Introduction
Ovarian cancer has a very high mortality rate, since
it tends to be asymptomic, which results in the vast majority of
patients with ovarian cancer being diagnosed in advanced stages
(stage III/IV) (1,2). The 5-year survival rate of patients
with early stage cancer ranges from 50–95%, but it is <25% for
those with advanced stage disease (3,4). Given
our knowledge about the steep decrease in survival rates relative
to the stage at which the disease is diagnosed, it is reasonable to
suggest that early detection remains the most promising approach
with which to improve the long-term survival of ovarian cancer
patients. Therefore, considerable efforts have been focused on the
identification of diagnostic biomarkers for early detection of
ovarian cancer (5,6).
CA125 has been used as a serum marker of ovarian
cancer for monitoring responses to chemotherapy, detecting disease
recurrence, distinguishing malignant from benign pelvic masses, and
potentially improving the designs of clinical trials. However,
CA125 has proven to be a poor diagnostic tumor biomarker for early
stage ovarian cancer (7). It is
elevated above reference levels in only 50% of clinically
detectable early stage disease, and is not infrequently elevated in
patients with benign ovarian tumors (8,9). In
addition, CA125 levels are falsely elevated in pregnant women and
women with detectable intraperitoneal pathologies that may alter
the clearance of the antigen (10–12).
Therefore, attempts have been made to combine or replace CA125 with
other markers, and investigators have evaluated the ability of some
established markers to improve the identification and prognosis of
ovarian cancer (8,13,14),
thus indicating that the addition of one or several markers to
CA125 would improve diagnostic and prognostic performance if
sensitivity were improved without a loss in specificity. However,
because the measurement of serum concentration of each putative
biomarker with individual ELISAs requires considerable time, cost,
and sample volumes in order to assess the combined effects of
several markers, new methods or technologies for multiplexing must
be developed.
The Luminex 100 bead-based system is a recently
developed technology that provides multiplexing in a solution
phase, resulting in it being particularly flexible and
nondestructive for protein analysis. Each set of up to 100 uniquely
color-coded polystyrene microspheres can be anchored with a
different capture antibody. The use of detection antibodies labeled
with biotin and streptavidin-R-phycoerythrin allows quantification
of antigen-antibody reactions that occur on the microsphere surface
through the measurement of the relative fluorescence intensity.
Therefore, the system is capable of measuring up to 100 analytes
simultaneously in a small sample volume (<50 μl).
In this study, we measured four serum biomarkers of
ovarian cancer, CA125, hemoglobin, haptoglobin, and apolipoprotein
E, using a multimarker bead-based immunoassay system, and evaluated
the combined effect of the four biomarkers for the diagnosis of
ovarian cancer compared with those of the individual markers
alone.
Materials and methods
Patients and samples
All patients were enrolled at St. Mary’s Hospital of
Catholic Medical School during the period from January 2001 to July
2007, according to the procedures approved by the Institutional
Review Board of The Catholic University of Korea. This study was
based on analyses of serum collected from patients with ovarian
cancer (n=69) and normal healthy females (n=76). Patient serum was
harvested before surgery or chemotherapy, and was then incubated
for 30 min at room temperature, followed by centrifugation at 3,000
rpm for separation. The serum was stored at −70°C until they were
used in experiments; frequent freezing and thawing were avoided.
The stages and grades of tumors from the ovarian cancer patients
were assigned according to the guidelines provided by the
International Federation of Gynecology and Obstetrics (FIGO), and
the enrolled groups were then divided according to age.
Conjugation of primary antibodies with
microspheres
Four different kinds of microspheres
(1×106 microspheres for each antibody, Biosource,
Camarillo, CA) were prepared in each tube, and were then
resuspended well by vortexing and sonication, followed by
centrifugation for 2 min at 8,000 rpm. Supernatants were discarded,
and the pellets were saved and washed once with 100 μl saline.
Monobasic sodium phosphate (80 μl of 100 mM) (pH 6.2,
Sigma-Aldrich, St. Louis, MO), 10 μl of 50 mM Sulfo-NHS (Pierce
Biotechnology, Rockford, IL) and 10 μl of 50 mM EDC (Pierce
Biotechnology) were added, and the solution was then incubated for
20 min at room temperature. After centrifugation (8,000 rpm, 2
min), the pellets were saved and washed twice with 250 μl of 50 mM
MES (pH 5.0, Sigma-Aldrich). After the removal of the supernatant,
500 μl of MES was added to each tube including different
microspheres. Following the addition of 0.5 μg of each antibody
[anti-CA125 (Fitzgerald Industries International, Inc., Concord,
MA), anti-hemoglobin (Abcam, Cambridge, MA), anti-haptoglobin
(Abcam), anti-apolipoprotein E (Fizgerald Industries International,
Inc.)] in each tube, the tubes were incubated for 2 h on a shaker,
which was protected from light. After the incubation,
antibody-bound microspheres were pelleted by centrifugation for 2
min at 8,000 rpm, and 500 μl of 1% BSA buffer was then added. After
additional incubation for 30 min at room temperature, the
microspheres were washed twice with 1% BSA buffer and then stored
at 4°C under protection from light.
