Introduction
Colorectal cancer (CRC) is one of the three leading
causes of cancer-related death among men and women worldwide.
Although the 5-year survival rate for patients with localized CRC
approaches 90%, the spread of disease to distant sites decreases
the 5-year survival rate to 10% (1,2). The
liver is the primary site of hematogenous metastases in colorectal
cancer. Already at the time of diagnosis of the primary tumor,
approximately 20% of patients present with synchronous liver
metastases, and another 20–30% of patients will develop liver
metastases after resection of the primary tumor (1). The cause of death for CRC patients is
the development of metastatic lesions at sites distant from the
primary tumor.
Tumor progression towards metastasis is often
depicted as a multistage process in which malignant cells spread
from the tumor of origin to colonize distant organs (3–5).
Approximately a century ago, Paget (6) postulated in his ‘seed and soil’
hypothesis that successful interactions of tumor cells (seeds) with
the microenvironment of a particular target organ (soil) leads to
formation of distant metastases in specific organs. Cancer’s
predilection for selecting organs is likely explained by this
theory that metastatic organs provide the optimal conditions for
disseminated tumor cells to colonize, survive and proliferate.
Recent data have shown that soluble attractant
molecules called chemokines support the metastasis of certain
cancers to certain organs (7). The
chemokines comprise a family of small basic chemotactic proteins
whose effects are mediated by binding to G-protein-coupled
receptors. They were originally identified by their ability to
induce migration of leukocytes. Gradients of chemokines have been
proposed to attract tumor cells with matching chemokine receptors
to specific sites analogous to the directed homing of leukocytes
(8–10). It is becoming increasingly clear
that the chemokine network plays an important role in cancer
through its effect on the growth and metastasis of tumor cells as
well as in manipulating host-tumor interactions (11).
CC chemokine ligand 7 (CCL7), also known as monocyte
chemotactic protein-3 (MCP-3), is identified from osteosarcoma
supernatant (12). It is expressed
and secreted by monocytes, fibroblasts, platelets, colonic
epithelial cells and some malignant tumor cells (13–15).
By binding to CCR1, CCR2 and CCR3, CCL7 acts on the immune cells
and activates monocytes, lymphocytes, dendritic cells, natural
killer cells, and granulocytes (16,17).
There are evidence suggesting that CCL7 plays an important role in
immune cells infiltrating in tumor cells and CCL7 gene transferred
into tumor cells elicits antitumor effect such as reducing
tumorigenicity and inhibiting tumor growth (17–20).
However, limited data are available about the expression of CCL7 in
CRC, and the role of CCL7 in liver metastasis of CRC has not been
studied.
In this study, we used RT2
ProfilerTM PCR array to identify genes that might play a
significant role in liver metastasis in CRC and then we evaluated
the expression of CCL7 and its receptors, CCR1, CCR2 and CCR3 in
CRC-induced liver metastasis.
Materials and methods
Patient selection and tissue samples
Six primary colorectal carcinomas and their matched
metastatic carcinoma from the liver were included in RT2
Profiler™ PCR array to identify possible target genes which show
differential gene expression between primary and metastatic sites.
All were synchronous cases in which primary and metastatic tumors
were found at the same time. All tumors were fresh frozen
specimens.
For validating study of CCL7, 30 patients with
primary CRC and their liver metastases were selected. Patients’
normal colon and liver tissues were selected to compare with the
cancer tissues. Of the 30 primary and liver-metastatic CRCs, 17
were synchronous cases and 13 were metachronous cases in which
metastatic tumors were found after surgery on primary tumors.
Tissue samples were obtained from the archives of the Department of
Pathology of the Samsung Medical Center. Patients were selected
from consecutively identified cases as long as their paraffin
blocks were available. The investigation was approved by the
Institutional Review Board (no. 2009-09-023).
