Introduction
Photodynamic therapy (PDT) is an oxygen-mediated,
minimally invasive therapeutic modality. It involves the
administration of a tumor-localizing photosensitizer that is
subsequently activated with light of a specific wavelength, thus
causing highly selective photodynamic destruction of tumor cells.
The evident advantage of PDT over other conventional cancer
treatments such as chemotherapy and radiotherapy is its minimal
invasiveness, selective targeting, and reduced toxicity, which
allows for repeated treatment (1).
Currently, PDT is being successfully used for the treatment of
early lung cancers (2,3) and in dermatology for the treatment of
non-melanoma skin cancers and precancerous diseases (4). PDT has also been successfully employed
to treat early carcinomas of the oral cavity and larynx to preserve
normal tissue and improve cure rates (5). Though therapeutic responses are
encouraging, recurrences have been noticed due to incomplete PDT,
which consequently triggers tumor angiogenesis. Non-homogeneous
light distribution, incomplete photosensitizer dosage, and
tissue/tumor dynamics are some of the factors that impose
constraints on the efficacy of PDT. Moreover, as PDT-induced
oxidative stress can cause hypoxic conditions in the surviving
tumor cells, it can elicit the expression of angiogenic growth
factors and cytokines as an adaptive response (6,7).
Hypoxia is also known to reduce tumor sensitivity to radiation
therapy and chemotherapy that is associated with decreased local
tumor control (8,9). Thus, further study of controlling
unwanted growth-stimulatory pathways after PDT is desirable to
minimize the risk of harmful adverse effects.
Selenium is an important antioxidant nutrient that
supports the production of enzymes that protect cells against
oxidant stress (10). Since
oxidants can damage DNA, leading to potentially carcinogenic
mutations, good selenium status has clearly anti-mutagenic
potential. Epidemiological studies have pointed to the decrease of
risks for certain cancers in people who have relatively high
selenium intakes or who live in regions of the world where soil
selenium levels are relatively high. The anti-carcinogenic effects
of selenium against leukemia and cancers of the colon, rectum,
pancreas, breasts, ovaries, prostate, bladder, lung, and skin have
been reported (11).
Anti-carcinogenic mechanisms in various cancers are related to
reactive oxygen species (ROS) produced by redox cycling,
modification of protein-thiols, and methionine mimicry. Principal
selenium metabolites, such as hydrogen selenide, methylselenol, and
selenomethionine execute anti-carcinogenic effects (12). Selenium compounds also can cause
acute toxicity due to DNA strand breaks by generating superoxide at
high concentrations of mainly inorganic forms. But many studies
using different forms and doses of selenium have been reported;
selenium doses involved in chemoprevention and tumor growth
inhibition remain to be elucidated (13).
To induce effective anti-tumor response with low
adverse effects, we attempted to co-treat with PDT and selenium.
Combination therapy using low concentrations of photosensitizer and
selenium may effectively reduce angiogenesis and recurrence of
cancer cells by PDT and selenium toxicity at a high concentration.
Additionally, apoptosis of cancer cells and continuous inhibition
of cancer cell development may be induced more effectively.
Materials and methods
Ethics statement
All procedure of animal research were provided in
accordance with the Laboratory Animals Welfare Act, the Guide for
the Care and Use of Laboratory Animals and the Guidelines and
Policies for Rodent experiment provided by the IACUC (Institutional
Animal Care and Use Committee) in School of Medicine, The Catholic
University of Korea (permit no: CUMC-2008-0062-02).
Cell culture and tumor model
A murine cell line, TC-1 (ATCC CRL-2785) was
cotransformed by HPV-16 E6/E7 oncoproteins and c-Has-Ras and was
cultured in RPMI-1640 medium (Gibco BRL, Rocksville, MD)
supplemented with 5% fetal bovine serum (FBS; Gibco BRL).
Streptomycin/penicillin (Gibco BRL), L-glutamine (Gibco BRL), 2.2
mg/ml sodium bicarbonate (Sigma, St. Louis, MO), and 0.4 mg/ml G418
disulfate (Duchefa, The Netherlands) were added to the culture
medium, and the cells were maintained at 37°C in a 5%
CO2 humid environment.
Specific pathogen-free female C57BL/6 mice 6–7 weeks
of age were purchased from Orient Bio (Seongnam, Korea) and
maintained in the specific pathogen-free animal facility. Five to
six mice were housed per cage under standard conditions. Mice were
fed standard laboratory chow and water. The depilated lower dorsa
of the mice were injected subcutaneously with 5×105 TC-1
cells to generate the tumor model. Tumor growth was documented
regularly by Vanier calipers in three orthogonal dimensions. Tumors
were used for experimentation 10–12 days after inoculation when
reaching surface diameters of 10–12 mm and thicknesses of 5–6
mm.
Photodynamic theraphy (PDT) and selenium
treatment
The photosensitizer, Radachorin, was purchased from
RADA-PHARMA group (RADA-PHARMA, Moscow, Russia) and was diluted in
PBS buffer to make a 1 mg/ml stock solution. The light source was a
diode laser with a 662±2 nm wavelength (ALOD-01, Alcom Medica Ltd.,
St.-Petersburg, Russia). TC-1 cells were treated with Radachlorin
(0.1, 0.15, or 0.2 μg/ml) for 12 h. Cells were washed with PBS
buffer and then irradiated with 6.25 J/cm2 of light.
Selenious acid (SELA, Sigma) was dissolved in PBS buffer and
filter-sterilized.
MTT assay
Cell proliferation was determined using the methyl
thiazolyl tetrazolium (MTT) assay. TC-1 cells were subjected to
PDT, selenium, or PDT plus selenium and further incubated at 37°C
and 5% CO2 in a humidified incubator. After incubation,
MTT solution was added to each well. After 4 h, DMSO was added, and
then absorbance was measured at 670 nm in an automated
spectrophotometer microtiter plate reader (Spectra Max 340,
Molecular Devices, CA, USA).
Flow cytometry analysis
For cell cycle analysis, propidium iodide (PI)
staining was performed. Cells were collected, washed twice with
PBS, and then re-suspended in PI buffer (0.1% sodium citrate,
Triton X-100, and 5 μg/ml propidium iodide). RNase A (250 μg/ml)
was added to each cell sample, and the cells were stained for 10
min in the dark. Flow cytometry was performed on a FACScan
automated system (Becton-Dickinson, Sunnyvale, CA, USA), and data
were analyzed using the CellQuest software package and ModFit LT
2.0 program.
