Introduction
Lung cancer is the leading cause of
malignancy-related mortality worldwide (1). Metastatic dissemination is amongst the
major determinants of adverse prognosis, affecting ≤50% of the
patients presenting with small cell (SCLC) and non-small cell
(NSCLC) lung carcinomas (2).
Despite the advancements achieved in the systemic
treatment of lung cancer, the 5-year survival probability in
patients with stage IV disease remains <5% (3). Metastatic spread to vital organs such
as the brain or the liver confers significant morbidity in patients
with advanced lung cancer, ultimately leading to a worsening of
clinical symptoms, organ failure and death (4).
It has long been recognized that the process of
metastatic diffusion to distant organs does not happen at random,
but rather reflects a complex interplay between the neoplastic cell
clone and the target tissue (5).
The invasion of the bloodstream by cancer cells is not sufficient
to produce a secondary growth, that rather depends on their ability
to survive in the bloodstream, arrest and extravasate into the
target organ and secure independent growth capacity (6).
Among the factors that make the metastatic diffusion
of cancer cells a highly selective process, an increasingly
relevant role has been recognized for the chemokine system, a
complex network of >50 different soluble glycoproteins and 20
G-protein coupled trans-membrane receptors that has been involved
in the promotion of neoangiogenesis (7) as well as in the acquisition of
invasive properties by tumour cells (8).
It has been shown that the constitutive production
of different subtypes of chemokines may represent the molecular
correlate which influences the preferential spread of tumour cells
in specific anatomical sites of the body. It has been suggested
that the chemokine secretion signature of a given tissue combined
with the selective expression of the matched receptors by tumour
cell clones might explain the site-specific spread of metastatic
foci observed in solid tumours (9).
This concept is scientifically valid in lung cancer
where the interaction between chemokine receptor CXCR4 and its
ligand CXCL12 has been discovered to promote the metastatic
potential in NSCLC both in vitro and in vivo(10), whereas the overexpression of CCR7 in
primary lung cancer specimens is a predictor of a significant
lymphotropic behavior (11).
Recently, an increasing scientific interest has been
devoted to the study of the chemokine receptor CX3CR1, a seven
spanning transmembrane protein that uniquely interacts with CX3CL1
or Fractalkine (FKN). It has been shown that tumour cell clones
expressing CX3CR1 are induced to adhere to neuronal cells through
the activation of β-integrins and focal adhesion kinase in
pancreatic cancer (12). Likewise,
overexpression of CX3CR1 in the primary tumour confers a 10-fold
increased risk for the development of brain metastases in
node-positive breast cancer patients (13).
As the role of CX3CR1 in the metastatic spread of
bronchogenic carcinoma is poorly defined, we designed this study in
order to evaluate whether the expression of CX3CR1 in primary lung
cancers specimens predicted the pattern of metastatic spread. In
addition we evaluated the expression of CX3CR1 in metastatic lung
cancer deposits. For this purpose, we adopted a tissue microarray
(TMA) analysis approach taking advantage of an isogeneic collection
matched primary and metastatic archival lung cancers obtained from
post-mortem (PM) examinations of previously untreated lung cancer
patients.
Materials and methods
Patients
The reports of 12,580 PM examinations performed at
the Hammersmith Hospital between January 1970 and December 2005
were reviewed (Ethics Reference: 06/Q0406/154). Lung cancer was
identified in 499 cases of whom 213 patients had not received any
antemortem therapy for their cancer and had also undergone a
complete post-mortem examination. Clinicopathological variables
such as gender, age at death, tumour staging together with the
number and distribution of metastatic sites were recorded. The
absence of any chemotherapy or radiotherapy treatment was confirmed
by a review of PM reports and medical notes. Tumour staging
followed the TNM criteria 7th edition (14). Hematoxylin and eosin (H&E)
slides from these cases were reviewed by a board certified
pathologist with expertise in pulmonary pathology (FAM) in order to
confirm histotype classification as well as metastatic
distribution. The quality of the primary lung and metastatic PM
tissue was preliminary evaluated on newly cut H&E sections. In
order to evaluate the suitability of the tissue for
immunohistochemical studies, well-preserved specimens were
immunostained for pan-cytokeratin MNF116 (Dako, Cambridge, UK) at
1:200 concentration after 0.1% trypsin in phosphate buffered saline
incubation for 10 min. PM specimens showing dubious or
unsatisfactory MNF116 staining were discarded. Based on these
quality criteria, we included a total number of 98 cases of primary
lung cancer with 134 matched metastatic deposits.
Tissue microarray and
immunohistochemistry
A syngeneic primary tumour/metastasis TMA was
prepared as previously described (15). Three 1-mm cores were obtained from
the most representative areas of the primary tumours and matched
secondary lesions and re-embedded in microarray blocks. Antigen
retrieval was carried out using standard procedures: briefly, the
sections were de-paraffinized in xylene, rehydrated in graded
alcohols and heated in a microwave oven at 900 W for 20 min in
citrate buffer at pH 6.0 (16).
