits (Dako, Glostrup, Denmark) were used for
immunohistochemistry and the subsequent procedures were performed
according to the manufacturer’s protocols. Immunostaining without
primary antibodies served as a negative control. The sections were
counterstained with Mayer hematoxylin. Two pathologists blinded to
the original group classification reviewed all of the slides. The
slides were evaluated for intensity and area of staining. The
intensity scales were (-) for invisible or trace staining in a
focal area, (+) for visible staining in a moderate area, and (++)
for dense, strong staining in an extensive area. Three different
sections per specimen were used for the analysis. The average value
of a sample was considered to be the immunohistochemical activity
of the sample. The difference between the groups was analyzed by
the independent samples t-test and significance was set at
P<0.05.
Results
Antisense TGF-β1 ODN decreases the levels
of TGF-β1 and cellular growth
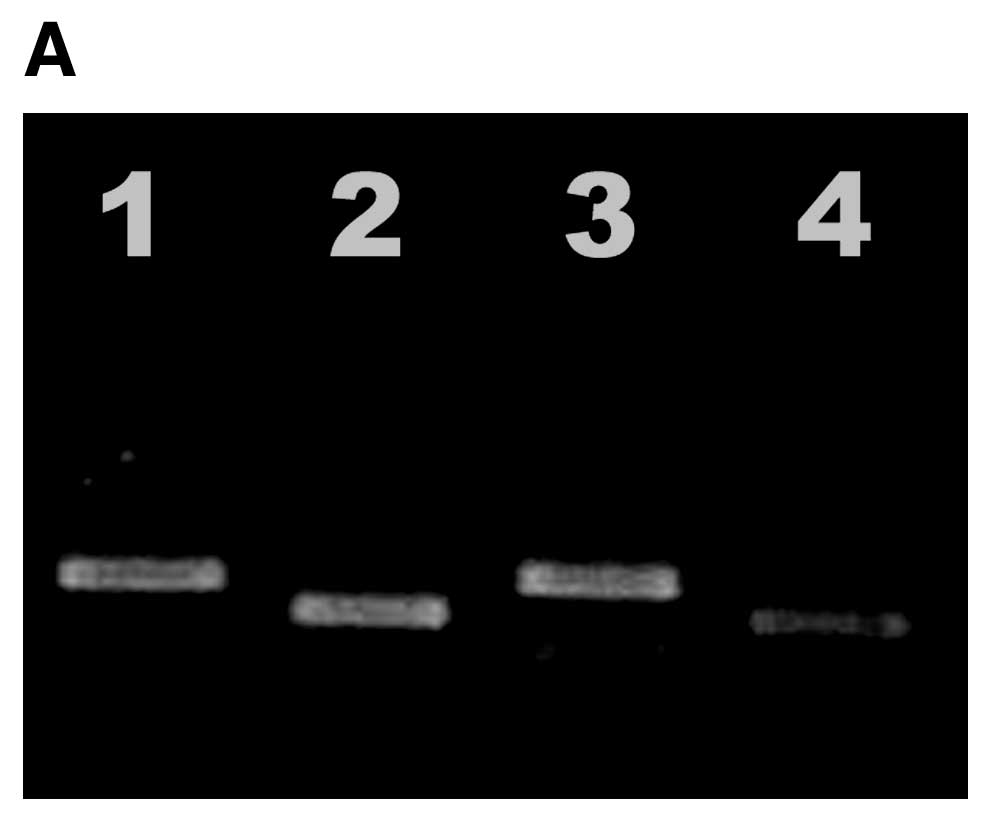
As shown in Fig. 1A,
RT-PCR showed that antisense TGF-β1 ODN decreased the level of
TGF-β1 mRNA. Western blot analysis experiments showed that
antisense TGF-β1 ODN decreased the level of TGF-β1 protein
(Fig. 1B). In addition, cellular
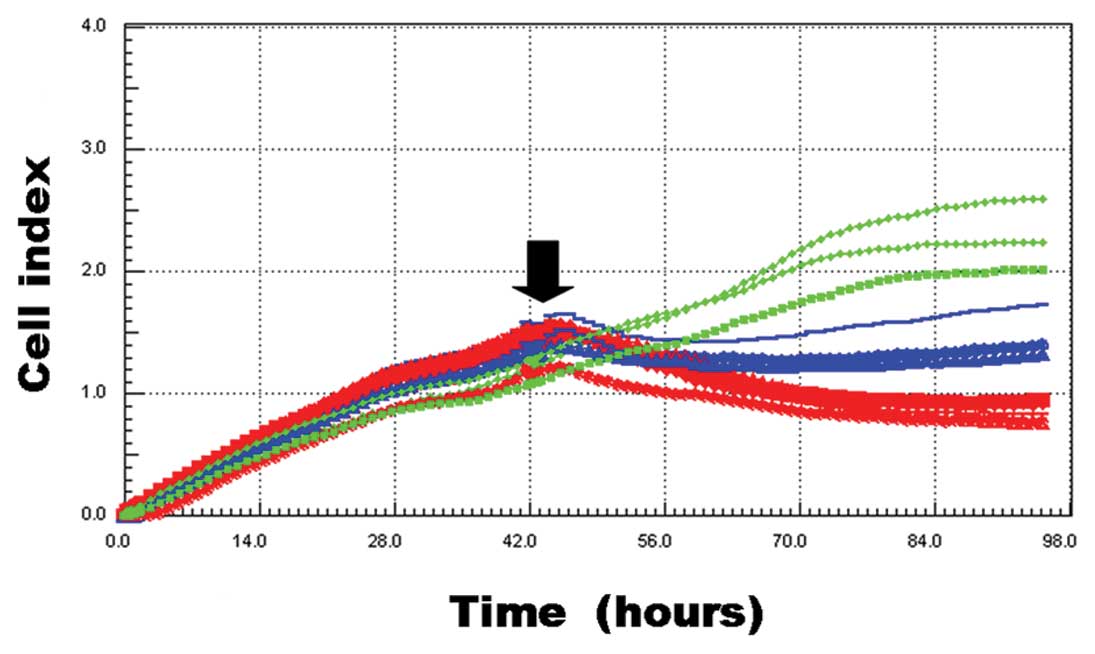