To determine apoptosis of TC-1 cells following
treatments of PDT and/or selenium, TC-1 cells were collected at 48
h after all treatments and washed twice with PBS. The cells were
stained with PI and annexin V-FITC. Annexin V-FITC staining was
performed with an apoptosis detection kit (Invitrogen, Camarillo,
CA, USA) as described by the manufacturer's instructions. The cells
were analyzed using a FACScan flow cytometer (Becton-Dickinson) at
488 nm laser light excitation. The cell populations were observed
in a dot plot graphic. The population of apoptotic cells was
represented as annexin V-positive and PI-negative cells, the
population of necrotic cells was represented as annexin V-negative
and PI-positive cells, and the population of late
apoptotic/necrotic cells were represented as annexin V-positive and
PI-positive cells.
Real-time quantitative PCR
(QPCR)/microarray analysis
Total RNA was isolated using TRIzol (Invitrogen Life
Technologies, Carlsbad, CA) from TC-1 cells previously treated with
PDT, selenium, or PDT plus selenium for 48 h. Reverse transcription
was performed using the RT2-First Strand Kit (SuperArray Bioscience
Corp., Frederick, MD) according to the manufacturer's protocol.
Gene profiling was performed using the RT2-profiler PCR array Mouse
Signal Transduction Pathway Finder PCR Array (89 genes including 5
housekeeping genes). The reactions were carried out in a Stratagene
Mx 3000P real-time PCR system (Stratagene, La Jolla, CA). The
values were obtained for the threshold cycle
(Ct) for each gene and normalized using
the average of the 5 housekeeping genes on the same array. The
resulting values were reported as fold change; only genes showing
3-fold or greater change were considered.
Statistical analyses
Genes that showed differences in their expression
levels ≥3.0 fold, were selected for analyses (gene ontology
analysis, hierarchical cluster analysis, functional cluster
analysis, biological pathway analysis). Hierarchical clustering
(Gene Cluster v3.0) and display programs (TreeView) were also used
for analysis (http://rana.stanford.edu/software). We performed
unsupervised hierarchical clustering based on the most variably
expressed genes using the Euclidean distance as the similarity
metric and the average linkage method as the between-cluster
distance metric. A t-test was also performed to find genes that had
changed between PDT alone and selenium alone, and PDT plus selenium
treatment. Supervised clustering of experimental samples was
performed by reducing the number of genes by statistical analysis.
To classify the gene expression profiles, functional analyses and
KEGG (Kyoto Encyclopedia for Genes and Genomes) pathway analyses
(http://www.genome.jp/kegg/pathway.html) were carried
out as previously described (14,15).
To perform a KEGG analysis, differentially expressed genes of each
treatment group were used for the calculation of their attribution
to pre-defined KEGG signaling pathways and analyzed by pair-wise
comparisons. Different number of genes were seen in a given
pathway. The Ingenuity Pathway Analysis software (IPA, Ingenuity
Systems, Mountain View, CA) was utilized to identify networks of
interacting genes and other gene ontology functional groups. The
significantly up-regulated and down-regulated functional activities
were analyzed according to the biological processes, cellular
components and molecular function ontology. Semantically consistent
pathway relationships were modeled based on a continual, formal
extraction from the public domain literature (www.ingenuity.com/products/pathways_knowledge.html).
Measurement of tumor size in TC-1 cells
implanted in C57BL/6 mice
Mice were divided into 4 groups of at least 5
animals per group. Tumor-bearing mice were given intravenous and
intraperitoneal injections of Radachlorin and selenium,
respectively. The first group was treated with selenium (dose: 2