Before immunostaining, slides were cooled at room temperature and
endogenous peroxidase activity was suppressed by incubation with a
3% solution of H2O2 for 5 min. The primary
antibody anti-CX3CR1 (Abcam ab8021, Cambridge, UK) was incubated
overnight at the concentration 1:350.
The TMA sections were incubated with the secondary
antibody for 1 hour at room temperature and then processed using
the Polymer-HRP kit (BioGenex, San Ramon, CA, USA) with development
in diaminobenzidine and Mayer’s hematoxylin counterstaining. A
pancreatic ductal adenocarcinoma tissue sample was used as external
positive control during each reaction to confirm its specificity.
Omission of the primary antibody and pre-absorption of the primary
with the immunizing peptide were used as negative control
reactions. This resulted in absence of staining in all cases.
CX3CR1 expression was scored as positive if tumour
cells showed clear cytoplasmic immunostain as described before
(17). Positivity in a single TMA
core was considered enough to classify the case as positive. Two
observers (F.A.M. and R.J.S.) blinded to the clinical data scored
all the cases and results were found to be consistent.
Statistical analysis
Pearson’s χ2 or Fisher’s exact tests were
used to elucidate any significant associations between categorical
variables as appropriate. Associations were considered
statistically significant at p-value of p<0.05. Analysis was
performed using SPSS software version 11.5 (SPSS Inc., Chicago, IL,
USA) and GraphPad PRISM (GraphPad software Inc., La Jolla, CA,
USA).
Results
Patients and tumour characteristics
The clinicopathological features reconstructed from
the 98 PM included in the study are summarized in Table I. Briefly, the majority of patients
were diagnosed with NSCLC (66%). At the time of death, 76% of the
subjects had evidence of distant metastases. SCLC cases displayed a
more advanced spread to locoregional lymph nodes (26/29 cases, 90%
versus 41/65, 63%; p<0.001) and to the liver (21/29, 72% versus
31/65 p=0.02) when compared to NSCLC.
 | Table IClinicopathological features of the
patients included in the study. |
Table I
Clinicopathological features of the
patients included in the study.
| Patient
characteristic | N=98 |
|---|
| Age at death, median
(range) | 70 (43–93) |
| Gender, M/F | 73/25 |
| Histopathological
classification |
| NSCLC | 65 (66) |
| Squamous cell
carcinoma | 30 (31) |
| Adenocarcinoma | 32 (33) |
| Mixed histology | 3 (2) |
| SCLC | 29 (30) |
| Large cell
neuroendocrine carcinomas | 4 (4) |
| TNM stage |
| II–III | 21 (21) |
| IVa | 3 (3) |
| IVb | 74 (76) |
| SCLC stage |
| Limited
disease | 4 (14) |
| Extensive
disease | 25 (86) |
The expression of CX3CR1 in primary lung
cancer and matched metastatic deposits
The frequency of CX3CR1 expression in the primary
tumours was significantly higher in NSCLC (41/65, 63%) compared to
SCLC (2/29, 7% p<0.001). Histological subclassification revealed
large cell neuroendocrine carcinoma (4/4, 100%) and squamous cell
carcinomas (27/30, 93%) as being mostly CX3CR1 expressors when
compared to adenocarcinomas (16/32, 50%, p<0.001). The
relationship between CX3CR1 immunopositivity in the primary tumour
and the clinicopathological characteristics of our patient cohort
is reported in Table II. The
overall proportion of CX3CR1 immunopositive metastatic deposits was
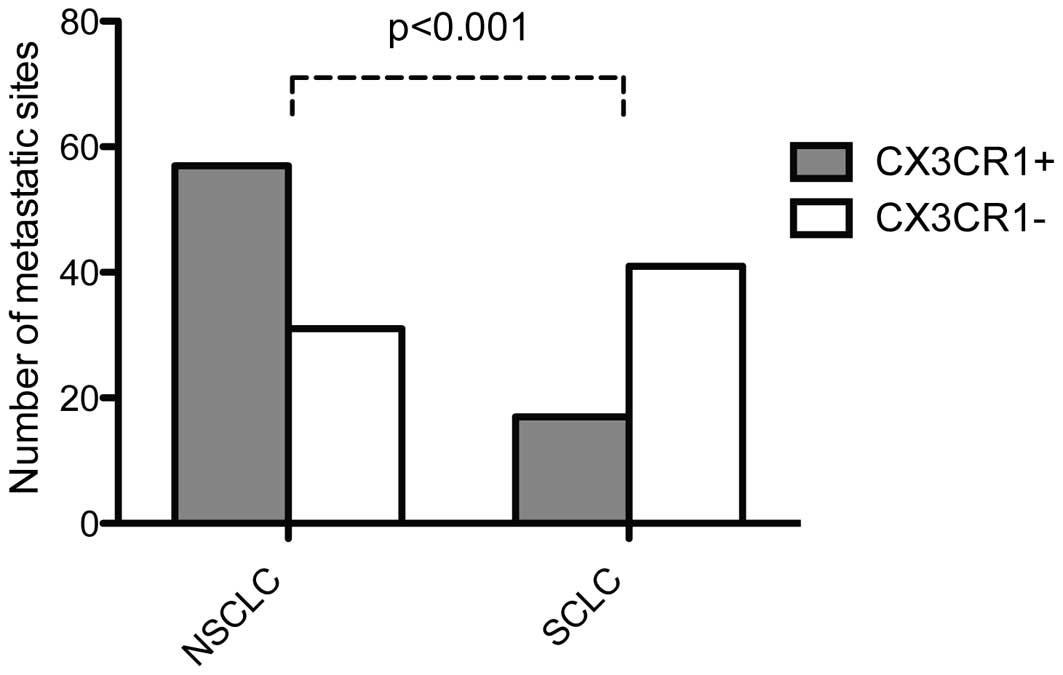
higher in NSCLC (57/88, 65%) compared to SCLC (17/58, 29%,
p<0.001) as shown in Fig. 1.