growth was significantly inhibited after antisense TGF-β1 ODN
treatment (Fig. 2). Forty-eight
hours after drug administration, the cell index of the antisense
TGF-β1 ODN group was 0.87±0.08. That of the DMEM and sense TGF-β1
ODN groups were 2.28±0.35 and 1.38±0.16, respectively. When we
compared the cell index of the antisense TGF-β1 ODN group to that
of the DMEM group, the difference was statistically significant
(P=0.003). The difference between the sense TGF-β1 ODN group and
the antisense TGF-β1 ODN group was also statistically significant
(P<0.001).
Antisense TGF-β1 ODN reduces mass
size
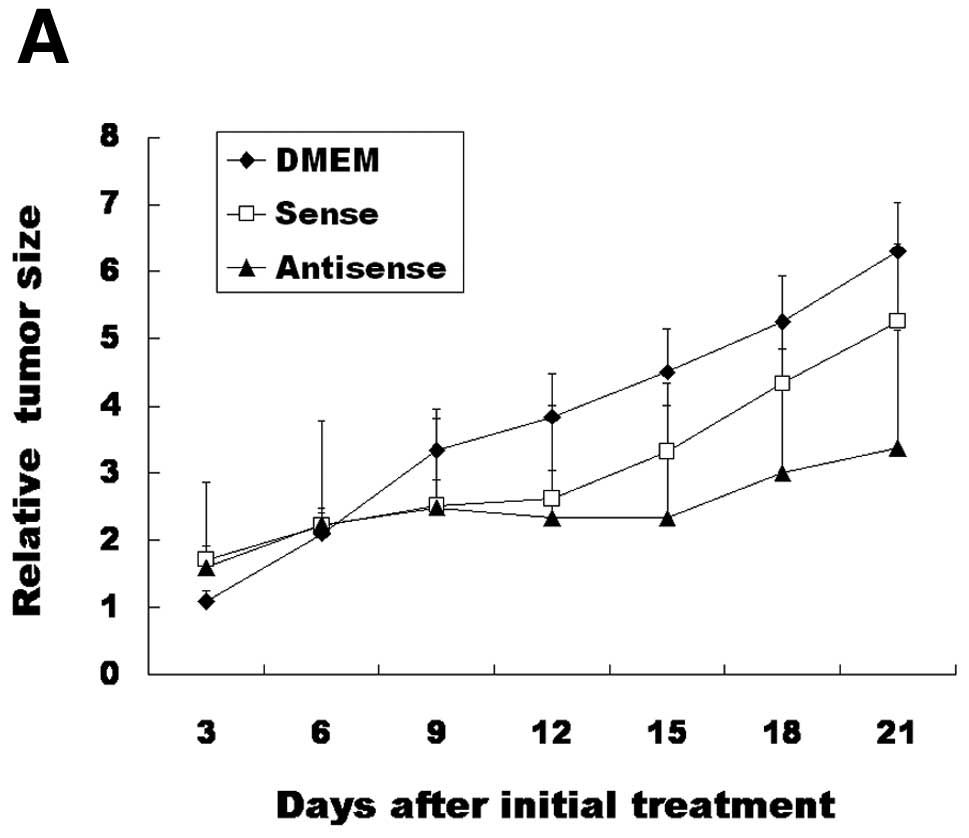
When we compared relative tumor size 21 days after
the initial treatment, the DMEM group, sense ODN group and
antisense ODN group had relative tumor mass sizes of 6.30±0.72,
5.26±1.15, and 3.37±1.76, respectively (Fig. 3A). There was a significant
difference between the DMEM group and antisense ODN group
(P=0.022). However, there were no significant differences between
the other groups (P>0.05). When we compared relative body weight
21 days after the initial treatment, the DMEM group, sense ODN
group and antisense ODN group had relative body weights of
0.99±0.13, 1.02±0.01 and 0.93±0.19, respectively (Fig. 3B). There were no significant
differences between groups (P>0.05). With respect to survival,