μg/kg body weight), the second group was treated with Radachlorin
(dose: 10 mg/kg), and the third group was treated with both
Radachlorin and selenium. The final two groups were untreated
controls. When tumors were ~8–10 mm in mean tumor diameter,
Radachlorin (10 mg/kg) was injected into the tail veins of mice 3 h
before irradiation. The tumor was irradiated at a fluence of 300
J/cm2. Following light treatment, selenum was given
daily by intraperitoneal injection (2 μg/kg bw).
One control group was only irradiated (no
administration of photosensitizer), and the other did not receive
any photosensitizer or irradiation. The mice were maintained, and
the tumor volumes were measured at two or three day intervals for
23 days. Tumor growth was measured twice a week using calipers and
recorded as volume diameter [longest surface length (a), width (b),
and height (c), a × b × c].
Statistical analysis
Statistical analysis included ANOVA and the
Student's t-test. The values for the different groups were
compared. P-values of less than 0.05 or 0.01 were considered
significant.
Results
Growth inhibitory effect in tumor cells
by PDT, selenium, or PDT plus selenium
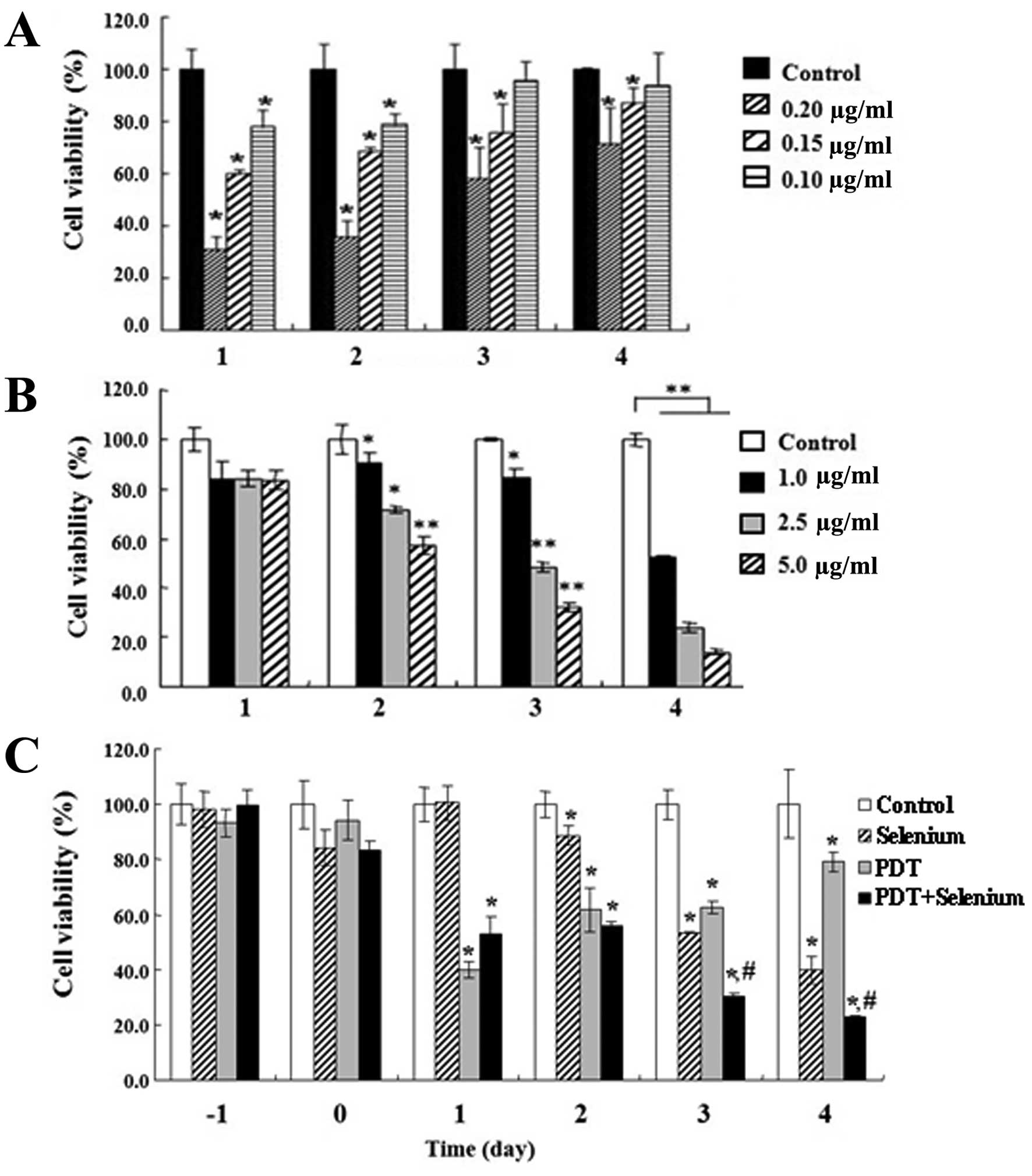
We performed cell viability assays (MTT assays) to
examine effects of PDT in growth inhibition of TC-1 cells. TC-1
tumor cells were treated with various concentrations of Radachlorin
as a photosensitizer and maintained for 12 h. The cells were washed
with PBS and then irradiated by 6.25 J/cm2 of light with
a 662±2 nm wavelength emitted from a diode laser. Cell viability
was determined by MTT assay. TC-1 cell growth was significantly
inhibited in a Radachlorin dose-dependent manner. However, the cell
growth that was inhibited by PDT recovered over time after 3–4 days
(Fig. 1A). In particular, at 4 days
after PDT therapy, growth of TC-1 cells treated with 1 μg/ ml
Radachlorin PDT recovered up to ~90%. We examined the effect of
selenium in TC-1 cell growth inhibition. Selenium is known to
possess anticancer properties at low concentration, but it can be
genotoxic and possibly carcinogenic at high concentrations. TC-1
cell growth was determined by MTT assay for different
concentrations of selenium (Fig.
1B). TC-1 cell growth was significantly suppressed in a
selenium dose-dependent manner, and cell growth recovery was not
observed, unlike that seen with PDT administration.
To effectively inhibit TC-1 growth using the
benefits of PDT and selenium treatment, we attempted combination
therapy consisting of PDT treatment with a low concentrations of
both Radachlorin and selenium. TC-1 cells incubated with 0.15 μg/ml
Radachlorin for 24 h, and selenium (1 μg/ml) were added to the
cultured medium immediately before light treatment. Co-treatment of
PDT plus selenium significantly reduced TC-1 cell growth compared
to PDT or selenium alone (Fig. 1C).
Despite the low concentrations of Radachlorin and selenium, TC-1
cell growth was continuously inhibited according to time, and
growth recovery of TC-1 cells after PDT was not observed at 3 and 4
days after treatment. These results indicate that co-treatment of
PDT and selenium is able to effectively suppress growth of TC-1
tumor cells.
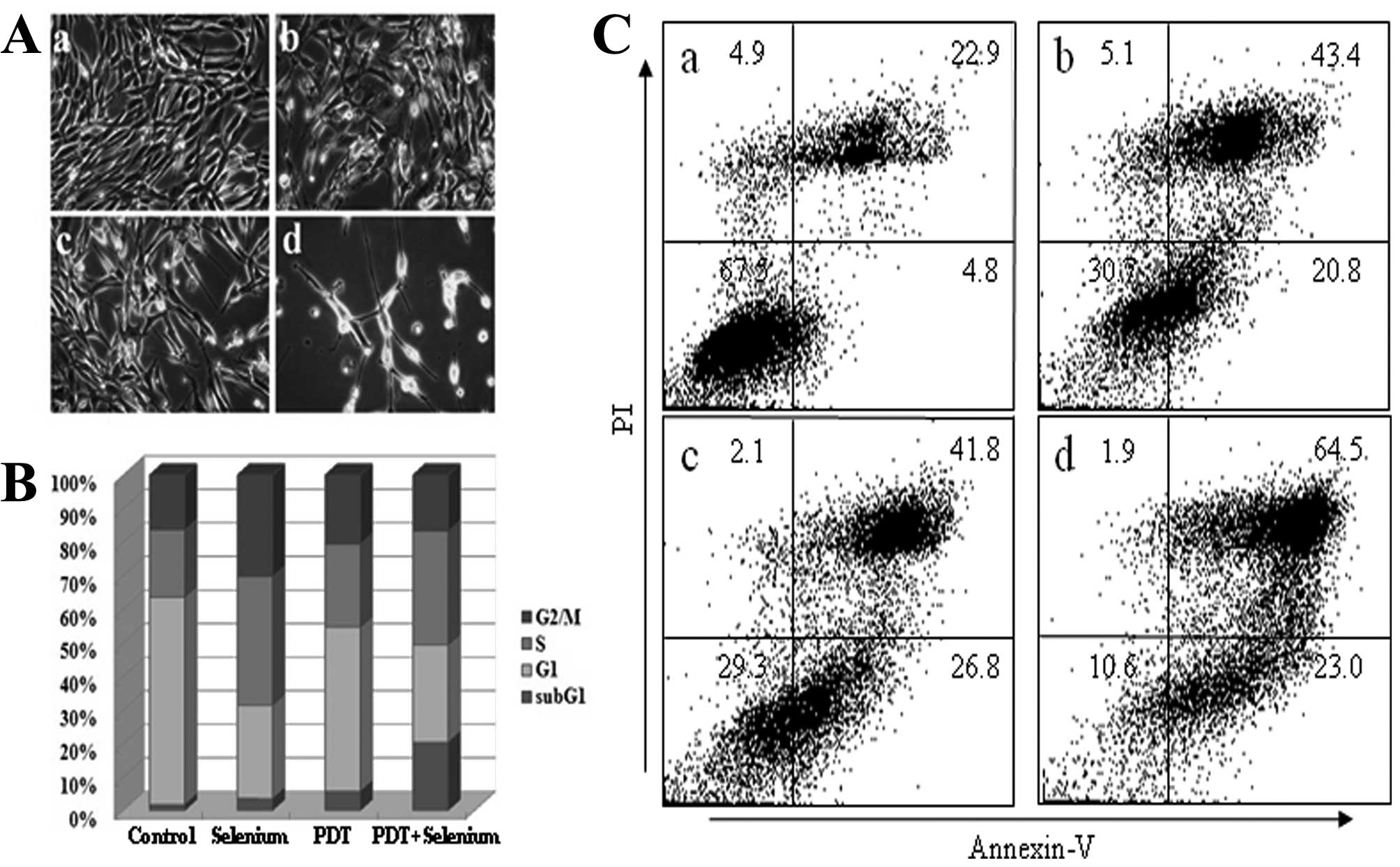
Apoptotic morphology of TC-1 cells by
co-treatment with PDT and selenium
We characterized TC-1 cell growth inhibition by
co-treatment of PDT and selenium. TC-1 cells were treated with 0.15
μg/ml Radachlorin PDT and/or 1 μg/ml selenium for 2 days. The cell
morphology was observed by microscopy (Fig. 2A). TC-1 cell growth was more
inhibited with co-treatment of PDT and selenium than PDT or
selenium alone. Co-treatment of PDT and selenium more greatly
induced the distorted morphology of TC-1 cells and generated many
apoptotic bodies. We performed cell cycle analysis to identify the
cell cycle distribution (Fig. 2B).