 | Table IIThe relationship between CX3CR1
expression in the primary tumour and clinicopathological features
at post-mortem examination. |
Table II
The relationship between CX3CR1
expression in the primary tumour and clinicopathological features
at post-mortem examination.
|
CX3CR1-positive |
CX3CR1-negative | P-value |
|---|
| Gender, M/F | 38/9 | 35/16 | NS |
| Age |
| <65/≥65 | 18/33 | 10/37 | NS |
| Stage |
| <IVb/IVb | 16/31 | 8/43 | 0.03a |
| Metastatic
spread |
| <6/≥6
sites | 42/5 | 37/14 | 0.04a |
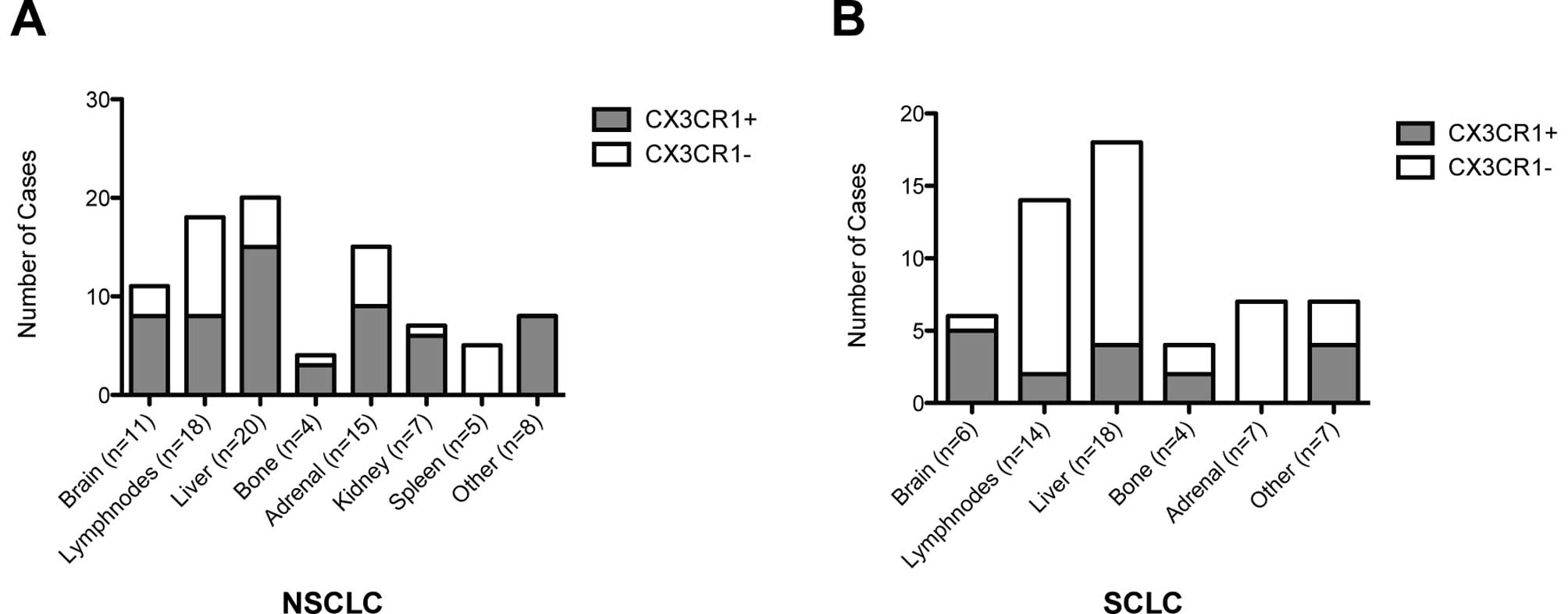
The distribution of CX3CR1 expression across the
sampled metastatic sites is reported in Fig. 2. In NSCLC, atypical sites of
metastatic spread including thyroid, heart, small bowel and skin
were all positive to CX3CR1 (8/8, 100%), followed by >75% of the
kidney, liver, bone and brain deposits. All the sampled splenic
metastases from NSCLC primaries were negative for CX3CR1 (5/5). In
SCLC cases, 83% (5/6) of the CNS metastatic sites were positive to
CX3CR1 whereas only a minority of the other sites including
locoregional lymph nodes (2/14, 14%) and liver (4/14, 28%)
expressed this chemokine receptor. Adrenal gland metastases arising
from SCLC were all negative for CX3CR1 (7/7).