RT2 Profiler PCR array
Surgical specimens of six primary colorectal cancer
tissues and their matched metastatic cancer to liver tissues were
obtained and frozen at −80°C until use. Selected frozen tissues
were stained with H&E to improve visualization. Necrotic tumor
tissues and intervening normal tissues were removed. Total RNAs
were extracted from frozen tissues with Nucleospin RNA kit. cDNA
was synthesized using an RT2 First Strand Synthesis kit
(Super Array Bioscience, Frederick, MD, USA) and was analyzed using
the Human tumor metastasis PCR array (Table I) and the RT2
SYBR-Green/Rox PCR Master Mix [APMM012C and PA-012-24, respectively
(Super Array Bioscience)]. Data were normalized using multiple
housekeeping genes and analyzed by comparing 2−ΔCt of
the normalized sample. PCR was performed on ABI 7300 Real-Time PCR
System (Applied Biosystems, Inc., Foster City, CA, USA).
 | Table IGenes differentially expressed between
primary and liver-metastatic CRC specimens. |
Table I
Genes differentially expressed between
primary and liver-metastatic CRC specimens.
| Symbol | Gene name | Description | Average-fold
changea | P-value |
|---|
| CCL7 | FIC/MARC | Chemokine (C-C motif)
ligand 7 | 9.26 | 0.0006 |
| FN1 |
CIG/DKFZp686F10164 | Fibronectin 1 | 4.40 | 0.0039 |
| CXCR4 |
CD184/D2S201E | Chemokine (C-X-C
motif) receptor 4 | 2.22 | 0.0421 |
| CST7 | CMAP | Cystatin F
(leukocystatin) | 1.82 | 0.0491 |
| MGAT5 | GNT-V | Mannosyl
(α-1,6-)-glycoprotein β-1,6-N-acetyl-glucosaminyltransferase | 1.77 | 0.0407 |
| IL1B |
IL-1/IL1-β | Interleukin 1, β | −6.18 | 0.0075 |
| MMP10 |
SL-2/STMY2 | Matrix
metallopeptidase 10 | −5.40 | 0.0105 |
| MMP2 |
CLG4/CLG4A | Matrix
metallopeptidase 2 | −4.07 | 0.0213 |
| MMP13 | CLG3 | Matrix
metallopeptidase 13 | −4.58 | 0.0352 |
| CTSK |
CTS02/CTSO | Cathepsin K | −2.29 | 0.0434 |
Immunohistochemical staining and
scoring
Immunohistochemical studies were carried out on
formalin-fixed, paraffin-embedded, 4 μm-thick tissue sections.
Rabbit anti-human polyclonal CCL7 antibody (GenWay Biotech, Inc.,
San Diego, CA, USA) was used at a dilution of 1:1000 for 30 min.
Tissue sections were deparaffinized three times in xylene for a
total of 15 min and subsequently rehydrated. Antigen retrieval was
carried out at 97°C, PTLink (Dako, Glostrup, Denmark) for 20 min in
citrate buffer (pH 6.0) or EDTA buffer (pH 8.0). Then,
immunostaining was performed using Bond Max autoimmunostainer
(Leica Biosystems, Melbourne, Australia). Briefly, after blocking
the endogenous peroxidase activity with 3% hydrogen peroxidase for
5 min, the primary antibody incubation was carried out for 15 min.
The antigen-antibody reaction was detected using Bond™ Polymer
Refine Detection, DS9800 (Vision BioSystems, Melbourne, Australia).
Counterstaining was performed with Mayer’s hematoxylin.
CCL7 expressions were estimated using both staining
intensity and proportion. Intensity was scored as follows: 0, no
staining; 1, weak staining; 2, moderate staining; and 3, strong
staining. Proportion was scored as follows: 0, no staining; 1,
positive area between 1–25%; 2, positive area between 26–50%; 3,
positive area between 51–75%; and 4, positive area between 76–100%.
Quantitative analyses of the CCL7 expressions were determined by
immunoreactivity score which was determined as intensity score
multiplied by proportion score. Cancer cell and stroma were graded
each apart.
Quantitative real-time RT-PCR for CCL7,
CCR1, CCR2 and CCR3 mRNA expression
Total RNA was extracted from paraffin blocks using
MasterPure™ Complete DNA and RNA Rurification kit (Epicentre
Biotechnologies) according to the manufacturer’s instructions.
Amplification of mRNA was performed and then it was transcribed
from double-stranded cDNA using SuperScript™ III Reverse
transciptase (Invitrogen).