the mean survival time of the DMEM group was 17.0±3.0 days
(Fig. 3C). The mean survival time
of the sense ODN and antisense ODN groups were 18.0±3.0 days and
18.0±3.0 days, respectively. When survival time was compared
between groups, there were no significant differences
(P>0.05).
Antisense TGF-β1 ODN therapy decreases
PCNA and MMP-2 expression in a tumor of the xenograft model
The PCNA and MMP-2 expression patterns are presented
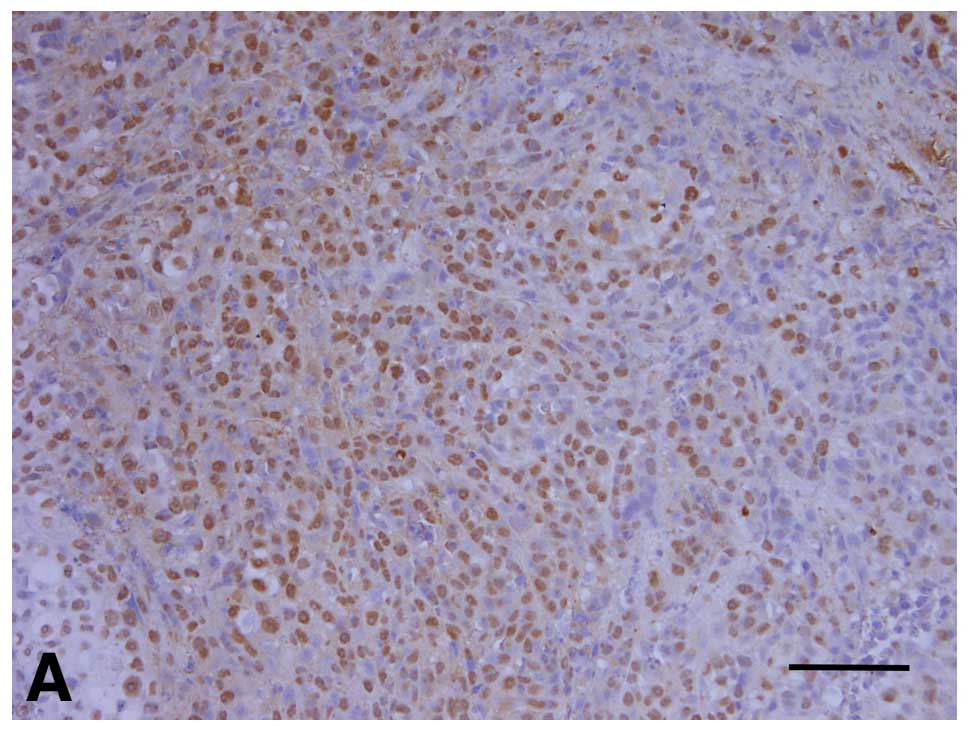
in Table I. The DMEM group and
sense ODN group had relatively high expression levels of PCNA
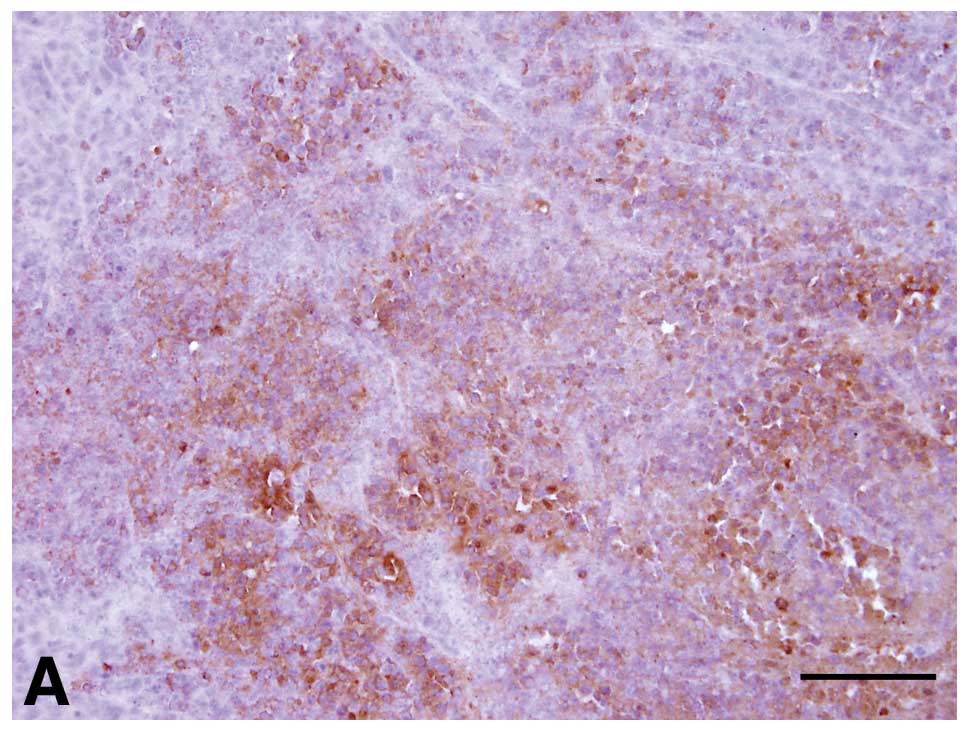
(Fig. 4A and B). In the DMEM group,
sections from all mice showed strong expression of PCNA (5/5 mice).
Two sections from animals in the sense ODN group showed visible
PCNA staining, and sections from the 3 other mice showed strong
staining. In contrast, in the antisense ODN group, the expression
of PCNA was negligible in 3/5 mice (− degree of staining) and
visible in 2/5 mice (+ degree of staining) (Fig. 4C). When the expression of PCNA in
the antisense ODN group was compared to that of the DMEM group, the
difference was statistically significant (P=0.003). However, there
were no statistically significant differences between the other
groups (P>0.05).
 | Table IImmunoreactivity of PCNA and
MMP-2. |