The relationship between the expression of CX3CR1 in
the primary tumour and the distribution of metastatic deposits is
reported in Table III. Primary
tumours lacking the expression of CX3CR1 were more likely to have
spread to the brain (p=0.03) and liver (p=0.04) and histological
subanalysis demonstrated this distribution to be typical of lung
adenocarcinoma (p=0.01), but not of other histotypes. CX3CR1 status
in the primary tumour correlated with N3 lymph node spread (p=0.04)
and a significant association between CX3CR1 positivity within the
primary tumour and in the secondary lymphatic spread was noted
(p=0.03).
 | Table IIIThe relationship between CX3CR1
expression across the different histotypes of primary lung cancer
and the site-specific metastatic spread. |
Table III
The relationship between CX3CR1
expression across the different histotypes of primary lung cancer
and the site-specific metastatic spread.
| ADK | | SCC | | SCLC | | Overall | |
|---|
|
| |
| |
| |
| |
|---|
| CX3CR1 | | CX3CR1 | | CX3CR1 | | CX3CR1 | |
|---|
|
| |
| |
| |
| |
|---|
| + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Brain |
| + | 1 | 9 | 0.01a | 1 | 2 | NS | 1 | 5 | NS | 6 | 16 | 0.03a |
| − | 14 | 8 | | 22 | 5 | | 1 | 22 | | 41 | 35 | |
| Liver |
| + | 4 | 13 | 0.01a | 5 | 2 | NS | 2 | 19 | NS | 21 | 34 | 0.04a |
| − | 11 | 4 | | 13 | 10 | | 0 | 8 | | 26 | 17 | |
| Lymph nodes N3 |
| + | 6 | 9 | NS | 8 | 1 | NS | 2 | 21 | NS | 18 | 31 | 0.04a |
| − | 9 | 8 | | 15 | 6 | | 0 | 6 | | 29 | 20 | |
| Adrenal |
| + | 7 | 11 | NS | 3 | 2 | NS | 2 | 11 | NS | 17 | 24 | NS |
| − | 6 | 8 | | 20 | 5 | | 0 | 16 | | 30 | 27 | |
| Kidney |
| + | 1 | 6 | NS | 3 | 1 | NS | 1 | 3 | NS | 6 | 10 | NS |
| − | 14 | 11 | | 20 | 6 | | 1 | 24 | | 41 | 41 | |
| Spleen |
| + | 2 | 2 | NS | 3 | 0 | NS | 0 | 2 | NS | 5 | 4 | NS |
| − | 13 | 15 | | 20 | 7 | | 2 | 25 | | 42 | 47 | |
| Other |
| + | 0 | 1 | NS | 2 | 0 | NS | 0 | 1 | NS | 3 | 2 | NS |
| − | 15 | 16 | | 7 | 21 | | 2 | 26 | | 44 | 49 | |
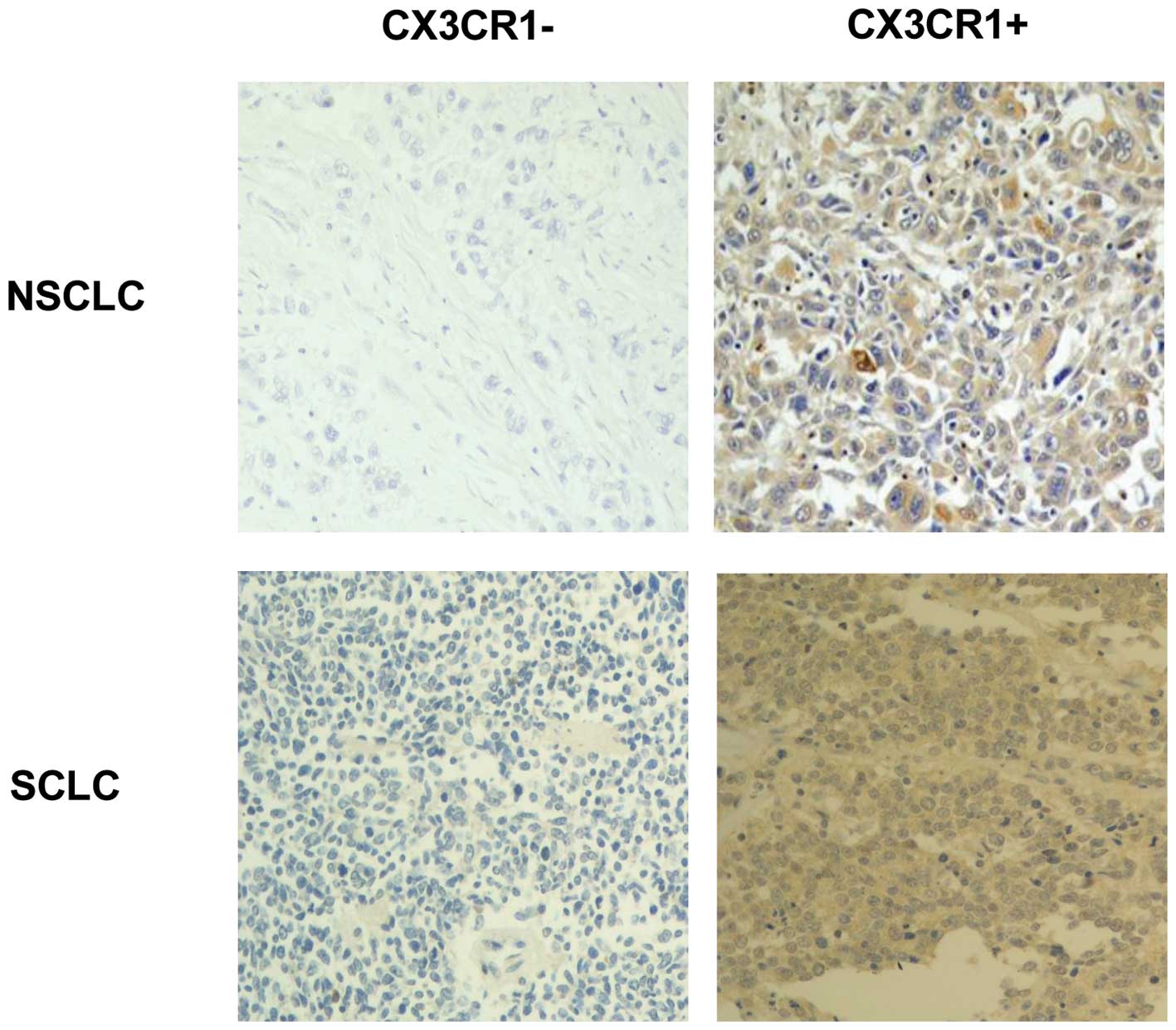
CX3CR1 positivity within the liver and adrenal
deposits was associated with NSCLC histology (p=0.001 and p=0.01
respectively). Representative sections of CX3CR1-positive and
-negative tumours are shown in Fig.
3.
Discussion
Previous research has shown that the differential
expression of chemokines and their receptors can determine
invasiveness and angiogenic potential of tumours and consequently