Quantitative real-time RT PCR was performed in
triplicate in 384-well plates; each 10 μl reaction consisted of 5
μl of Power SYBR®-Green PCR Master Mix (Applied
Biosystems), 0.25 μl of 10 μM concentrated Primer, Probe sets of
CCL7 (Bioneer Oligo Synthesis Report), CCR1 (Bioneer Oligo
Synthesis Report), CCR2 (Bioneer Oligo Synthesis Report), CCR3
(Bioneer Oligo Synthesis Report) and GAPDH (Bioneer Oligo Synthesis
Report). CCL7 primers were: sense 5′-TGCT CAGCCAGTTGGGATTA-3′ and
antisense 5′-GGACAGTG GCTACTGGTGGT′3′. CCR1 primers were: sense
5′-CTGG T TGGAAACATCCTGGT-3′ and antisense 5′-GGAAGCGTG
AACAGGAAGAG-3′. CCR2 primers were: sense 5′-CCCCA
GTCACCTGCTGTTAT-3′ and antisense 5′-GCTTCTTTGG GACACTTGCT-3′. CCR3
primers were: sense 5′-GTGTTC ACTGTGGGCCTCTT-3′ and antisense
5′-GTGACGAGGA AGAGCAGGTC-3′. GAPDH primers were: sense 5′-GCACC
GTCAAGGCTGAGAA-3′ and antisense 5′-AGCATCGCCC CACTTGATT-3′. The
real-time PCR analysis was performed on an Applied Biosystems Prism
7900 Sequence Detection System (Applied Biosystems).
Statistical analysis
Ct values were normalized for the deviations against
the average of five housekeeping genes: B2M, RPRT1,
HPRT1, GAPDH and ACTB. The differential gene
expression was estimated as: ΔCt = Ct(liver metastases)
- Ct(colon primary), and fold-change =
2(−ΔCt). Quantitative analysis of the array data on the
primary colon and the metastatic liver tumors was carried out by
the Web-based PCR array data analysis software provided by the
manufacturer (http://www.sabiosciences.com).
The Volcano plot was plotted according to the values
of fold-change and P-value between primary and liver-metastatic
tumor. The Volcano plot considered genes that had ≥4-fold change
and a P-value <0.05 in the t-test was selected.
Statistical analyses were conducted to compare CCL7,
CCR1, CCR2 and CCR3 mRNA expression levels between primary CRC and
liver-metastatic carcinoma using the paired t-test or Wilcoxon
signed ranks test. P-value of <0.05 was considered statistically
significant. PASW statistical software version 17 (SPSS Inc.,
Chicago, IL, USA) was used for all statistical analyses.
Results
Identification of differentially
expressed genes between primary and liver-metastatic CRC using
RT2 Profiler PCR array
The mean expressions of CCL7, fibronectin, CXCR4,
CST7 and MGAT5 were significantly higher in liver-metastatic tumor,
whereas those of IL1B, MMP10, MMP2, MMP13 and CTSK were lower
compared with the primary tumor (Table
I). When metastatic tumor was compared with primary tumor,
Volcano plot analysis generated a gene list of 6 genes, of which 2
genes were upregulated and 4 genes were downregulated (Fig. 1). There was a remarkable
upregulation of CCL7 in liver-metastatic tumor tissues (average
fold-change, 9.26). Therefore, CCL7 was selected for validation of
the following studies.