The proportion of cells in the sub-G1 phase increased in TC-1 cells
following co-treatment with PDT and selenium compared with those
treated with PDT or selenium alone. To ascertain whether TC-1 tumor
cells were apoptotically induced by combination treatment, we
performed FACS analysis after TC-1 cells were treated with 0.15
μg/ml Radachlorin PDT and/or 1 μg/ml of selenium for 2 days
(Fig. 2C). The proportion of cells
in apoptosis was significantly higher in TC-1 cells following the
combination treatment of PDT plus selenium compared to either
single therapy. These data show that combination treatment induces
a higher level of apoptosis in TC-1 tumor cells compared with
treatment of PDT or selenium alone.
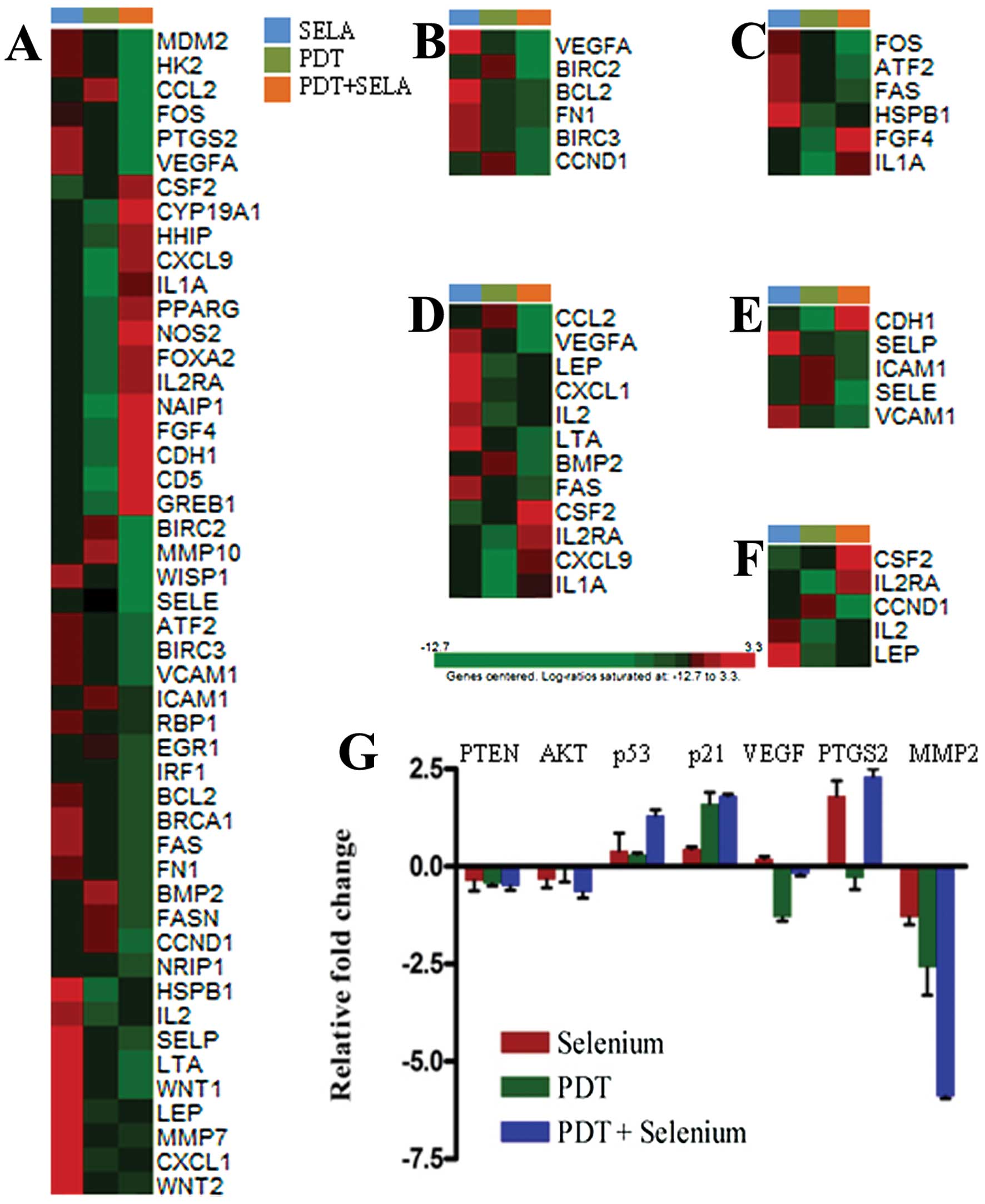
Change in gene expression of TC-1 cells
by combination therapy
Apoptosis, the process of programmed cell death in
eukaryotic cells, causes expression changes in various genes, as
well as changes in cell morphology. Therefore, we performed
real-time reverse transcription PCR assays to identify genes with
significant changes in expression in TC-1 cells treated with PDT
plus selenium. Co-treatment of PDT plus selenium compared with
treatment of PDT or selenium alone induced many changes in gene
expression in TC-1 cells (Table I).