Table I
Immunoreactivity of PCNA and
MMP-2.
| DMEM group | TGF-β1 sense
group | TGF-β1 antisense
group |
|---|
|
|
|
|
|---|
| 0 | + | ++ | 0 | + | ++ | 0 | + | ++ |
|---|
| PCNA | 0 | 0 | 5 | 0 | 2 | 3 | 3 | 2 | 0 |
| MMP-2 | 0 | 1 | 4 | 1 | 2 | 2 | 2 | 2 | 1 |
The DMEM group showed strong expression of MMP-2 in
the sections from 4 mice (80.0%) (Fig.
5A). The sense ODN group showed slightly reduced expression of
MMP-2 compared to the DMEM group (Fig.
5B). However, in the antisense ODN group, the expression of
MMP-2 was negligible in 2/5 mice (− degree of staining) and visible
in 2/5 mice (+ degree of staining) (Fig. 5C). When the expression of MMP-2 in
the antisense ODN group was compared with that in the DMEM group,
the differences were statistically significant (P=0.046). However,
there were no statistically significant differences between the
other groups (P>0.05).
Discussion
TGF-β1 is a potent inhibitor of growth for many cell
types (15), including normal human
oral keratinocytes (16). However,
TGF-β1 expression in neoplasia increases tumorigenicity, invasion
and drug resistance (17). In this
study, TGF-β1 knockdown by antisense ODN in SCC-9 inhibited tumor
cell growth both in vitro and in vivo. In addition,
antisense TGF-β1 ODN decreased the levels of PCNA and MMP-2 in a
tumor xenograft model.