Labeling biotins on the secondary
antibodies
For labeling biotins on the secondary antibodies, a
biotin labeling kit (Alpha Diagnostics International Inc., San
Antonio, TX) was used according to the manufacturer’s protocol.
Briefly, biotin was added at a ratio of 1:10 (biotin:antibody).
After incubation for 1 h at room temperature under protection from
light, dialysis was performed with phosphate-buffered saline
(PBS).
Analysis of samples by multiplex liquid
array system, Luminex 100
The serum from healthy normal control and ovarian
cancer patients were diluted to 1:100 in a buffer including 1% BSA
(Sigma-Aldrich) and 0.05% Tween-20 (Sigma-Adrich). Fifty μl of each
diluted serum were plated on a 1.2-μm filter plate (96- well), to
which 2,500 of each antibody-bound microsphere were added in 50 μl.
After incubation for 2 h at room temperature under protection from
light, they were washed twice with PBS buffer including 0.05%
Tween-20. Streptavidin-R-phycoerythrin (100 μl of 0.4 μg)
(Sigma-Aldrich) was added to each well, and plates were then
incubated for 30 min, followed by two washes with PBS containing
0.05% Tween-20. The identification of antibody-bound microspheres
and the screening of antigen-antibody-bound microspheres were
carried out by using Luminex 100 (Luminex Corp., Houston, TX)
according to the manufacturer’s protocol. Ranges of the
concentrations of each antigen for standard curves were 10–250 U/ml
for CA125, 1–1000 μg/ml for hemoglobin, 0.1–100 μg/ml for
haptoglobin, and 0.5–50 ng/ml for apolipoprotein E. The data were
analyzed by the BeadView program (Upstate, Charlottesville,
VA).
Statistical analysis
The analysis of variance (ANOVA) test was used to
assess the statistical significance of differences between the
normal individuals and ovarian cancer patients. SigmaPlot (v12.0,
Systat, Chicago, IL) and SAS (v9.1, SAS Institute, Cary, NC) was
used for statistical analysis to determine the sensitivity,
specificity, and the receiver operator characteristic (ROC)
curve.
Results
Serum levels of ovarian tumor markers in
normal control and ovarian cancer groups
The characteristics of patients and serum levels of
ovarian cancer markers are shown in Table I. Concentration of serum biomarkers
such as CA125, hemoglobin, haptoglobin, and apolipoprotein E in