Fold changes were relatively high in TC-1 tumor cells co-treated
with PDT plus selenium compared with those treated with PDT or
selenium alone. To identify signal transduction pathways related to
significantly changed genes by co-treatment, they were classified
as shown in Table II. Using the
signal transduction pathway SuperArray, FOS, HK2, CCL2, MDM2,
PTGS2, VEFGA, VCAM1, BIRC2, and BIRC3 were very significantly
downregulated at least 10-fold in TC-1 cells following
co-treatment. These genes were included in gene groups closely
related to the NFκB, p53, and phospholipase C pathways.
Furthermore, MDM2, BIRC2, and BIRC3 are anti-apoptosis related
genes, and FOS, HK2, VCAM1, CCL2, PTGS2, WISP1, MMP10, and WISP1
are tumor survival or promoting genes. Upregulated genes by
co-treatment had relatively low fold changes compared with the fold
changes of downregulated genes and were related to cell survival
and proliferation mechanisms. These results indicate that the
combination of PDT plus selenium can induce a significant tumor
suppression response. To detect the differences in the functional
profiles, KEGG pathway analyses were carried out. The enhanced cell
growth inhibition and antitumor effects were significantly related
with gene expression levels of focal adhesion, MAPK pathway,
cytokine-cytokine interaction, and cell adhesion pathway (Fig. 3A-E). Significantly down-regulated
molecular functions for the combination treatment group was focal
adhesion pathway. Each representative gene in the pathway was
tested by quantitative PCR (Fig.
3G). These included genes coding for PTEN, AKT, p53, p21, VEGF,
PTGS2, and MMP2. Jak-STAT pathway was also studied in the gene
sets, but not strictly correlated with the enhanced cell growth
inhibition (Fig. 3F).
 | Table IRelative expression of target genes in
TC-1 cells. |
Table I
Relative expression of target genes in
TC-1 cells.
| Fold change |
|---|
|
|
|---|
| Gene symbol | Selenium | PDT | PDT + selenium |
|---|
| ATF2 | −1.1 | −1.8 | −9.5 |
| BCL2 | −1.2 | −2.0 | −5.7 |
| NAIP1 | 6.4 | −1.2 | 24.4 |
| BIRC2 | −3.4 | −2.7 | −84.0 |
| BIRC3 | −2.4 | −3.6 | −19.9 |
| BMP2 | −1.3 | 1.4 | −3.4 |
| BRCA1 | −1.8 | −3.5 | −10.7 |
| CCL2 | −3.6 | −1.9 | −3748.3 |
| CCND1 | −1.0 | 1.4 | −3.7 |
| CD5 | 7.8 | 1.1 | 34.5 |
| CDH1 | 4.2 | −1.6 | 20.6 |
| CSF2 | 3.2 | 4.9 | 14.8 |
| CXCL1 | 4.8 | 1.4 | 1.5 |
| CXCL9 | 8.3 | −1.2 | 16.5 |
| CYP19A1 | 4.9 | −1.2 | 46.8 |
| EGR1 | −2.1 | −2.0 | −3.9 |
| FAS | −1.3 | −2.6 | −5.5 |
| FASN | −1.5 | −1.1 | −3.8 |
| FGF4 | 5.7 | −1.2 | 32.6 |
| FN1 | −2.7 | −4.4 | −9.9 |
| FOS | −1.7 | −1.9 | −1391.1 |
| FOXA2 | 5.2 | −1.2 | 15.1 |
| GREB1 | 5.7 | −1.2 | 26.3 |
| HHIP | 2.1 | −1.3 | 6.2 |
| HK2 | −1.4 | −1.8 | −498.7 |
| HSPB1 | 23.7 | 1.3 | 6.3 |
| ICAM1 | −3.3 | −2.6 | −3.7 |
| IL1A | 10.6 | 1.1 | 16.2 |
| IL2 | 6.0 | 1.1 | 2.2 |
| IL2RA | 4.6 | −1.2 | 13.3 |
| IRF1 | −3.8 | −3.8 | −5.9 |
| LEP | 6.1 | −1.2 | −1.1 |
| LTA | 5.0 | −1.0 | −4.4 |
| MDM2 | 1.0 | −1.3 | −8792.2 |
| MMP10 | 1.5 | 2.7 | −17.2 |
| MMP7 | 7.2 | −1.2 | −1.8 |
| NOS2 | 7.5 | 1.3 | 25.2 |
| NRIP1 | −1.3 | −1.3 | −3.7 |
| PPARG | 4.5 | −1.2 | 10.8 |
| PTGS2 | 1.2 | −1.5 | −1597.9 |
| RBP1 | −1.8 | −2.6 | −3.5 |
| SELE | 5.2 | 5.3 | −1.7 |
| SELP | 8.4 | 1.1 | −3.0 |
| VCAM1 | −1.4 | −2.1 | −10.9 |
| VEGFA | −1.2 | −2.4 | −1642.9 |
| WISP1 | −1.3 | −2.6 | −28.1 |
| WNT1 | 8.5 | 2.0 | −2.3 |
| WNT2 | 3.9 | −1.2 | −1.8 |
 | Table IIEffects of co-treatment of PDT and
selenium in signal transduction pathway. |
Table II
Effects of co-treatment of PDT and
selenium in signal transduction pathway.