Many drugs have been designed to modify the TGF-β1
signaling pathway (18). However,
systemic administration of a TGF-β1 blocker will have undesired
effects on normal cells (19).
Thus, targeting of TGF-β1 should be specific for cancer cells.
Local drug delivery has been tried in many tumor models.
Additionally, antisense ODN is an effective tool for the
suppression of a specific gene (20). However, slow uptakes into cells and
rapid degradation in the cytoplasm have been obstacles to the
systemic use of ODN for the treatment of disease (21). If antisense ODN is delivered locally
to the tumor, unwanted suppression of TGF-β1 in normal cells may be
avoided. In addition, use of the proper vehicle will increase
transfection rates. In this study, antisense ODN packed in
liposomes caused suppression of both target gene expression and
SCC-9 growth (Figs. 1 and 2). Local administration of antisense
TGF-β1 ODN did not cause weight loss of experimental animals
compared to control animals treated with DMEM (Fig. 3B). Antisense TGF-β1 ODN also showed
therapeutic effects in a xenograft model (Fig. 3A).
When siRNA is used, gene silencing is very specific
to the target sequence (22). Like
designing the siRNA, selection of the proper target sequence is a
vital component of developing the therapeutic applications of ODN.
We confirmed the gene silencing effect of the antisense ODN using
RT-PCR and western blot analysis (Fig.
1). The antisense ODN caused significantly lower tumor cell
growth compared to the corresponding sense ODN and DMEM treatments
(Fig. 2). As siRNA and antisense
ODN capitalize on similar methods of gene silencing (20,21),
the presented antisense ODN design may also be applicable to
siRNA.
Expression levels of PCNA and MMP-2 were decreased
by antisense TGF-β1 ODN therapy (Figs.
4 and 5). MMPs are highly
expressed in cancer cells showing EMT, and EMT may facilitate
cancer progression toward a more invasive phenotype (23). TGF-β1 expression is higher in
metastatic tumors compared to primary tumors in a tumor-bearing
murine model (24). In human OSCC,
increased MMP levels correlate with increases in the TGF-β1
signaling pathway (25). TGF-β1 can
increase the expression of MMP-2 in cancer cells (4,26).
TGF-β1 frequently co-localizes with MMP-2 in OSCC, and MMP-2 is
highly expressed in OSCC with lymph node metastasis (12). Therefore, decreases in MMP-2
expression observed after antisense TGF-β1 ODN treatment in the
xenograft model might be due to lower levels of TGF-β1 caused by
the antisense TGF-β1 ODN. Though metastasis was not evaluated in
this study, tumors from animals treated with antisense TGF-β1 ODN
showed significantly less MMP-2 expression compared to those
treated with DMEM (Fig. 5).
In conclusion, local delivery of antisense TGF-β1
ODN resulted in significantly slower tumor growth and no
significant differences in weight loss compared to animals that
were similarly administered DMEM. However, antisense TGF-β1 ODN did
not prolong survival compared to DMEM. To confirm the effect of
this treatment on survival, long-term observation is required. In
addition, synergistic effects of combining antisense TGF-β1 ODN
with conventional chemotherapeutic agents warrants further
investigation.
Acknowledgements
This study was supported by a grant from the
Next-Generation BioGreen21 Program (Center for Nutraceutical &
Pharmaceutical Materials no. PJ009013), Rural Development
Administration, Republic of Korea.
References
|
1
|
Kim ES, Kies M and Herbst RS: Novel
therapeutics for head and neck cancer. Curr Opin Oncol. 14:334–342.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong SD, Hong SP, Lee JI and Lim CY:
Expression of matrix metalloproteinase-2 and -9 in oral squamous
cell carcinomas with regard to the metastatic potential. Oral
Oncol. 36:207–213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lionetti P, Pazzaglia A, Moriondo M, et
al: Differing patterns of transforming growth factor-β expression
in normal intestinal mucosa and in active celiac disease. J Pediatr
Gastroenterol Nutr. 29:308–313. 1999.
|
|
4
|
Ellenrieder V, Hendler SF, Ruhland C,
Boeck W, Adler G and Gress TM: TGF-β-induced invasiveness of
pancreatic cancer cells is mediated by matrix metalloproteinase-2
and the urokinase plasminogen activator system. Int J Cancer.
93:204–211. 2001.
|
|
5
|
Sier CFM, Kubben FJGM, Ganesh S, et al:
Tissue level of matrix metalloproteinases MMP-2 and MMP-9 are
related to the overall survival of patients with gastric carcinoma.