serum from healthy normal control and ovarian cancer patients was
simultaneously measured by a multiplex liquid array system using
microbeads coated with capture antibodies and biotin-labeled
antibodies against each of the tumor markers and
streptavidin-R-phycoerythrin. The serum levels of all four tumor
markers were significantly higher in ovarian cancer patients than
that in normal controls (Fig. 1A).
First, we compared the serum levels of these four tumor markers
according to the tumor stages (Fig.
1B). The serum levels of CA125 were gradually elevated with
tumor stage. Also, the other three tumor markers were significantly
increased in ovarian cancers compared with that in normal controls.
Next, we attempted to compare the serum levels of four tumor
markers according to histologic types of ovarian cancer (Fig. 1C). The serum levels of CA125,
haptoglobin, and apolipoprotein E were the highest in serous type
compared with those in the other types, as the case numbers for
patients with clear cell and granulosa cell histology were too
small.
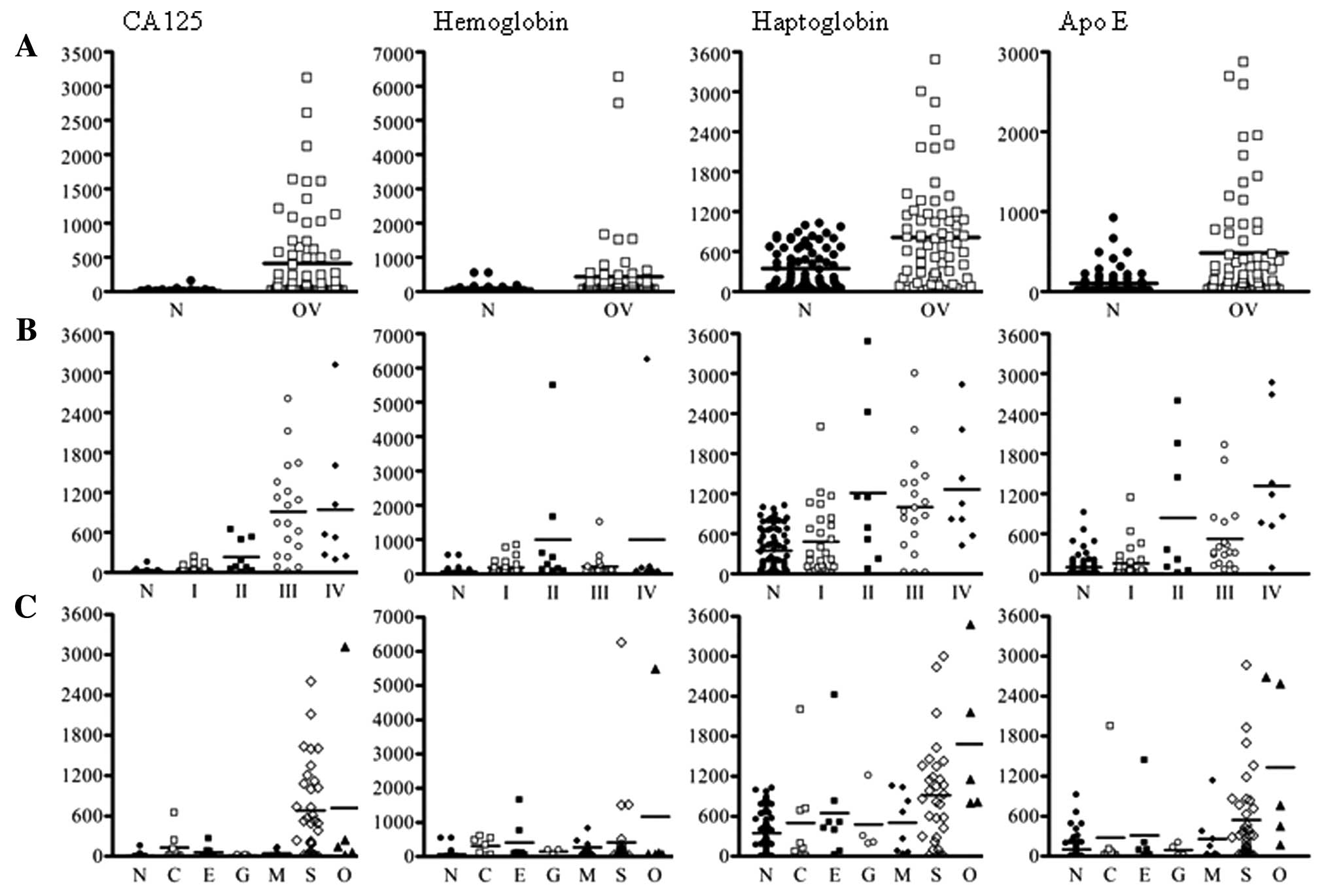 | Figure 1Scatter plots of concentrations of
CA125, hemoglobin, haptoglobin, and apolipoprotein E. (A) Normal
controls and ovarian cancer patients; (B) tumor stages in ovarian
cancer patients; (C) different histological subtypes in normal
controls and ovarian cancer patients. N, normal controls; C, clear
cell; E, endometrioid; G, granulosa cell; M, mucinous; S, serous;
O, other. |
 | Table IConcentration of serum markers with
clinicopathological findings in ovarian cancer patients. |
Table I
Concentration of serum markers with
clinicopathological findings in ovarian cancer patients.
| Characteristics | N (%) | CA125 (U/ml) | Hemoglobin
(μg/ml) | Haptoglobin
(μg/ml) | Apolipo E
(ng/ml) |
|---|
| Healthy normal
(control) | 76 (100%) | 11.5a | 69.0 | 347.6 | 100.1 |
| Ovarian cancer
patients | 69 (100%) | | | | |
| Age (years), mean
(range) | 50.1±14.0
(17–82) | | | | |
| FIGO stage |
| I | 29 (42.0%) | 42.3 | 188.8 | 479.0 | 157.0 |
| II | 9 (13.0%) | 230.9 | 992.7 | 1209.5 | 838.6 |
| III | 19 (27.5%) | 909.4 | 212.8 | 996.5 | 523.4 |
| IV | 9 (11.6%) | 943.7 | 995.6 | 1263.9 | 1318.5 |
| Histological
subtype |