Expression of CCL7
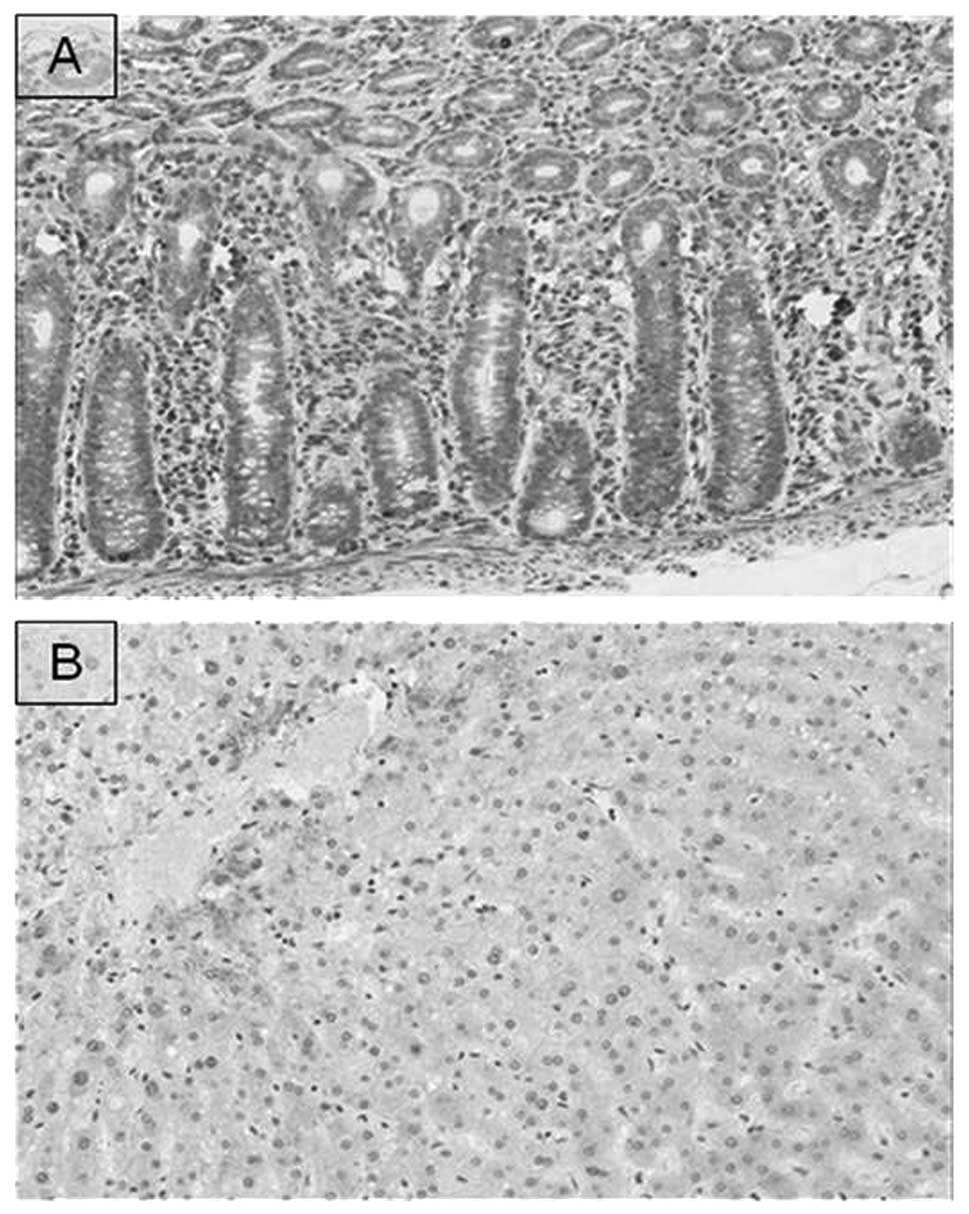
We observed the CCL7 expression in normal colon and
liver tissues (Fig. 2).
Immunohistochemical staining revealed that CCL7 was expressed in
the cytoplasm of normal colonic epithelial cells, fibroblasts and
inflammatory cells, but CCL7 was not expressed in the normal
hepatocytes.