is associated with a worse survival outcome in NSCLC (7,18) as
well as in SCLC (19,20). In addition, the activation of
specific chemokine signaling pathways may predict the organ
orientation of the metastatic spread (21).
The expression of CX3CR1 has been previously
reported in several tumours such as breast (13), prostate (22), pancreatic (12), ovarian cancer (23) and B-cell lymphoma (17). However, the precise role of this
receptor in the malignant progression of lung cancer has not been
addressed by previous research.
In our study we sought to determine whether CX3CR1
may represent a molecular factor involved in the metastatic spread
of lung cancer. To test this hypothesis, we examined whether CX3CR1
expression in the primary tumour supported the metastatic site
predilection of lung cancer cells on a series of untreated lung
cancers. Interestingly, we found that primary tumours lacking
CX3CR1 expression displayed a higher proportion of liver and brain
metastatic deposits, and this was particularly significant in lung
adenocarcinoma, a histotype with peculiar neurotropic and
hepatotropic potential.
Several published reports suggest that the aberrant
activation of CX3CR1 is a molecular trait promoting the
organotropic spread of primary tumours of the breast, pancreas and
prostate to other vital organs including liver and brain.
However, in the specific context of lung cancer, we
found that primary adenocarcinomas staining positively for CX3CR1
had a lesser tendency to diffuse to the liver and the brain. In
keeping with the results described for hepatocellular carcinoma,
where positivity to FKN and CX3CR1 reflects a less aggressive
course of the disease (24), our
findings let us infer that this molecular pathway can variably
influence the process of metastatic spread across different tumour
histotypes.
Since the molecular phenotype of lung primary
tumours can differ significantly from that of their metastatic
counterparts (25), we subsequently
evaluated the distribution of CX3CR1 expression across the various
sampled metastatic sites to further characterize its role in the
systemic spread of the disease. Interestingly, the disproportion in
CX3CR1 positivity we observed in the primaries was conserved in the
matched metastatic deposits, in which NSCLC displayed higher
expression rates over SCLC in all the sampled districts with the
exception of the brain.