| Serous | 33 (47.8%) | 681.7 | 417.8 | 916.9 | 541.3 |
| Mucinous | 10 (14.5%) | 42.4 | 269.8 | 506.6 | 255.6 |
| Clear cell | 9 (13.0%) | 129.6 | 305.8 | 498.0 | 276.7 |
| Endometrioid | 8 (11.6%) | 58.9 | 402.7 | 648.6 | 312.9 |
| Granulosa
cell | 4 (5.8%) | 13.6 | 147.8 | 478.2 | 91.7 |
| Other | 5 (7.2%) | 718.6 | 1167.3 | 1684.4 | 1334.0 |
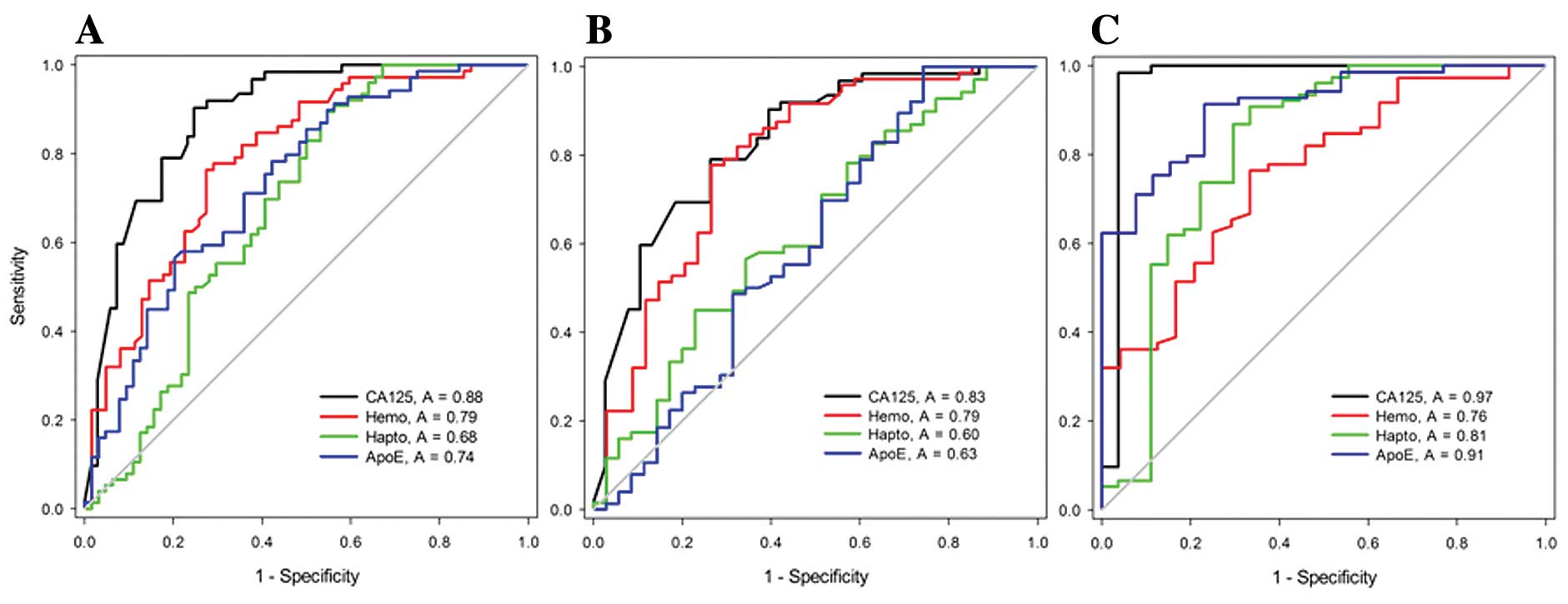
Comparison of the sensitivity and
specificity between four tumor markers alone and the combination of
four markers for the diagnosis of ovarian cancer
We compared the sensitivity and specificity between
each marker alone and the four markers in combination in order to
diagnose ovarian cancer using receiver operating characteristic
(ROC) analysis. In this study, we used cut-off values of 35 U/ml,
71.6 μg/ml, 1,007 μg/ml, and 248.4 ng/ml for CA125, hemoglobin,
haptoglobin, and apolipoprotein E, respectively, for better
diagnostic accuracy for the samples tested. By using these cut-off
values, we were able to minimize the rates of false-positive and
false-negative findings in the differentiation of normal controls
from subjects with ovarian cancer. The sensitivity and specificity
of individual markers with CA125, hemoglobin, haptoglobin, and
apolipoprotein E were 88.5 and 75.3%, 80.0 and 66.1%, 68.0 and
59.4%, and 73.6 and 59.4%, respectively (Fig. 2A).
The sensitivity and specificity of the individual
markers for early-stage (stages I and II) were 82.9 and 63.2%, 78.9
and 70.2%, 62.8 and 48.6%, and 59.6 and 57.1%, respectively
(Fig. 2B). And the sensitivity and
specificity of the individual markers for late-stage (stages III
and IV) were 96.5 and 96.3%, 75.9 and 66.7%, 81.2 and 70.4%, and
90.8 and 76.9%, respectively (Fig.
2C). The sensitivities and specificities for discriminating
between ovarian cancer and healthy tissue are shown in Table II. For CA125 alone, at 90% (95% CI,
82–96%) specificity, overall sensitivity was 59% (95% CI, 46–72%),
45% (95% CI, 32–58%) for stages I+II, and 98% (95% CI, 91–99%) for
stages III+IV. At 95% (95% CI, 82–96%) specificity, overall
sensitivity was 41% (95% CI, 32–58%) for all stages, 36% (95% CI,
24–48%) for stages I+II and 96% (95% CI, 88–99%) for stages
III+IV.
 | Table IISensitivities and specificities. |
Table II
Sensitivities and specificities.