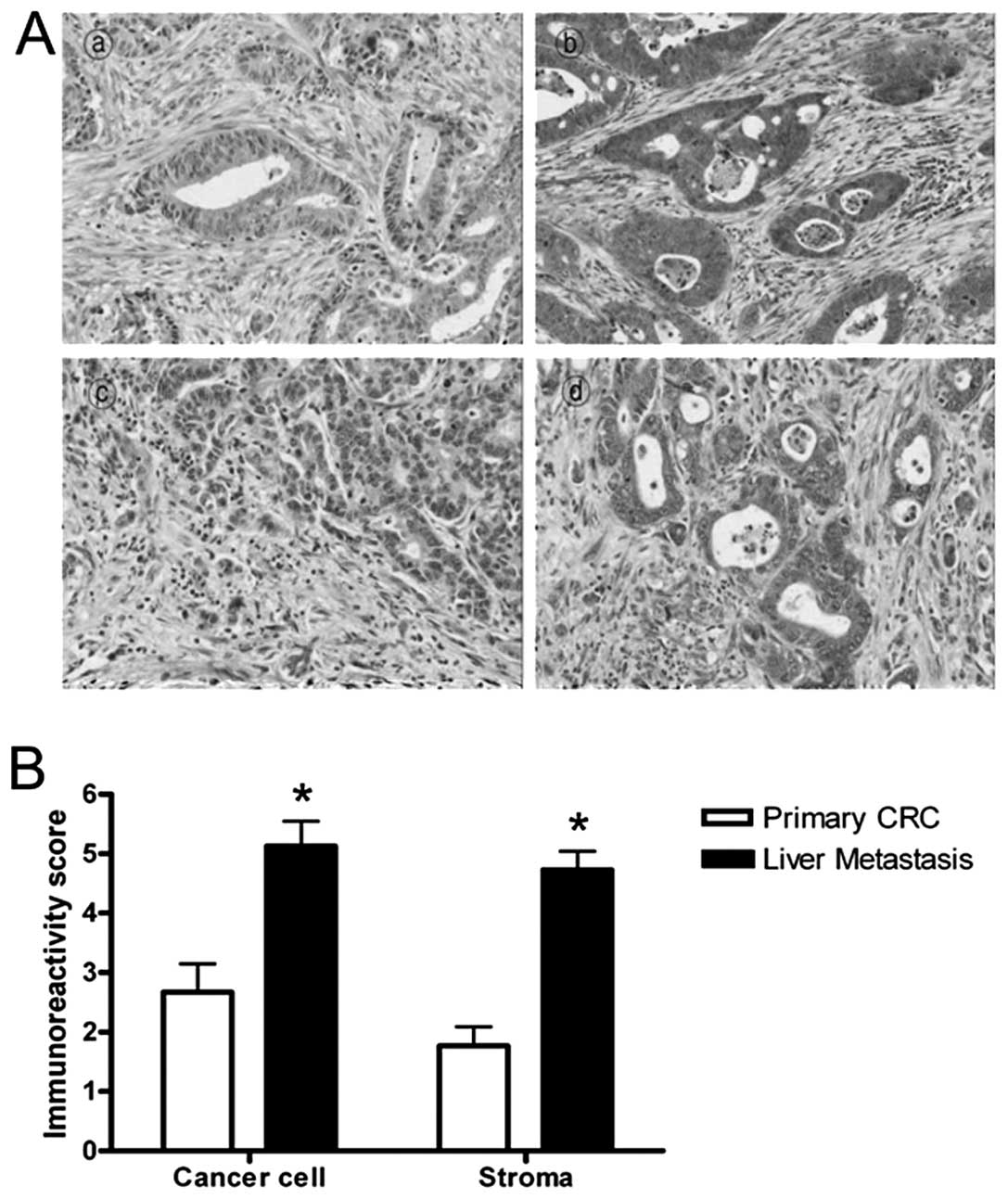
We compared the CCL7 expression in primary CRC with
its matched liver-metastatic cancer. The immunohistochemical
staining revealed that CCL7 is expressed in both primary and
liver-metastatic cancer tissues (Fig.
3A). The CCL7 positive staining was located mostly in
epithelial and fibromuscular stromal cells. Quantitative analysis
using the immunoreactivity score showed that levels of CCL7
expression of liver-metastatic tissues were greater than those of
primary CRC tissues in both cancer cell and stroma (Fig. 3B, P<0.001). These results
demonstrated that CCL7 protein was more highly expressed in most
liver-metastatic tissues compared to primary CRC tissues suggesting
that CCL7 may play a critical role in liver metastasis of CRC.