Br J Cancer. 74:413–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wakefield LM and Roberts AB: TGF-β
signaling: positive and negative effects on tumorigenesis. Curr
Opin Genet Dev. 12:22–29. 2002.
|
|
7
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kovasc EJ: Fibrogenic cytokines: The role
of immune mediators in the development of scar tissue. Immunol
Today. 12:17–23. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
11
|
Kim SG, Kim MH, Chae CH, Jung YK and Choi
JY: Downregulation of matrix metalloproteinases in hyperplastic
dental follicles results in abnormal tooth eruption. BMB Rep.
41:322–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JY, Rotaru H and Kim SG: The clinical
significance of the expression of TGF-β1 and MMP-2 related to the
regional lymph node metastasis in the oral squamous cell carcinoma.
J Korean Oral Maxillofac Surg. 33:199–203. 2007.
|
|
13
|
Park YW, Kim SG, Choi JY and Lee SK:
Recapitulating orthotopic tumor model through establishment of a
parotid gland tumor with lung metastasis using HeLa cell injection
into nude mice. Oncol Rep. 23:701–708. 2010.PubMed/NCBI
|
|
14
|
Carlsson G, Gullberg B and Hafström L:
Estimation of liver tumor volume using different formulas - an
experimental study in rats. J Cancer Res Clin Oncol. 105:20–23.
1983.PubMed/NCBI
|
|
15
|
Roberts AB and Sporn MB: The transforming
growth factors. Peptide Growth Factors and their Receptors I. Sporn
MB and Roberts AB: Springer; Berlin: pp. 419–472. 1990, View Article : Google Scholar
|
|
16
|
Prime SS, Matthews JB, Patel V, et al:
TGF-β receptor regulation mediates the response to exogenous ligand
but is independent of the degree of cellular differentiation in
human oral keratinocytes. Int J Cancer. 56:406–412. 1994.
|
|
17
|
Aiping H: Drug resistance and gene
amplification potential regulated by transforming growth factor β1
gene expression. Cancer Res. 55:1758–1762. 1995.PubMed/NCBI
|
|
18
|
Yingling JM, Blanchard KL and Sawyer JS:
Development of TGF-β signaling inhibitors for cancer therapy. Nat
Rev Drug Discov. 3:1011–1022. 2004.
|
|
19
|
Moore LD, Isayeva T, Siegal GP and
Ponnazhagan S: Clin Cancer Res. 14:4961–4970. 2008. View Article : Google Scholar
|
|
20
|
Woolf TM, Melton DA and Jennings CGB:
Specificity of an antisense oligonucleotides in vivo. Proc Natl
Acad Sci USA. 89:7305–7309. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thierry AR, Rahman A and Dritschilo A:
Liposomal delivery as a new approach to transport antisense
oligonucleotides. Gene Regulation: Biology of Antisense RNA and
DNA. Erickson R and Izant JF: Raven Press Ltd; New York: pp.
147–161. 1992
|
|
22
|
Cheng K, Yang N and Mahato RI: TGF-β1 gene
silencing for treating liver fibrosis. Mol Pharm. 6:772–779.
2009.
|
|
23
|
Wilkins-Port CE and Higgins PJ: Regulation
of extracellular matrix remodeling following transforming growth
factor-beta1/epidermal growth factor-stimulated
epithelial-mesenchymal transition in human premalignant
keratinocytes. Cells Tissues Organs. 185:116–122. 2007. View Article : Google Scholar
|
|
24
|
Dasgupta S, Bhattacharya-Chatterjee M,
O’Malley BW Jr and Chatterjee SK: Tumor metastasis is an orthotopic
murine model of head and neck cancer: possible role of TGF-beta 1
secreted by the tumor cells. J Cell Biochem. 97:1036–1051. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L, Diamond ME, Ottaviano AJ, Joseph
MJ, Ananthanarayan V and Munshi HG: Transforming growth factor-β1
promotes matrix metalloproteinase-9-mediated oral cancer invasion
through Snail expression. Mol Cancer Res. 6:10–20. 2008.
|
|
26
|
Munshi HG, Wu YI, Mukhopadhyay S, et al:
Differential regulation of membrane type 1-matrix metalloproteinase
activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of
metalloproteinases 2 expression controls transforming growth
factor-beta 1-induced pericellular collagenolysis. J Biol Chem.
279:39042–39050. 2004. View Article : Google Scholar
|