| CA125 | Hemoglobin | Haptoglobin | Apolipo E | All markers |
|---|
|
|
|
|
|
|
|---|
| Patient group | SNa | SPb | SN | SP | SN | SP | SN | SP | SN | SP |
|---|
| All stages | 99 | 42 | 99 | 13 | 99 | 32 | 99 | 16 | 99 | 24 |
| 95 | 62 | 95 | 42 | 95 | 36 | 95 | 27 | 95 | 70 |
| 90 | 75 | 90 | 52 | 90 | 43 | 90 | 44 | 90 | 87 |
| 59 | 90 | 36 | 90 | 7 | 90 | 7 | 90 | 86 | 90 |
| 41 | 95 | 32 | 95 | 6 | 95 | 16 | 95 | 75 | 95 |
| Stage I+II | 99 | 13 | 99 | 15 | 99 | 26 | 99 | 11 | 99 | 29 |
| 95 | 45 | 95 | 44 | 95 | 26 | 95 | 14 | 95 | 29 |
| 90 | 61 | 90 | 56 | 90 | 29 | 90 | 23 | 90 | 71 |
| 45 | 90 | 32 | 90 | 8 | 90 | 17 | 90 | 76 | 90 |
| 36 | 95 | 22 | 95 | 4 | 95 | 16 | 95 | 68 | 95 |
| Stage III+IV | 99 | 89 | 99 | 8 | 99 | 44 | 99 | 23 | 99 | 97 |
| 95 | 96 | 95 | 33 | 95 | 52 | 95 | 46 | 95 | 97 |
| 90 | 96 | 90 | 38 | 90 | 67 | 90 | 77 | 90 | 100 |
| | | 36 | 90 | 7 | 90 | 71 | 90 | 100 | 90 |
| | | 36 | 95 | 7 | 95 | 62 | 95 | 100 | 95 |
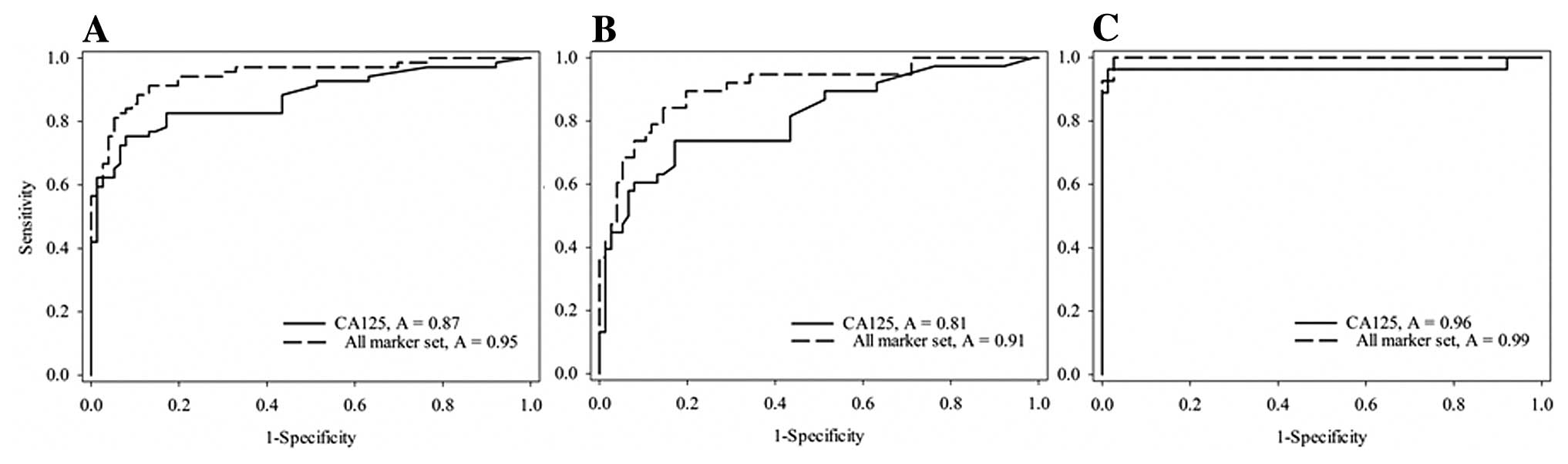
When CA125 was combined with the biomarkers
(hemoglobin, haptoglobin, and apolipoprotein E), the overall
sensitivity and specificity were significantly improved in the ROC
curve, which showed 95 and 75% sensitivity and specificity,
respectively (Fig. 3A). At a very
high sensitivity of 99% there was a loss in specificity to 24% for
all four tumor markers compared to 42% for CA125 alone. At this
sensitivity, however, the specificity was higher for all markers in
the stage I+II patient group (Fig.
3B). The strength of the markers at this high sensitivity is
restricted to the stage III+IV group with a specificity at 97%
(Fig. 3C). At 95% specificity for
all stages the sensitivity increased to 75% compared to 41% for
CA125 alone. For stage I+II increased the sensitivity to 68% from
36% for CA125 alone. For stage III+IV the corresponding values
were, respectively, 100 and 95%, suggesting that the four biomarker
set classified early-stage cancers with 68% sensitivity and
late-stage cancers with 100% sensitivity at 95% specificity, which
was significantly higher than CA125 alone.
Discussion
In the present study, we evaluated, for the first
time, a new combination of four known biomarkers of ovarian cancer,
CA125, hemoglobin, haptoglobin, and apolipoprotein E, in an attempt
to improve the sensitivity and specificity of the diagnosis of
ovarian cancer. Moreover, this study effectively presented the
validation of the use of a multiplex liquid assay system for the
simultaneous detection of several biomarkers for the diagnosis of
ovarian cancer.
CA125 has been a potentially useful marker for
diagnosis and prognosis after treatment (surgery or conventional
therapies) of ovarian cancer, but it is often not elevated in
clinically-detected ovarian cancers, and is also frequently
elevated in women with benign ovarian cancers (15–17).