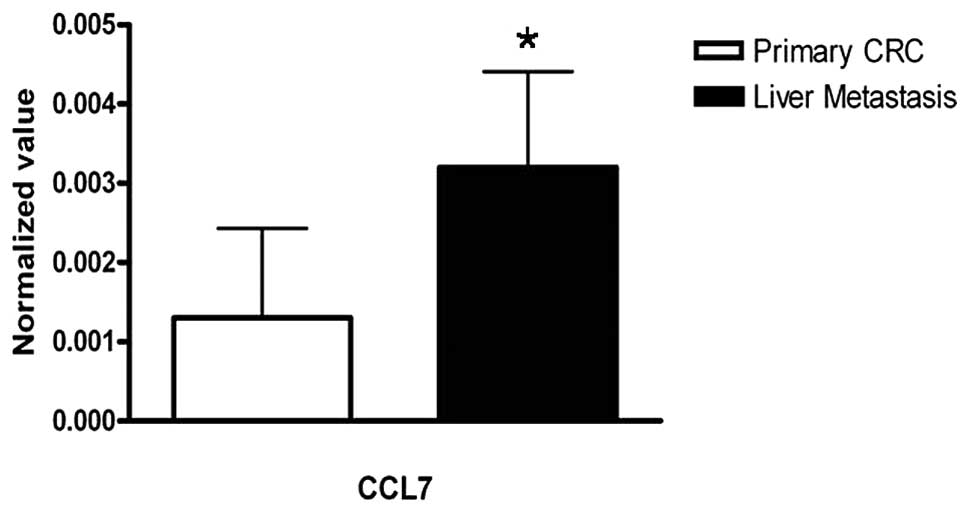
Quantitative real-time RT-PCR was used to verify the
expression of CCL7 mRNA in primary CRC tissues and liver-metastatic
tissues. CCL7 mRNA expression was significantly higher in
liver-metastatic tissues than in primary CRC tissues (Fig. 4, P<0.001).
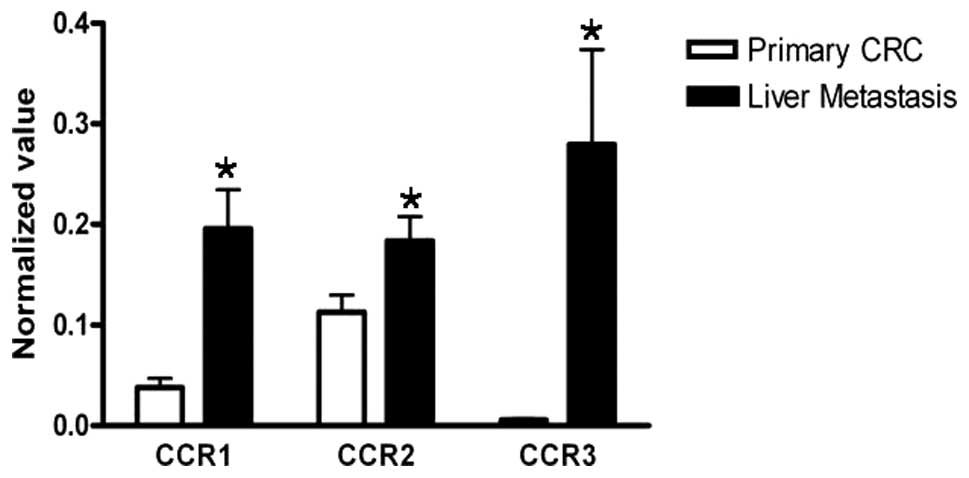
Expression of CCR1, CCR2 and CCR3
Because CCL7 has been known to act through specific
receptors, CCR1, CCR2 and CCR3, we investigated the expression of
CCR1, CCR2 and CCR3. We analyzed the mRNA expressions of
CCR1, CCR2, and CCR3 in the primary CRC
tissues and their corresponding liver-metastatic tissue samples by
using real-time RT-PCR. The results revealed that all the
expressions of CCR1, CCR2 and CCR3 in
liver-metastatic tissues were significantly higher than those in
the corresponding primary CRC tissues (Fig. 5, P=0.001, P=0.033, and P<0.001,
respectively).
Discussion
In this study, we performed RT2 Profiler
PCR array to identify biomarkers expressed differentially between
primary CRC and liver metastasis and then CCL7 was selected
as a possible biomarker related to the liver metastasis of CRC. In
order to evaluate the possible relation of CCL7 with liver
metastasis of CRC, we analyzed expression levels of CCL7 and its
receptors CCR1, CCR2, and CCR3 in 30 primary CRC specimens with
their corresponding liver metastasis tissues. The results from
real-time RT-PCR analysis showed that mRNA expressions of both
CCL7 and its receptors CCR1, CCR2 and
CCR3 were higher in liver metastasis tissues than those in
their corresponding primary CRC tissues. By immunohistochemical
staining, we found that CCL7 was expressed in the normal colonic
epithelium, colon cancer cells, and liver-metastatic cells, but not
in normal hepatocytes.