Strikingly, all the atypical sites of metastatic
diffusion including myocardium, thyroid, small bowel and skin were
positive to CX3CR1 in NSCLC, whereas SCLC adrenal secondaries did
not express the receptor. As previous research in NSCLC has shown
that CXC chemokines and their receptors are frequently subject to
epigenetic silencing in primary tumours compared to normal
bronchial epithelium (26), we may
suppose that a reversal of such epigenetic control may be
responsible for the significant degree of CX3CR1 positivity
observed in the metastatic disease.
Furthermore, the high expression rates observed in
specific districts of metastatic spread such as the liver and the
central nervous system (CNS) support the hypothesis that the
acquisition of CX3CR1 positivity does not happen at random, but may
rather represent a site-selective molecular event conferring growth
advantage to the metastasized cell clone.
FKN is largely and constitutively expressed in the
CNS (27) including in activated
brain endothelial cells (28). It
is hypothesized that FKN expression within the target tissue could
provide a chemoattracting gradient promoting trans-endothelial
migration of metastasizing tumour cells (29). According to this model, expression
of CX3CR1 by epithelial tumours may favor the neurotropic
dissemination of micrometastatic foci (30).
A similar mechanism is inherent in the pathogenesis
of liver metastatic diffusion, a renowned poor prognostic trait in
lung cancer (31), whereby the
expression of FKN by hepatic sinusoidal cells may promote liver
specific homing of pulmonary adenocarcinoma cells (24).
Since the activation of CX3CR1 is known to increase
the phosphorylation of master regulators of cancer cell
proliferation such as AKT and ERK1/2 (30), we speculate that the contribution of
the FKN/CX3CR1 axis in the progression of lung cancer may be that
it provides survival advantage signals to the metastasized tumour
cells which secures independent growth potential within the brain
parenchyma.
On the other hand, given the recognized role of FKN
in the promotion of angiogenesis (32), it is reasonable to presume that the
increased proportion of CX3CR1 positivity observed within the brain
deposits may reflect a locally enhanced pro-angiogenic environment.
It has been previously demonstrated that brain metastases do not
share the same angiogenic phenotype of the corresponding primary
NSCLC. In particular, the stronger production of vascular
endothelial growth factor (VEGF) observed in the brain secondary
tumours (25) may be mirrored by
the consistent and potentially angiogenesis-driven up-regulation of
CX3CR1 as reported in our study.
Irrespective of the mechanisms involved, the results
of the present study suggest that CX3CR1 is involved in the
clinical progression of NSCLC, being expressed in the majority of
NSCLC metastatic deposits as well as in a predominant proportion of
SCLC brain metastases.
Our data clearly show that the expression of FKN
receptor, CX3CR1, is differentially distributed across the diverse
histological subgroups of primary lung cancer, with almost
two-thirds of the NSCLC being classified as receptor-positive.
Moreover, CX3CR1 expression in the primary tumour correlates with
advanced lymphatic spread and with the extent of metastatic
dissemination independent of histology. Since oligometastatic
patients have significantly better outcomes compared to patients
with widespread metastatic diffusion (33), such an observation indirectly
supports the role of CX3CR1 in driving the clinical behaviour of
the disease, a concept that is further reinforced by the
significant association found between receptor expression and
tumour stage.
Although limited by the small sample size, our study
is also the first to provide preliminary evidence that CX3CR1
expression may be involved in the pathophysiology of large cell
neuroendocrine carcinomas an original finding that warrants further
and more complete research in an adequately powered case
series.
Since our study included advanced cases at the
terminal stage of the disease we were unable to determine whether
CX3CR1 positivity in the primary tumour can vary as a function of
stage. On the other hand, our study design is peculiar in that it
allowed us to find that up to three quarters of brain metastatic
counterparts spread from both SCLC and NSCLC primaries expressed
CX3CR1. Taken together, these findings suggest that this pathway
might be a positive regulator in the progression of lung
cancer.
We believe that the translational implications of
this study are multifaceted. Firstly, routine pathological
evaluation of CX3CR1 status in resected specimens of lung
adenocarcinoma may represent a useful biomarker in the assessment
of the metastatic potential of these tumours and allow for risk
stratification of patients after curative treatment. Despite
metastatic dissemination to the liver and the CNS is regarded as a
common event in the natural history of bronchogenic carcinoma,
there is a lack of predictors for the development of metastatic
spread to these districts.
The CNS in particular represents the first and most
common site of relapse after definitive treatment of non-small cell
lung cancer (NSCLC) (34),
eventually affecting ≤55% of the patients with locally advanced
disease (35). Regardless of the
histopathological classification, intracranial diffusion is
invariably associated with reduced survival (33,36).
Although a number of clinical predictors of
metastatic spread to the CNS including nodal status and
non-squamous histology (37) have
been identified, the precise molecular mechanisms contributing to
the neurotropic potential of NSCLC have not been fully
elucidated.
Since CX3CR1-negative adenocarcinomas were more
frequently associated with neurotropic spread, clinicians may
incorporate this information to estimate the risk of intracranial
progression and guide treatment decisions accordingly. Although
such conclusion cannot be directly inferred from our retrospective
data, prospective studies evaluating the impact of CX3CR1 on
progression-free and overall survival are warranted.