| A, Down-regulated
genes ≤10-fold |
|---|
|
|---|
| Related signal
pathway | Gene symbol | Selenium | PDT | PDT + selenium |
|---|
| Calcium and
PKC | FOS | −1.7 | −1.9 | −1391.1 |
| CREB | FOS | −1.7 | −1.9 | −1391.1 |
| Estrogen | BRCA1 | −1.8 | −3.5 | −10.7 |
| Insulin | HK2 | −1.4 | −1.8 | −498.7 |
| Jak/Src | MMP10 | 1.5 | 2.7 | −17.2 |
| LDL | VCAM1 | −1.4 | −2.1 | −10.9 |
| CCL2 | −3.6 | −1.9 | −3748.3 |
| Mitogenic | FOS | −1.7 | −1.9 | −1391.1 |
| NFκB | VCAM1 | −1.4 | −2.1 | −10.9 |
| BRIC3 | −2.4 | −3.6 | −19.9 |
| BRIC2 | −3.4 | −2.7 | −84.0 |
| P53 | MDM2 | 1.1 | −1.3 | −8792.2 |
| PLC | VCAM1 | −1.4 | −2.1 | −10.9 |
| FOS | −1.7 | −1.9 | −1391.1 |
| PTG2 | −1.2 | −1.5 | −1597.9 |
| Stress | FOS | −1.7 | −1.9 | −1391.1 |
| Wnt | WISP1 | −1.4 | −2.6 | −28.1 |
| VEGFA | −1.3 | −2.4 | −1642.9 |
|
| B, Up-regulated
genes ≤10-fold |
|
| Related signal
pathway | Gene symbol | Selenium | PDT | PDT + selenium |
|
| Selenium Calcium
and PKC | CSF2 | 3.2 | 4.9 | 14.8 |
| IL2RA | 4.6 | −1.18 | 13.34 |
| CREB | CYP19A1 | 4.9 | −1.18 | 46.79 |
| Estrogen | GREB1 | 5.7 | −1.18 | 26.38 |
| Hedgehog | FOXA2 | 5.2 | −1.18 | 15.12 |
| Jak-Stat | NOS2 | 7.5 | 1.3 | 25.25 |
| CXCL9 | 8.3 | −1.18 | 16.54 |
| LDL | CSF2 | 3.2 | 4.9 | 14.8 |
| NFκB | NOS2 | 7.5 | 1.3 | 25.25 |
| IL1A | 10.6 | 1.09 | 16.2 |
| PLC | NOS2 | 7.5 | 1.3 | 25.25 |
| Wnt | FGF4 | 5.7 | −1.18 | 32.63 |
| CDH1 | 4.2 | −1.55 | 20.65 |
| PPARG | 4.5 | −1.18 | 10.84 |
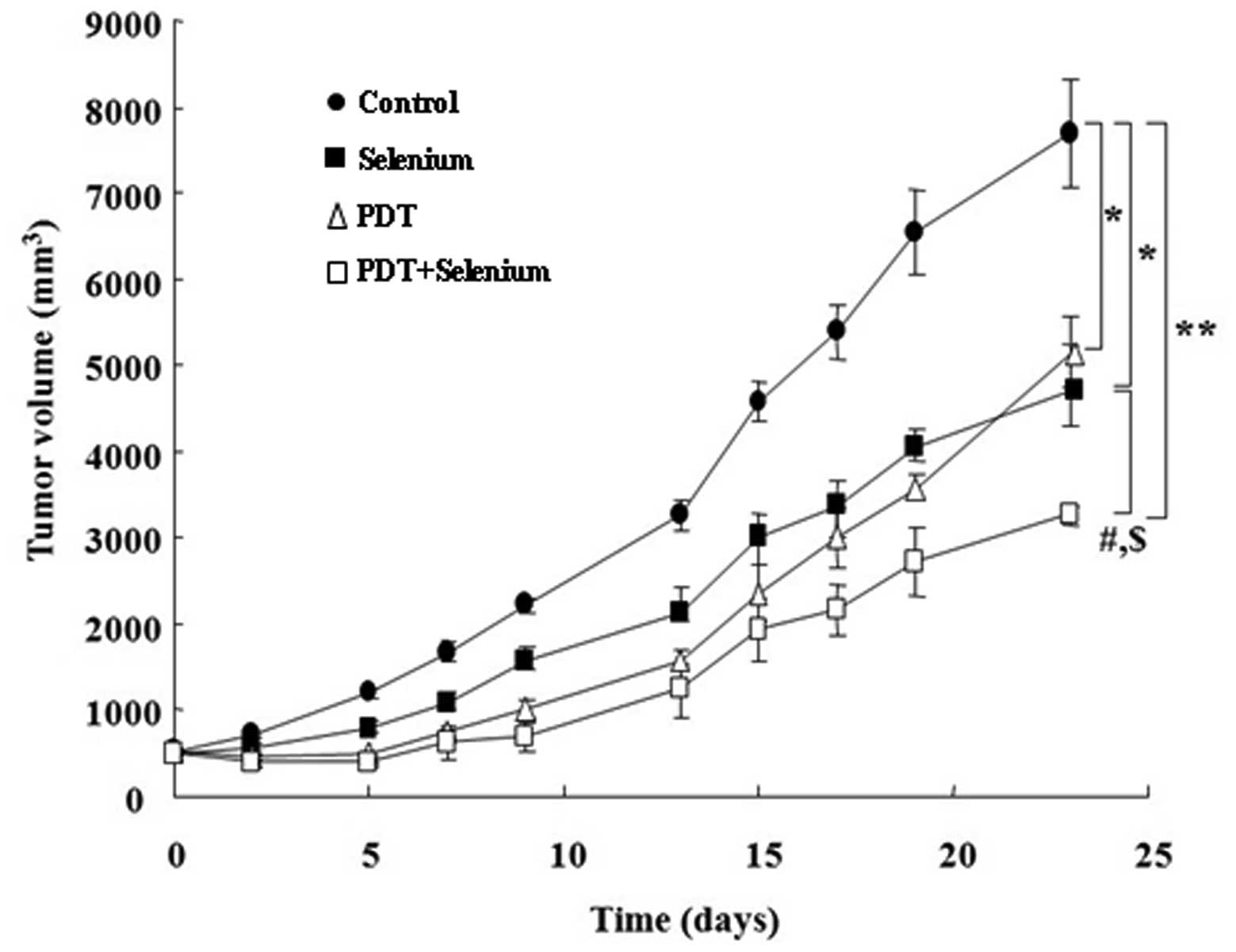
Suppression of tumor development in TC-1
cells implanted in C57L/6 mice
We measured tumor sizes of TC-1 tumor cell-implanted
C57BL/6 mice to determine in vivo effects of PDT and
selenium combination therapy. TC-1 cell xenografted C57BL/6 mice
were grouped in four different groups: control, PDT, selenium, and
PDT plus selenium groups (Fig. 4).
PDT effectively inhibited in tumor development for a short period
(approximately 15 days), but inhibition of tumor growth by PDT
seemed to be recovered after ~20 days. After 20 days, growth rates
of tumors following PDT treatment compared with growth rates
following selenium treatment were relatively increased. In
contrast, combination treatment with PDT plus selenium clearly
reduced tumor diameters, and this tumor suppression was maintained
for 23 days. This result suggests that combination therapy with PDT
plus selenium in the tumor-bearing mouse model is effective for
in vivo tumor suppression as well as for in vitro
tumor suppression.
Discussion
Photodynamic therapy (PDT) is a relatively recent
clinical treatment applied against patients' lesions, including
various cancers, and it has already been recognized as a very
promising therapeutic method (16).