A mechanism most recently ascribed to organ-specific
cancer metastasis is the paradigm of chemokine-mediated cell
migration. Since chemokine signaling results in the directional
migration and specific arrival of cells at a target destination,
chemokines have emerged as crucial molecules in the metastatic
process. From the initial studies by Muller et al(7), numerous articles have been published
regarding chemokine/chemokine receptor interactions with
metastasis. Various studies implicate chemokines CXCL12, CCL20,
CCL19, and CCL21 and their corresponding receptors in the
tumorigenic process and metastatic homing of tumor cells. An
association between the CCL20 and its receptor CCR6 expression in
the colorectal cancer progression and liver metastasis is
previously reported, suggesting involvement of CCL20/CCR6 chemokine
receptor pair in the promotion of colorectal cancer liver
metastasis (21–23). In recent years the CC-chemokines
CCL2 and CCL7 attracted considerable interest as inflammatory
mediators that play pleiotropic tumorigenic roles in breast cancer
homing to bone and metastases growth through osteoblast induction
(24). A recent study demonstrated
that CCL2 expressed by both metastatic breast cancer tumors and
stroma plays a critical role in tumor cell extravasation and
metastatic seeding that is mediated via inflammatory monocyte
recruitment (25). Morrison et
al(26) showed that cleavage of
CCL2 and CCL7 by MMP-13 generates forms of the chemokines that are
potent receptor antagonists in a breast cancer bone metastasis
model. Jung et al(27)
reported that CCL7 promoted the invasion and migration of oral
squamous cell carcinoma cells (OSCC), and the invasiveness was
inhibited by treatment with CCL7 neutralizing antibody. They also
demonstrated that inhibiting CCR1 and CCR3 reduces CCL7-induced
OSCC cell migration, suggesting that CCL7 promotes cancer cell
migration through those receptors in OSCC cells. These data
prompted us to comparatively investigate CCL7 and its receptors
expression profiles in liver metastases in colorectal cancer. In
this study, we observed significantly higher CCL7 expression in the
liver metastases tissues compared with their corresponding primary
CRC tissues. Furthermore, CCL7 receptors, CCR1, CCR2 and CCR3, were
also overexpressed in the liver metastases suggest that the
malignant status of a colorectal cancer cell might be correlated
with CCL7 and its receptors expression.
Since the identification of chemokines as key
targets in cancer metastasis has emerged as research topic, the
investigation of the CCL7 as novel target in liver metastasis of
CRC may be of potential clinical value for the prevention of
hepatic recurrences. Therefore, further studies to investigate the
functions of CCL7 in liver metastasis of CRC and the mechanism of
interaction between CCL7 and its receptors CCR1, CCR2, and CCR3
should be conducted.
Acknowledgements
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(no. 2010-0025652), a grant of the Korea Healthcare technology
R&D Project, Ministry for Health and Welfare Affairs, Republic
of Korea (A092255), Samsung Biomedical Research Institute grant
(#SBRI C-B0-318-1) and grants from IN-SUNG Foundation for Medical
Research (C-A9-852-1).
References
|
1
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar
|
|
2
|
Kim J, Takeuchi H, Lam ST, Turner RR, Wang
HJ, Kuo C, Foshag L, Bilchik AJ and Hoon DS: Chemokine receptor
CXCR4 expression in colorectal cancer patients increases the risk
for recurrence and for poor survival. J Clin Oncol. 23:2744–2753.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta GP and Massague J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
7
|
Muller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL,
et al: Involvement of chemokine receptors in breast cancer
metastasis. Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zlotnik A: Chemokines and cancer. Int J
Cancer. 119:2026–2029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JH, Cho YS, Lee JY, Kook MC, Park JW,
Nam BH and Bae JM: The chemokine receptor CCR4 is expressed and
associated with a poor prognosis in patients with gastric cancer.
Ann Surg. 249:933–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar
|
|
12
|
Van Damme J, Proost P, Lenaerts JP and
Opdenakker G: Structural and functional identification of two
human, tumor-derived monocyte chemotactic proteins (MCP-2 and
MCP-3) belonging to the chemokine family. J Exp Med. 176:59–65.