A second, but not less important implication of our
study relates to the role of the FKN/CX3CR1 axis as an emerging
therapeutically meaningful pathway (38). Our study in fact provides
preliminary evidence supporting a potential role for specific
inhibitors of FKN/CX3CR1 in the setting of advanced lung carcinoma
and possibly, as an adjuvant strategy to treat micrometastatic
disease.
Clearly, the retrospective and single center nature
of our study should be considered as a limitation when evaluating
our results. Moreover, as our study was on post-mortem specimens,
we could not assess the impact of CX3CR1 expression on patients’
survival and staging at diagnosis. However, despite the small
number of subjects included, the uniqueness of our case series has
to be highlighted for two reasons. Firstly, all the enrolled
patients did not receive systemic or radiation treatment which
eliminates the possibility of treatment-induced changes in the
expression of our marker. Secondly, collection of matched primary
and multiple metastatic tissue is rarely achievable, in prospective
studies.
In conclusion, we have shown that CX3CR1 is
differentially expressed across NSCLC and SCLC, two histological
subgroups of lung cancer with very different pathophysiology,
clinical behavior and treatment strategies. We have indicated that
CX3CR1 upregulation is a molecular trait shared by secondary brain
tumours irrespective of histological categorization. Based on these
results, CX3CR1 could not only represent a determinant in the
biological aggressiveness of NSCLC but also a potential therapeutic
target for advanced disease.
Moreover, negativity to CX3CR1 identifies a specific
subgroup of primary tumours with a preferential tendency to
metastasize to the brain and the liver, which suggests that CX3CR1
may be a potential biomarker of site-specific organotropic
spread.
Acknowledgements
We would like to acknowledge John Brennan and Paul
Nya for their help in the retrieval of tissue specimens. D.J.P.
received support from Fondazione De Agostini for PhD studies.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Cersosimo RJ: Lung cancer: a review. Am J
Health Syst Pharm. 59:611–642. 2002.PubMed/NCBI
|
|
3
|
Fry WA, Phillips JL and Menck HR: Ten-year
survey of lung cancer treatment and survival in hospitals in the
United States: a national cancer data base report. Cancer.
86:1867–1876. 1999.PubMed/NCBI
|
|
4
|
Sawaya R: Considerations in the diagnosis
and management of brain metastases. Oncology (Williston Park).
15:1144–1165. 2001.PubMed/NCBI
|
|
5
|
Langley RR and Fidler IJ: Tumor cell-organ
microenvironment interactions in the pathogenesis of cancer
metastasis. Endocr Rev. 28:297–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
7
|
Strieter RM, Belperio JA, Burdick MD,
Sharma S, Dubinett SM and Keane MP: CXC chemokines: angiogenesis,
immunoangiostasis, and metastases in lung cancer. Ann NY Acad Sci.
1028:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh S, Sadanandam A and Singh RK:
Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis
Rev. 26:453–467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zlotnik A, Burkhardt AM and Homey B:
Homeostatic chemokine receptors and organ-specific metastasis. Nat
Rev Immunol. 11:597–606. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phillips RJ, Burdick MD, Lutz M, Belperio
JA, Keane MP and Strieter RM: The stromal derived
factor-1/CXCL12-CXC chemokine receptor 4 biological axis in
non-small cell lung cancer metastases. Am J Respir Crit Care Med.
167:1676–1686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takanami I: Overexpression of CCR7 mRNA in
nonsmall cell lung cancer: correlation with lymph node metastasis.
Int J Cancer. 105:186–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marchesi F, Piemonti L, Fedele G, et al:
The chemokine receptor CX3CR1 is involved in the neural tropism and
malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res.
68:9060–9069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andre F, Cabioglu N, Assi H, et al:
Expression of chemokine receptors predicts the site of metastatic
relapse in patients with axillary node positive primary breast
cancer. Ann Oncol. 17:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marshall HM, Leong SC, Bowman RV, Yang IA
and Fong KM: The science behind the 7th edition Tumour, Node,
Metastasis staging system for lung cancer. Respirology. 17:247–260.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dhillon T, Mauri FA, Bellezza G, et al:
Overexpression of the mammalian target of rapamycin: a novel
biomarker for poor survival in resected early stage non-small cell
lung cancer. J Thorac Oncol. 5:314–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi SR, Key ME and Kalra KL: Antigen
retrieval in formalin-fixed, paraffin-embedded tissues: an
enhancement method for immunohistochemical staining based on
microwave oven heating of tissue sections. J Histochem Cytochem.
39:741–748. 1991. View Article : Google Scholar
|
|
17
|
Andreasson U, Ek S, Merz H, et al: B cell
lymphomas express CX3CR1 a non-B cell lineage adhesion molecule.
Cancer Lett. 259:138–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
White ES, Flaherty KR, Carskadon S, et al:
Macrophage migration inhibitory factor and CXC chemokine expression
in non-small cell lung cancer: role in angiogenesis and prognosis.