The cut-off 35 U/ml for CA125 we used is generally accepted
(18). Due to the vulnerable points
of CA125 as a biomarker of ovarian cancer (19), combining one or more other tumor
markers with CA125 might improve the sensitivity and specificity of
the diagnosis of ovarian cancers or the earlier detection of such
cancers.
We have identified and verified hemoglobin-α and -β
as new serum biomarkers of ovarian cancer using proteomic
technologies and ELISA (20).
Following our identification of hemoglobin as an ovarian cancer
biomarker, four serum proteins, including hemoglobin β, was
identified as serum biomarkers of early stage ovarian cancer using
micro-LC-MS/MS and ELISA analysis (21). Consistent with these previous
reports, the serum level of hemoglobin in ovarian cancer was
significantly higher than that in normal controls in this study.
The level of hemoglobin was higher in stage II cancers, but was not
stage-dependent, while that of CA125 was much higher in late stages
(III and IV) of ovarian cancer than in early stages. These findings
suggest that combination of CA125 with hemoglobin could compensate
for the weakness of CA125 in the detection of early stage ovarian
cancer. However, to evaluate the validation of hemoglobin as a
biomarker for early detection, extended case numbers of early and
late stage cancers are required for comparison, as the numbers of
cases presented here are small.
In a manner similar to other acute-phase proteins,
haptoglobin also originates mainly from the liver, and elevation of
this peptide could be observed in infections, inflammation, and
various malignant diseases, including lung and bladder cancers
(22–24), leukemia (25), breast cancer (26), and urogenital tumors (27). Haptoglobin-α has been suggested as a
serum biomarker using surface-enhanced laser desorption and
ionization, and subsequently identified this as the α chain of
haptoglobin using ELISA (28). In
addition, it was reported that significantly elevated haptoglobin
concentrations were associated with poor survival rates in women
with ovarian cancer, and chemotherapeutic treatment reduced the
serum level of haptoglobin, thus indicating a role of haptoglobin
in the monitoring of patients undergoing chemotherapy (29), as well as in the diagnosis of
ovarian cancer (30,31).
Apolipoprotein E, was among the genes that were
highly up-regulated in ovarian cancer, as identified by serial
analysis of gene expression (SAGE); this was further validated
through immunohistochemical analysis (32). In addition, the expression of
apolipoprotein E was frequently detected in ovarian serous
carcinomas, the most common and lethal type of ovarian cancer, but
not in serous borderline tumors or normal ovarian surface
epithelium (33,34). Even though these findings strongly
suggested that apolipoprotein E might function as a potent
biomarker of ovarian cancer, the present study is the first trial
to use apolipoprotein E as a serum biomarker of ovarian cancer. Our
data show that the serum level of apolipoprotein E was elevated in
ovarian cancer patients, and was the highest in serous carcinoma.
These data were in agreement with the previously reported result
that apolipoprotein E was highly expressed in ovarian serous
carcinoma (35). Thus, these
combined results indicate that apolipoprotein E in serum might be a
product that is released from tumors, and could be a direct
predictor of ovarian cancer.
However, when applied individually, three of the
markers studied here did not surpass CA125 in their sensitivities
and specificities in the diagnosis of ovarian cancer. Combining
individual markers has been attempted by other researchers as one
strategy to enhance the overall ovarian cancer detection rate
(19,36–38).
We applied the combination of the three serum markers with CA125,
and compared the sensitivities and specificities between the
combination of the four markers and each marker alone. Results from
ROC curve analysis show that combining four biomarkers had a much
improved sensitivity over that of each biomarker alone. At a high
sensitivity of 90%, combining four biomarkers resulted in a slight
increase in the specificity to 87% compared to 75% for CA125 alone.
At a high specificity of 90%, there was a gain in sensitivity to
27% for the four biomarker set. At a 95% specificity for all stages
the sensitivity increased to 75% compared to 41% for CA125 alone.
The four biomarker set classified early-stage cancers with 68%
sensitivity and late-stage cancers with 100% sensitivity at 95%
specificity. The sensitivity and specificity of this panel for
stage III+IV are comparable to results with a four biomarker panel
selected from 96 candidate antigens measured by immunoassays with
multiplex techniques (39). The
high specificity and corresponding increases in sensitivity for all
four biomarkers have merit in ovarian cancer screening trials.
Thus, a large number of ovarian cancer patients and a healthy
control cohort would be required to further improve the specificity
and sensitivity of the combined biomarkers in both retrospective
and prospective clinical trials and lead to increased survival
(6,40).