PDT has been applied to improve early stage disease, including lung
cancer, brain cancers, head and neck cancers, and non-oncological
disorders, such as age-related macular degeneration (17). However, PDT can cause hypoxic
conditions in the surviving tumor cells due to oxidative stress
induction, and it can elicit tumor recurrence and angiogenesis
(8,9). Selenium is a dietary essential trace
element with biological roles and possesses anticancer properties
(11). However, selenium at low
concentrations has anti-carcinogenic properties in multiple
cancers, including those of the colon, prostate, breast, ovary, and
lung, whereas at high concentrations, selenium can generate
reactive oxygen species (ROS), which can induce DNA damage
(13). Therefore, we tried
combination therapy consisting of PDT and selenium to develop a
therapeutic method with low adverse effects. We used the TC-1
cervical cell line with overexpression of human papilloma virus
(HPV), the E6/E7 oncogene, and the Ha-ras oncogene. To
prepare the cervical cancer mouse model, the TC-1 cervical cell
line was transplanted into C57BL/6 mice. Combination therapy with
PDT and selenium was applied both in the TC-1 cell line in
vitro and tumor-bearing C57BL/6 mice in vivo.
TC-1 cell viability was significantly inhibited both
in PDT or selenium treatment, but cell growth inhibited by PDT
alone recovered over time. We tried to co-treat with Radachlorin
PDT plus selenium at low concentrations to prevent adverse effects,
such as tumor cell growth recovery and cell toxicity. Despite the
low concentrations of Radachlorin PDT and selenium, TC-1 cell
growth was effectively inhibited by co-treatment, and cell growth
recovery did not occur during the time of the experiment. To
determine cell arrest and cell apoptosis by combination therapy, we
performed cell cycle and apoptosis analyses using a FACS analyzer.
Co-treatment of Radachlorin PDT and selenium compared to
Radachlorin PDT or selenium alone induced greater apoptotic TC-1
cell morphology and increased the proportion of TC-1 cells in the
sub-G1 phase of the cell cycle. In FACS analysis to confirm TC-1
apoptosis by co-treatment, TC-1 cells following co-treatment were
observed to have a larger proportion of annexin V-positive and
PI-negative cells in a FACS analysis dot plot graph.
We supposed that combination therapy with
Radachlorin PDT and selenium should more effectively inhibit growth
of TC-1 cancer through higher TC-1 apoptosis induction. Therefore,
we tried to identify genes with significant expression changes as a
result of combination treatment. Overall, downregulated genes by
co-treatment with PDT and selenium had relatively higher fold
changes than those of upregulated genes. Co-treatment of PDT and
selenium downregulated expression of many genes related to the
NFκB, p53, and phospholipase C pathways. These genes included
groups of anti-apoptotic and anti-tumor genes. Upregulated genes by
co-treatment of PDT plus selenium were related with cell survival
and proliferation mechanisms. Upregulation of these genes may be a
repair mechanism for various types of damage induced by therapies.
Finally, we examined effects of combination therapy in tumor tissue
using the TC-1 cell-implanted mouse model. Combination treatment
with PDT plus selenium clearly reduced tumor diameters, and this
tumor suppression was maintained for a long time.
PDT induces apoptosis by production of singlet
oxygen (18), and selenium also
induces apoptosis at a low concentration. High concentrations of
selenium have pro-oxidant toxicity due to DNA strand breaks, and
PDT can cause tumor recurrence and angiogenesis by hypoxic
conditions. Therefore, we inspected the results for gene expression
change in TC-1 cells following co-treatment with low concentrations
of Radachlorin PDT and selenium. Genes with relatively high fold
changes in significantly downregulated genes were FOS, HK2, CCL2,
MDM2, PTGS2, and VEGFA. FOS, an FBJ murine osteosarcoma viral
oncogene homolog, is a transcription factor with oncogenic activity
and is frequently overexpressed in various tumor cells (19). HK2, hexokinase II, is known as an
enzyme catalyzing the initial metabolic step of glycolysis and is
predominantly expressed in many cancer cells. Downregulation of the
HK2 gene increases the sensitivity of cancer to anti-cancer agents
(20). CCL2, a chemokine (C-C
motif) ligand 2, is known as a chemoattractant for monocytes and is
expressed in at least nine of ten cervical cancer cell lines
(21). MDM2, transformed mouse 3T3
cell double minute 2, induces degradation of tumor suppressor p53
protein and inhibits apoptosis of cancer cells by p53 (22). PTGS2, prostaglandin-endoperoxide
synthase 2, also known as cyclooxygenase 2 (COX-2), is known to be
regulated by the human papillomavirus 16 oncogenes E6 and E7
through EGFR, and high PTGS2 expression is related to advanced
diseases, distant metastases, and decreased survival in cervical
cancer patients (23). VEGFA,
vascular endothelial growth factor A, is known to stimulate the
growth of endothelial cells and is a powerful inducer of
angiogenesis. VEGF prevents apoptosis of endothelial cells by the
lack of serum and induces expression of anti-apoptotic proteins
BCL-2 and A1 (24,25). The significant expression reduction
of these genes that are closely related to cancer development is
consistent with the results for proliferation inhibition and
apoptosis of TC-1 tumor cells by combination therapy with PDT and
selenium.
BRCA1, MMP10, VCAM1, BIRC2, BIRC3, and WISP1 were
also significantly down-regulated in TC-1 cells with co-treatment
of PDT and selenium. Deficiency of BRCA1, breast cancer associated
gene 1, causes abnormalities in the S-phase checkpoint, the G2/M
checkpoint, and centrosome duplication and induces inhibition of
cell proliferation and apoptosis (26). Matrix metalloproteinases (MMPs) are
involved in several steps of cancer development, and MMP10 is
overexpressed in head and neck SCCs (27). Expression of VCAM1, vascular cell
adhesion molecule 1, in tumor cells decreases accumulation of T
cells in the tumor site by promoting T-cell migration away from
tumors. VCAM1-expressing tumor cells are able to avoid immune
attack (28). BIR2 and BIRC3,
baculoviral IAP repeat-containing 2 and 3, are known to accelerate
tumorigenesis as anti-apoptotic genes (29,30).
WISP-1, WNT1 inducible signaling pathway protein 1, is strongly
expressed in breast tumors developing in WNT-1 transgenic mice, and
overexpression in normal rat kidney fibroblasts induces their
transformaiton (31,32). As these genes were also related to
cell cycle and transformation of tumors, downregulation of these
genes would effectively inhibit development of TC-1 cancer
cells.