Clin Cancer Res. 9:853–860. 2003.PubMed/NCBI
|
|
19
|
Burger M, Glodek A, Hartmann T, et al:
Functional expression of CXCR4 (CD184) on small-cell lung cancer
cells mediates migration, integrin activation, and adhesion to
stromal cells. Oncogene. 22:8093–8101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartmann TN, Burger JA, Glodek A, Fujii N
and Burger M: CXCR4 chemokine receptor and integrin signaling
co-operate in mediating adhesion and chemoresistance in small cell
lung cancer (SCLC) cells. Oncogene. 24:4462–4471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raynaud CM, Mercier O, Dartevelle P, et
al: Expression of chemokine receptor CCR6 as a molecular
determinant of adrenal metastatic relapse in patients with primary
lung cancer. Clin Lung Cancer. 11:187–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shulby SA, Dolloff NG, Stearns ME, Meucci
O and Fatatis A: CX3CR1-fractalkine expression regulates cellular
mechanisms involved in adhesion, migration, and survival of human
prostate cancer cells. Cancer Res. 64:4693–4698. 2004. View Article : Google Scholar
|
|
23
|
Kim M, Rooper L, Xie J, Kajdacsy-Balla AA
and Barbolina MV: Fractalkine receptor CX3CR1 is expressed in
epithelial ovarian carcinoma cells and required for motility and
adhesion to peritoneal mesothelial cells. Mol Cancer Res. 10:11–24.
2012. View Article : Google Scholar
|
|
24
|
Matsubara T, Ono T, Yamanoi A, Tachibana M
and Nagasue N: Fractalkine-CX3CR1 axis regulates tumor cell cycle
and deteriorates prognosis after radical resection for
hepatocellular carcinoma. J Surg Oncol. 95:241–249. 2007.
View Article : Google Scholar
|
|
25
|
Jubb AM, Cesario A, Ferguson M, et al:
Vascular phenotypes in primary non-small cell lung carcinomas and
matched brain metastases. Br J Cancer. 104:1877–1881. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baird AM, Gray SG and O’Byrne KJ:
Epigenetics underpinning the regulation of the CXC
(ELR+) chemokines in non-small cell lung cancer. PLoS
One. 6:e145932011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizuno T, Kawanokuchi J, Numata K and
Suzumura A: Production and neuroprotective functions of fractalkine
in the central nervous system. Brain Res. 979:65–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hurst LA, Bunning RA, Couraud PO, et al:
Expression of ADAM-17, TIMP-3 and fractalkine in the human adult
brain endothelial cell line, hCMEC/D3, following pro-inflammatory
cytokine treatment. J Neuroimmunol. 210:108–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imaizumi T, Yoshida H and Satoh K:
Regulation of CX3CL1/fractalkine expression in endothelial cells. J
Atheroscler Thromb. 11:15–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nevo I, Sagi-Assif O, Meshel T, et al: The
involvement of the fractalkine receptor in the transmigration of
neuroblastoma cells through bone-marrow endothelial cells. Cancer
Lett. 273:127–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamazaki K, Sugio K, Yamanaka T, et al:
Prognostic factors in non-small cell lung cancer patients with
postoperative recurrence following third-generation chemotherapy.
Anticancer Res. 30:1311–1315. 2010.
|
|
32
|
Lee SJ, Namkoong S, Kim YM, et al:
Fractalkine stimulates angiogenesis by activating the
Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am J
Physiol Heart Circ Physiol. 291:H2836–H2846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oh Y, Taylor S, Bekele BN, et al: Number
of metastatic sites is a strong predictor of survival in patients
with nonsmall cell lung cancer with or without brain metastases.
Cancer. 115:2930–2938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mamon HJ, Yeap BY, Janne PA, et al: High
risk of brain metastases in surgically staged IIIA non-small-cell
lung cancer patients treated with surgery, chemotherapy, and
radiation. J Clin Oncol. 23:1530–1537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen AM, Jahan TM, Jablons DM, Garcia J
and Larson DA: Risk of cerebral metastases and neurological death
after pathological complete response to neoadjuvant therapy for
locally advanced nonsmall-cell lung cancer: clinical implications
for the subsequent management of the brain. Cancer. 109:1668–1675.
2007. View Article : Google Scholar
|
|
36
|
Simon GR and Turrisi A: Management of
small cell lung cancer: ACCP evidence-based clinical practice
guidelines (2nd edition). Chest. 132:S324–S339. 2007. View Article : Google Scholar
|
|
37
|
Besse B, Massard C, Haddad V, et al: ERCC1
influence on the incidence of brain metastases in patients with
non-squamous NSCLC treated with adjuvant cisplatin-based
chemotherapy. Ann Oncol. 22:575–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
D’Haese JG, Demir IE, Friess H and Ceyhan
GO: Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a
major and unique therapeutic potential. Expert Opin Ther Targets.
14:207–219. 2010.PubMed/NCBI
|