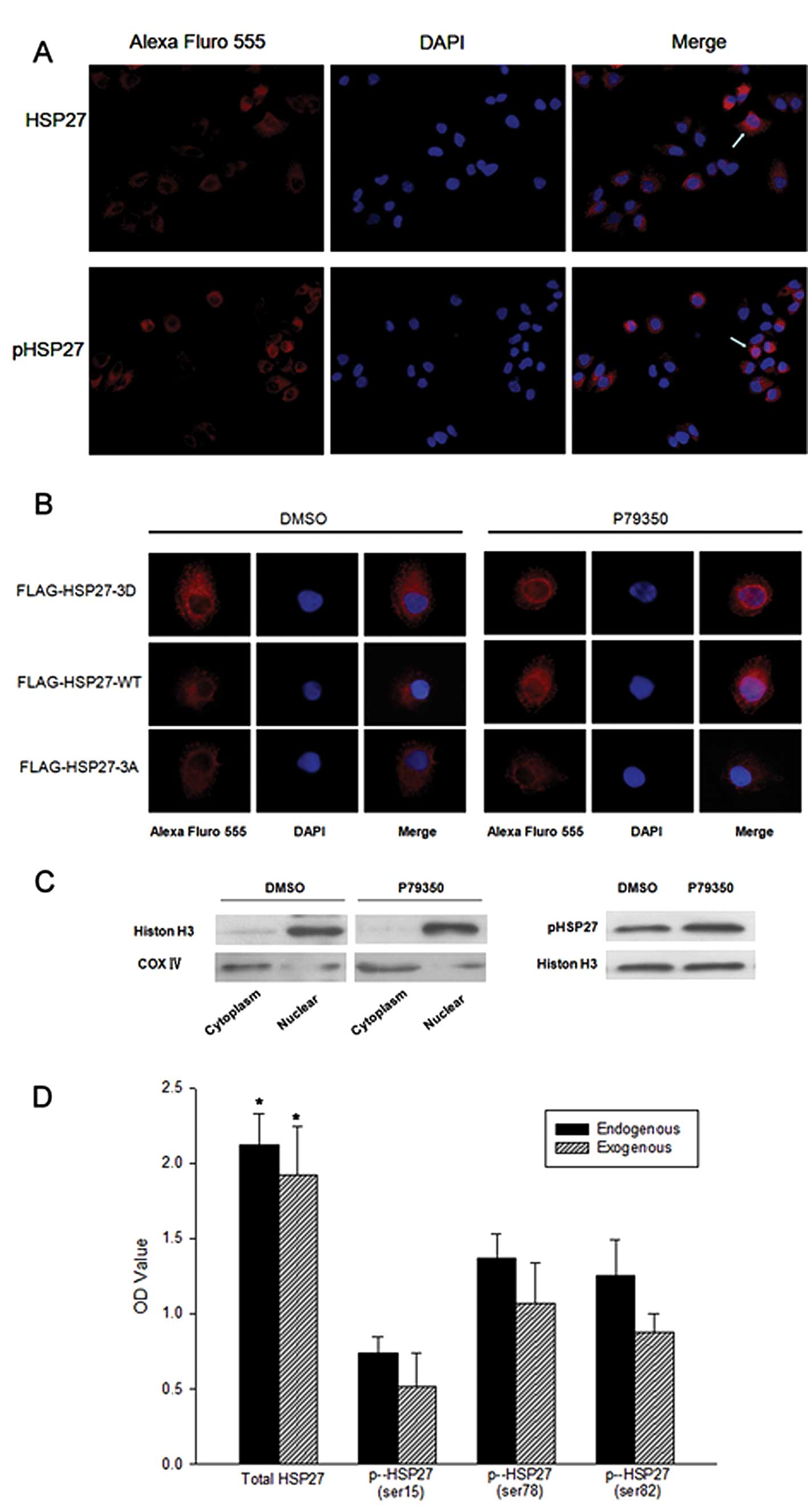
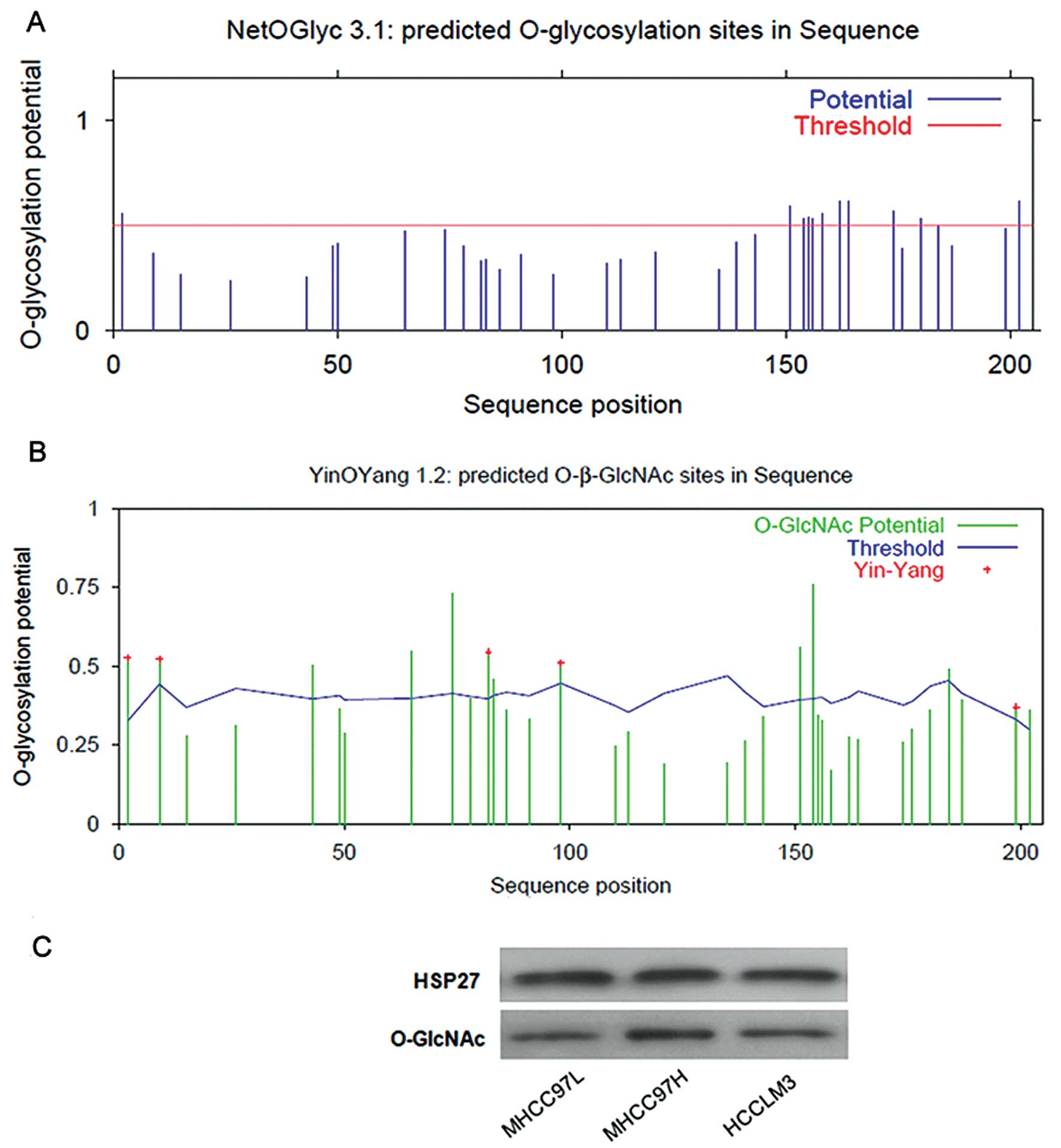
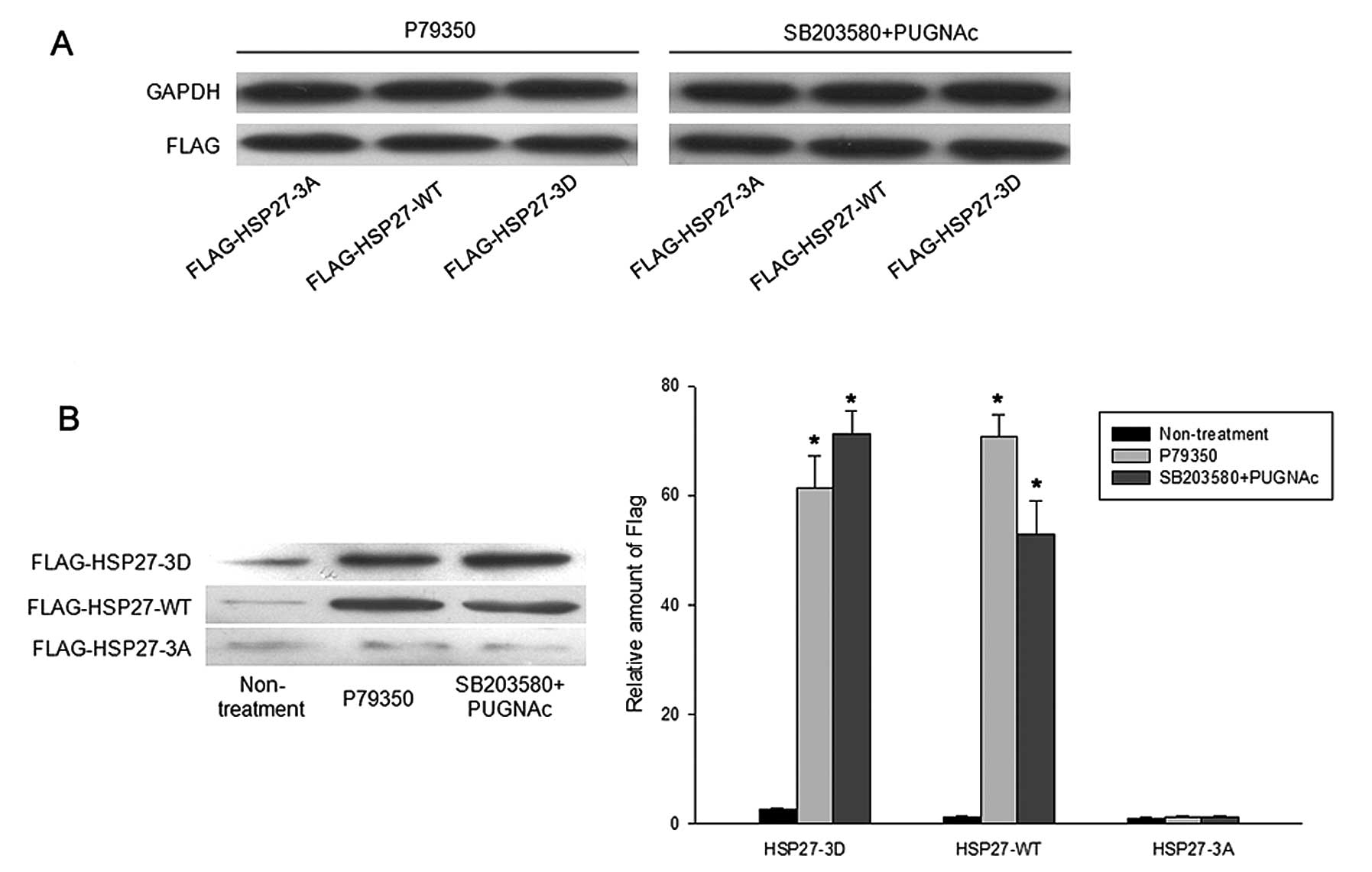
|
1
|
Sitia R and Molteni SN: Stress, protein
(mis)folding, and signaling: the redox connection. Sci STKE.
2004:pe272004.PubMed/NCBI
|
|
2
|
Isaacs JS, Xu WP and Neckers L: Heat shock
protein 90 as a molecular target for cancer therapeutics. Cancer
Cell. 3:213–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gimenez Ortiz A and Montalar Salcedo J:
Heat shock proteins as targets in oncology. Clin Transl Oncol.
12:166–173. 2010.PubMed/NCBI
|
|
4
|
Calderwood SK, Khaleque MA, Sawyer DB and
Ciocca DR: Heat shock proteins in cancer: chaperones of
tumorigenesis. Trends Biochem Sci. 31:164–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arrigo AP, Simon S, Gibert B, Kretz-Remy
C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C and
Vicart P: Hsp27 (HspB1) and αB-crystallin (HspB5) as therapeutic
targets. FEBS Lett. 581:3665–3674. 2007.
|
|
6
|
Garrido C, Brunet M, Didelot C, Zermati Y,
Schmitt E and Kroemer G: Heat shock proteins 27 and 70:
anti-apoptotic proteins with tumorigenic properties. Cell Cycle.
5:2592–2601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Concannon CG, Gorman AM and Samali A: On
the role of Hsp27 in regulating apoptosis. Apoptosis. 8:61–70.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hassan S, Biswas MH, Zhang C, Du C and
Balaji KC: Heat shock protein 27 mediates repression of androgen
receptor function by protein kinase D1 in prostate cancer cells.