In conclusion, we have shown that co-treatment with
PDT and selenium induces growth inhibition, apoptotic morphology
changes, and gene expression changes in TC-1 tumor cells. Treatment
with low concentrations of PDT and selenium in combination
sufficiently inhibited tumor cell proliferation without growth
recovery of cancer cells following PDT alone. These effects may be
due to significant down-regulation of anti-apoptotic genes and
tumor-promoting genes. These results indicated that combination
therapy of selenium and PDT could effectively induce a tumor
suppression response compared to PDT or selenium alone. Therefore,
we suggested that combination therapy using PDT and selenium can be
an approach to induce effective anti-cancer effects.
Acknowledgements
This study was supported by Business of
Globalization for Science and Technology funded by the Ministry of
Education, Science and Technology, Seoul, Republic of Korea (Grant
5-2012-A0154-00001).
References
|
1
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar
|
|
2
|
Moghissi K, Dixon K, Thorpe JA, Stringer M
and Oxtoby C: Photodynamic therapy (PDT) in early central lung
cancer: a treatment option for patients ineligible for surgical
resection. Thorax. 62:391–395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Usuda J, Kato H, Okunaka T, et al:
Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol.
1:489–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein A, Babilas P, Karrer S, Landthaler M
and Szeimies RM: Photodynamic therapy in dermatology - an update
2008. J Dtsch Dermatol Ges. 6:839–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Biel MA: Photodynamic therapy treatment of
early oral and laryngeal cancers. Photochem Photobiol.
83:1063–1068. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dougherty TJ, Gomer CJ, Henderson BW, et
al: Photodynamic therapy. J Natl Cancer Inst. 90:889–905. 1998.
View Article : Google Scholar
|
|
7
|
Gollnick SO, Evans SS, Baumann H, et al:
Role of cytokines in photodynamic therapy-induced local and
systemic inflammation. Br J Cancer. 88:1772–1779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harrison L and Blackwell K: Hypoxia and
anemia: factors in decreased sensitivity to radiation therapy and
chemotherapy? Oncologist. 9(Suppl 5): 31–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaupel P and Harrison L: Tumor hypoxia:
causative factors, compensatory mechanisms, and cellular response.
Oncologist. 9(Suppl 5): 4–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stadtman TC: Selenium biochemistry.
Mammalian selenoenzymes. Ann NY Acad Sci. 899:399–402. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Navarro-Alarcon M and Cabrera-Vique C:
Selenium in food and the human body: a review. Sci Total Environ.
400:115–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jackson MI and Combs GF Jr: Selenium and
anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr
Metab Care. 11:718–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Letavayova L, Vlckova V and Brozmanova J:
Selenium: from cancer prevention to DNA damage. Toxicology.
227:1–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pletcher SD, Macdonald SJ, Marguerie R, et
al: Genome-wide transcript profiles in aging and calorically
restricted Drosophila melanogaster. Curr Biol. 12:712–723. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang JL, Lin YW, Chen HM, et al: Calcium
prevents tumorigenesis in a mouse model of colorectal cancer. PLoS
One. 6:e225662011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson BC and Patterson MS: The physics,
biophysics and technology of photodynamic therapy. Phys Med Biol.
53:R61–R109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zawacka-Pankau J, Krachulec J, Grulkowski
I, Bielawski KP and Selivanova G: The p53-mediated cytotoxicity of
photodynamic therapy of cancer: recent advances. Toxicol Appl
Pharmacol. 232:487–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buytaert E, Dewaele M and Agostinis P:
Molecular effectors of multiple cell death pathways initiated by
photodynamic therapy. Biochim Biophys Acta. 1776:86–107.
2007.PubMed/NCBI
|
|
19
|
Milde-Langosch K: The Fos family of
transcription factors and their role in tumourigenesis. Eur J
Cancer. 41:2449–2461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng QP, Zhou JM, Zhou Q, Pan F, Zhong DP
and Liang HJ: Downregulation of the hexokinase II gene sensitizes
human colon cancer cells to 5-fluorouracil. Chemotherapy.
54:357–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers
PH, Kenter GG and Gorter A: The absence of CCL2 expression in
cervical carcinoma is associated with increased survival and loss
of heterozygosity at 17q11.2. J Pathol. 208:507–517. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hengstermann A, Linares LK, Ciechanover A,
Whitaker NJ and Scheffner M: Complete switch from Mdm2 to human
papillomavirus E6-mediated degradation of p53 in cervical cancer
cells. Proc Natl Acad Sci USA. 98:1218–1223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrario A, Von Tiehl K, Wong S, Luna M
and Gomer CJ: Cyclooxygenase-2 inhibitor treatment enhances
photodynamic therapy-mediated tumor response. Cancer Res.
62:3956–3961. 2002.PubMed/NCBI
|
|
24
|
Bequet-Romero M and Lopez-Ocejo O:
Angiogenesis modulators expression in culture cell lines positives
for HPV-16 oncoproteins. Biochem Biophys Res Commun. 277:55–61.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karamysheva AF: Mechanisms of
angiogenesis. Biochemistry (Mosc). 73:751–762. 2008. View Article : Google Scholar
|
|
26
|
Deng CX: BRCA1: cell cycle checkpoint,
genetic instability, DNA damage response and cancer evolution.
Nucleic Acids Res. 34:1416–1426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yen CY, Chen CH, Chang CH, et al: Matrix
metalloproteinases (MMP) 1 and MMP10 but not MMP12 are potential
oral cancer markers. Biomarkers. 14:244–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu TC: The role of vascular cell adhesion
molecule-1 in tumor immune evasion. Cancer Res. 67:6003–6006. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zender L, Spector MS, Xue W, et al:
Identification and validation of oncogenes in liver cancer using an
integrative oncogenomic approach. Cell. 125:1253–1267. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma O, Cai WW, Zender L, et al: MMP13,
Birc2 (cIAP1), and Birc3 (cIAP2), amplified on chromosome 9,
collaborate with p53 deficiency in mouse osteosarcoma progression.
Cancer Res. 69:2559–2567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khor TO, Gul YA, Ithnin H and Seow HF: A
comparative study of the expression of Wnt-1, WISP-1, survivin and
cyclin-D1 in colorectal carcinoma. Int J Colorectal Dis.
21:291–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie D, Nakachi K, Wang H, Elashoff R and
Koeffler HP: Elevated levels of connective tissue growth factor,
WISP-1, and CYR61 in primary breast cancers associated with more
advanced features. Cancer Res. 61:8917–8923. 2001.PubMed/NCBI
|