1992.PubMed/NCBI
|
|
13
|
Uguccioni M, D’Apuzzo M, Loetscher M,
Dewald B and Baggiolini M: Actions of the chemotactic cytokines
MCP-1, MCP-2, MCP-3, RANTES, MIP-1 alpha and MIP-1 beta on human
monocytes. Eur J Immunol. 25:64–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menten P, Proost P, Struyf S, Van Coillie
E, Put W, Lenaerts JP, Conings R, Jaspar JM, De Groote D, Billiau
A, et al: Differential induction of monocyte chemotactic protein-3
in mononuclear leukocytes and fibroblasts by interferon-alpha/beta
and interferon-gamma reveals MCP-3 heterogeneity. Eur J Immunol.
29:678–685. 1999. View Article : Google Scholar
|
|
15
|
Power CA, Clemetson JM, Clemetson KJ and
Wells TN: Chemokine and chemokine receptor mRNA expression in human
platelets. Cytokine. 7:479–482. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu LL, McVicar DW, Ben-Baruch A, Kuhns DB,
Johnston J, Oppenheim JJ and Wang JM: Monocyte chemotactic
protein-3 (MCP3) interacts with multiple leukocyte receptors:
binding and signaling of MCP3 through shared as well as unique
receptors on monocytes and neutrophils. Eur J Immunol.
25:2612–2617. 1995. View Article : Google Scholar
|
|
17
|
Hu JY, Li GC, Wang WM, Zhu JG, Li YF, Zhou
GH and Sun QB: Transfection of colorectal cancer cells with
chemokine MCP-3 (monocyte chemotactic protein-3) gene retards tumor
growth and inhibits tumor metastasis. World J Gastroenterol.
8:1067–1072. 2002.PubMed/NCBI
|
|
18
|
Wang JM, Deng X, Gong W and Su S:
Chemokines and their role in tumor growth and metastasis. J Immunol
Methods. 220:1–17. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujita M, Furukawa Y, Nagasawa Y, Ogawa M
and Nakamura Y: Down-regulation of monocyte chemotactic protein-3
by activated beta-catenin. Cancer Res. 60:6683–6687.
2000.PubMed/NCBI
|
|
20
|
Fioretti F, Fradelizi D, Stoppacciaro A,
Ramponi S, Ruco L, Minty A, Sozzani S, Garlanda C, Vecchi A and
Mantovani A: Reduced tumorigenicity and augmented leukocyte
infiltration after monocyte chemotactic protein-3 (MCP-3) gene
transfer: perivascular accumulation of dendritic cells in
peritumoral tissue and neutrophil recruitment within the tumor. J
Immunol. 161:342–346. 1998.
|
|
21
|
Rubie C, Oliveira V, Kempf K, Wagner M,
Tilton B, Rau B, Kruse B, Konig J and Schilling M: Involvement of
chemokine receptor CCR6 in colorectal cancer metastasis. Tumour
Biol. 27:166–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghadjar P, Coupland SE, Na IK, Noutsias M,
Letsch A, Stroux A, Bauer S, Buhr HJ, Thiel E, Scheibenbogen C and
Keilholz U: Chemokine receptor CCR6 expression level and liver
metastases in colorectal cancer. J Clin Oncol. 24:1910–1916. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rubie C, Frick VO, Wagner M, Weber C,
Kruse B, Kempf K, Konig J, Rau B and Schilling M: Chemokine
expression in hepatocellular carcinoma versus colorectal liver
metastases. World J Gastroenterol. 12:6627–6633. 2006.PubMed/NCBI
|
|
24
|
Bar-Shavit Z: The osteoclast: a
multinucleated, hematopoietic-origin, bone-resorbing osteoimmune
cell. J Cell Biochem. 102:1130–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morrison C, Mancini S, Cipollone J,
Kappelhoff R, Roskelley C and Overall C: Microarray and proteomic
analysis of breast cancer cell and osteoblast co-cultures: role of
osteoblast matrix metalloproteinase (MMP)-13 in bone metastasis. J
Biol Chem. 286:34271–34285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung DW, Che ZM and Kim J, Kim K, Kim KY,
Williams D and Kim J: Tumor-stromal crosstalk in invasion of oral
squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer.
127:332–344. 2010.PubMed/NCBI
|