Oncogene. 284:386–4396. 2009.PubMed/NCBI
|
|
9
|
Guo K, Kang NX, Li Y, Sun L, Gan L, Cui
FJ, Gao MD and Liu KY: Regulation of HSP27 on NF-κB pathway
activation may be involve d in metastatic hepatocellular carcinoma
cells apoptosis. BMC Cancer. 9:1002009.
|
|
10
|
Guo K, Liu Y, Zhou H, Dai Z, Zhang J, Sun
R, Chen J, Sun Q, Lu W, Kang X and Chen P: Involvement of protein
kinase C β-extracellular signal-regulating kinase1/2/p38
mitogen-activated protein kinase-heat shock protein 27 activation
in hepatocellular carcinoma cell motility and invasion. Cancer Sci.
99:486–496. 2008.
|
|
11
|
Bryantsev AL, Chechenova MB and Shelden
EA: Recruitment of phosphorylated small heat shock protein Hsp27 to
nuclear speckles without stress. Exp Cell Res. 313:195–209. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michaud S, Lavoie S, Guimond MO and
Tanguay RM: The nuclear localization of Drosophila Hsp27 is
dependent on a monopartite arginine-rich NLS and is uncoupled from
its association to nuclear speckles. Biochim Biophys Acta.
1783:1200–1210. 2008.PubMed/NCBI
|
|
13
|
Zhang D, Wong LL and Koay ES:
Phosphorylation of Ser78 of Hsp27 correlated with HER-2/neu status
and lymph node positivity in breast cancer. Mol Cancer. 6:522007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Den Engelsman J, Gerrits D, de Jong WW,
Robbins J, Kato K and Boelens WC: Nuclear import of αB-crystallin
is phosphorylation-dependent and hampered by hyperphosphorylation
of the myopathy-related mutant R120G. J Biol Chem. 280:37139–37148.
2005.
|
|
15
|
Geum D, Son GH and Kim K:
Phosphorylation-dependent cellular localization and
thermoprotective role of heat shock protein 25 in hippocampal
progenitor cells. J Biol Chem. 277:19913–19921. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked β-N-acetylglucosamine on nucleocytoplasmic
proteins. Nature. 446:1017–1022. 2007.
|
|
17
|
Slawson C and Hart GW: Dynamic interplay
between O-GlcNAc and O-phosphate: the sweet side of protein
regulation. Curr Opin Struct Biol. 13:631–636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wells L, Vosseller K and Hart GW:
Glycosylation of nucleocytoplasmic proteins: signal transduction
and O-GlcNAc. Science. 291:2376–2378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zachara NE and Hart GW: The emerging
significance of O-GlcNAc in cellular regulation. Chem Rev.
102:431–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adhikari AS, Sridhar Rao K, Rangaraj N,
Parnaik VK and Mohan Rao C: Heat stress-induced localization of
small heat shock proteins in mouse myoblasts: intranuclear lamin
A/C speckles as target for αB-crystallin and Hsp25. Exp Cell Res.
299:393–403. 2004.PubMed/NCBI
|
|
21
|
Lasa M, Mahtani KR, Finch A, Brewer G,
Saklatvala J and Clark AR: Regulation of cyclooxygenase 2 mRNA
stability by the mitogen-activated protein kinase p38 signaling
cascade. Mol Cell Biol. 204:265–4274. 2000.PubMed/NCBI
|
|
22
|
Hedges JC, Dechert MA, Yamboliev IA,
Martin JL, Hickey E, Weber LA and Gerthoffer WT: A role for
p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol
Chem. 274:24211–24219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benndorf R, Engel K and Gaestel M:
Analysis of small Hsp phosphorylation. Methods Mol Biol.
99:431–445. 2000.PubMed/NCBI
|
|
24
|
McClaren M and Isseroff RR: Dynamic
changes in intracellular localization and isoforms of the 27-kD
stress protein in human keratinocytes. J Invest Dermatol.
102:375–381. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vosseller K, Wells L and Hart GW:
Nucleocytoplasmic O-glycosylation: O-GlcNAc and functional
proteomics. Biochimie. 83:575–581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kouzarides T: Acetylation: a regulatory
modification to rival phosphorylation. EMBO J. 19:1176–1179. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nielsen H, Brunak S and von Heijne G:
Machine learning approaches for the prediction of signal peptides
and other protein sorting signals. Protein Eng. 12:3–9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Love DC and Hanover JA: The hexosamine
signaling pathway: deciphering the ‘O-GlcNAc code’. Sci STKE.
2005.re132005.PubMed/NCBI
|
|
29
|
Bryantsev AL, Kurchashova SY, Golyshev SA,
Polyakov VY, Wunderink HF, Kanon B, Budagova KR, Kabakov AE and
Kampinga HH: Regulation of stress-induced intracellular sorting and
chaperone function of Hsp27 (HspB1) in mammalian cells. Biochem J.
407:407–417. 2007. View Article : Google Scholar : PubMed/NCBI
|