We performed a multimarker bead-based immunoassay
for the detection of these biomarkers in the serum from normal
control and ovarian cancer patients using a multiplex liquid assay
system, Luminex 100. This immunoassay system has several benefits
for the immunoassay using clinical samples compared with the
conventional enzyme-linked immunosorbent assay techniques and
proteomic based analyses (41). i)
This system requires only a small sample volume for simultaneous
detections for several markers, so in the event that several assays
for the detection of several markers should be required with very
small or rare samples from patients (39), this system is very beneficial. ii)
Time and cost can be saved due to multiplexing. iii) When new
biomarkers are identified from high-throughput screening and need
to be verified, they can be measured with previously established
markers at the same time and in the same well, resulting in
reducing experimental errors or variations. However, there are some
difficulties inherent to the set up for multiplexing. Since proper
concentrations of antibodies in bead-antibody conjugation according
to the antibodies present, the concentration of each antibody
should be experimentally determined. A good pair of capture
antibody and detection antibody should be determined, and
cross-reactivity among different antibodies for multiplexing should
be avoided. Several commercially available Luminex multiplex panels
were compared with conventional commercial ELISAs for measurement
of biomarkers in human plasma that are associated with obesity and
inflammation (42). The correlation
between Luminex multiplexed assays and ELISAs was good for some
analytes, but some with very low plasma concentrations showed low
assay sensitivity and poor correlations, thus suggesting that the
Luminex multiplex system might be considered when attempting to
detect analytes present at very low concentrations in serum.
Although the Luminex multiplex assay system has the complexities
mentioned above, this technology will be very useful and convenient
in clinical studies with a large number of samples once the most
appropriate conditions are determined.
The present study showed significant improvement of
sensitivity for the diagnosis of ovarian cancer when using a
combination of new serum biomarkers, including CA125, hemoglobin,
haptoglobin, and apolipoprotein E, using a multiplex liquid assay
system. Further studies are going to be extended to a large number
of ovarian cancer patients in early and late stages, as well as
healthy women, in order to confirm the validity of the combination
of these markers for the diagnosis at an early stage of ovarian
cancer.
Acknowledgements
The study was supported by the National Research
Foundation of Korea (NRF), Seoul, Republic of Korea (Grant no.
5-2011-A0154-00120).
References
|
1
|
Badgwell D and Bast RC Jr: Early detection
of ovarian cancer. Dis Markers. 23:397–410. 2007. View Article : Google Scholar
|
|
2
|
Bast RC Jr: Early detection of ovarian
cancer: new technologies in pursuit of a disease that is neither
common nor rare. Trans Am Clin Climatol Assoc. 115:233–248.
2004.PubMed/NCBI
|
|
3
|
Morice P, Brehier-Ollive D, Rey A, et al:
Results of interval debulking surgery in advanced stage ovarian
cancer: an exposed-non-exposed study. Ann Oncol. 14:74–77. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Integrated genomic analyses of ovarian
carcinoma. Nature. 474:609–615. 2011. View Article : Google Scholar
|
|
5
|
Donach M, Yu Y, Artioli G, et al: Combined
use of biomarkers for detection of ovarian cancer in high-risk
women. Tumour Biol. 31:209–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hensley ML: A step forward for two-step
screening for ovarian cancer. J Clin Oncol. 28:2128–2130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liede A, Karlan BY, Baldwin RL, Platt LD,
Kuperstein G and Narod SA: Cancer incidence in a population of
Jewish women at risk of ovarian cancer. J Clin Oncol. 20:1570–1577.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tcherkassova J, Abramovich C, Moro R, Chen
C, Schmit R and Gerber A: Combination of CA125 and RECAF biomarkers
for early detection of ovarian cancer. Tumour Biol. 32:831–838.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Einhorn N, Sjovall K, Knapp RC, et al:
Prospective evaluation of serum CA 125 levels for early detection
of ovarian cancer. Obstet Gynecol. 80:14–18. 1992.PubMed/NCBI
|
|
10
|
Aslam N, Ong C, Woelfer B, Nicolaides K
and Jurkovic D: Serum CA125 at 11–14 weeks of gestation in women
with morphologically normal ovaries. BJOG. 107:689–690. 2000.
|
|
11
|
Menon U and Jacobs IJ: Recent developments
in ovarian cancer screening. Curr Opin Obstet Gynecol. 12:39–42.
2000. View Article : Google Scholar
|
|
12
|
Predanic M: Differentiating tubal abortion
from viable ectopic pregnancy with serum CA-125 and beta-human
chorionic gonadotropin determinations. Fertil Steril. 73:522–525.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Bast RC Jr, Yu Y, et al: Three
biomarkers identified from serum proteomic analysis for the
detection of early stage ovarian cancer. Cancer Res. 64:5882–5890.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta D and Lis CG: Role of CA125 in
predicting ovarian cancer survival - a review of the
epidemiological literature. J Ovarian Res. 2:132009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jacobs I and Bast RC Jr: The CA 125
tumour-associated antigen: a review of the literature. Hum Reprod.
4:1–12. 1989.PubMed/NCBI
|
|
16
|
Kim KA, Park CM, Lee JH, et al: Benign
ovarian tumors with solid and cystic components that mimic
malignancy. AJR Am J Roentgenol. 182:1259–1265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Helzlsouer KJ, Bush TL, Alberg AJ, Bass
KM, Zacur H and Comstock GW: Prospective study of serum CA-125
levels as markers of ovarian cancer. JAMA. 269:1123–1126. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bast RC Jr, Klug TL, St John E, et al: A
radioimmunoassay using a monoclonal antibody to monitor the course
of epithelial ovarian cancer. N Engl J Med. 309:883–887. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke CH, Yip C, Badgwell D, et al:
Proteomic biomarkers apolipoprotein A1, truncated transthyretin and
connective tissue activating protein III enhance the sensitivity of
CA125 for detecting early stage epithelial ovarian cancer. Gynecol
Oncol. 122:548–553. 2011. View Article : Google Scholar
|
|
20
|
Woong-Shick A, Sung-Pil P, Su-Mi B, et al:
Identification of hemoglobin-alpha and -beta subunits as potential
serum biomarkers for the diagnosis and prognosis of ovarian cancer.
Cancer Sci. 96:197–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozak KR, Su F, Whitelegge JP, Faull K,
Reddy S and Farias-Eisner R: Characterization of serum biomarkers
for detection of early stage ovarian cancer. Proteomics.
5:4589–4596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beckman G, Eklund A, Frohlander N and
Stjernberg N: Haptoglobin groups and lung cancer. Hum Hered.
36:258–260. 1986. View Article : Google Scholar
|
|
23
|
Benkmann HG, Hanssen HP, Ovenbeck R and
Goedde HW: Distribution of alpha-1-antitrypsin and haptoglobin
phenotypes in bladder cancer patients. Hum Hered. 37:290–293. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdullah M, Schultz H, Kahler D, et al:
Expression of the acute phase protein haptoglobin in human lung
cancer and tumor-free lung tissues. Pathol Res Pract. 205:639–647.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitchell RJ, Carzino R and Janardhana V:
Associations between the two serum proteins haptoglobin and
transferrin and leukaemia. Hum Hered. 38:144–150. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Awadallah SM and Atoum MF: Haptoglobin
polymorphism in breast cancer patients form Jordan. Clin Chim Acta.
341:17–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dunzendorfer U, Jung K and Ohlenschlager
G: Transferrin, C3 complement, haptoglobin, plasminogen and alpha
2-microglobulin in patients with urogenital tumors. Eur Urol.
6:232–236. 1980.PubMed/NCBI
|
|
28
|
Ye B, Cramer DW, Skates SJ, et al:
Haptoglobin-alpha subunit as potential serum biomarker in ovarian
cancer: identification and characterization using proteomic
profiling and mass spectrometry. Clin Cancer Res. 9:2904–2911.
2003.PubMed/NCBI
|
|
29
|
Zhao C, Annamalai L, Guo C, et al:
Circulating haptoglobin is an independent prognostic factor in the
sera of patients with epithelial ovarian cancer. Neoplasia. 9:1–7.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diamandis EP: Analysis of serum proteomic
patterns for early cancer diagnosis: drawing attention to potential
problems. J Natl Cancer Inst. 96:353–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmed N, Barker G, Oliva KT, et al:
Proteomic-based identification of haptoglobin-1 precursor as a
novel circulating biomarker of ovarian cancer. Br J Cancer.
91:129–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hough CD, Sherman-Baust CA, Pizer ES, et
al: Large-scale serial analysis of gene expression reveals genes
differentially expressed in ovarian cancer. Cancer Res.
60:6281–6287. 2000.PubMed/NCBI
|
|
33
|
Chen YC, Pohl G, Wang TL, et al:
Apolipoprotein E is required for cell proliferation and survival in
ovarian cancer. Cancer Res. 65:331–337. 2005.PubMed/NCBI
|
|
34
|
Shih Ie M and Kurman RJ: Ovarian
tumorigenesis: a proposed model based on morphological and
molecular genetic analysis. Am J Pathol. 164:1511–1518.
2004.PubMed/NCBI
|
|
35
|
McIntosh MW, Drescher C, Karlan B, et al:
Combining CA 125 and SMR serum markers for diagnosis and early
detection of ovarian carcinoma. Gynecol Oncol. 95:9–15. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosen DG, Wang L, Atkinson JN, et al:
Potential markers that complement expression of CA125 in epithelial
ovarian cancer. Gynecol Oncol. 99:267–277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anderson GL, McIntosh M, Wu L, et al:
Assessing lead time of selected ovarian cancer biomarkers: a nested
case-control study. J Natl Cancer Inst. 102:26–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Palmer C, Duan X, Hawley S, et al:
Systematic evaluation of candidate blood markers for detecting
ovarian cancer. PLoS One. 3:e26332008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yurkovetsky Z, Skates S, Lomakin A, et al:
Development of a multimarker assay for early detection of ovarian
cancer. J Clin Oncol. 28:2159–2166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang B, Barekati Z, Kohler C, et al:
Proteomics and biomarkers for ovarian cancer diagnosis. Ann Clin
Lab Sci. 40:218–225. 2010.PubMed/NCBI
|
|
41
|
West-Norager M, Kelstrup CD, Schou C,
Hogdall EV, Hogdall CK and Heegaard NH: Unravelling in vitro
variables of major importance for the outcome of mass
spectrometry-based serum proteomics. J Chromatogr B Analyt Technol
Biomed Life Sci. 847:30–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu MY, Xydakis AM, Hoogeveen RC, et al:
Multiplexed analysis of biomarkers related to obesity and the
metabolic syndrome in human plasma, using the Luminex-100 system.
Clin Chem. 51:1102–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|