Introduction
Overactivation of the coagulation system is a widely
described pathology in cancer patients (1). Hypercoagulation of plasma in cancer
patients can initiate thrombosis and also promote the progression
of cancer. The risk of venous thromboembolism (VTE) is increased
several-fold in cancer patients (2). Many coagulation factors are involved
in the development of cancer and VTE (3–5).
Thrombin, an important coagulation factor, is a trypsin-like serine
protease which plays a pivotal role in both VTE and the progression
of cancer (6, 7). Local and systemic thrombin production
is increased in cancer patients, and thrombin is produced by both
tumor cells and tumor-associated platelets in the tumor
microenvironment (8–10).
Previous research has suggested that
thrombin-induced tumor invasion and metastasis are mainly mediated
by protease-activated receptor 1 (PAR1). PAR1 is highly expressed
in many cancer cells, especially in cancer cell lines with high
metastasis potential (11–13). PAR1 belongs to a group of seven
transmembrane receptors on the cell surface. The catalytic domain
and exosite I domain of thrombin are both essential for PAR1
activation. The thrombin exosite I domain interacts with the
hirudin (HV)-like sequence in the amino-terminal exodomain of PAR1,
and cleavage of the PAR1 amino-terminal exodomain by the thrombin
catalytic domain exposes a new amino-end, which serves as a
tethered ligand for the PAR1 receptor and leads to activation of
internal G-proteins.
It is widely recognized that integrins play a
significant role in cancer cell invasion and metastasis. A
relationship between thrombin and integrins has been reported in
various tumor cells (14–16). Thrombin can affect the expression or
distribution of integrins on the tumor cell surface (15–17).
The Arg-Gly-Asp (RGD)-containing snake venom peptide and synthetic
peptides can block thrombin-mediated tumor cell adhesion (15,18).
However, the mechanism by which thrombin activates integrins has
not yet been clearly described. To date, interactions between
thrombin and integrins have mainly been reported in endothelial
cells. However, there is considerable debate regarding the roles of
the thrombin active site and RGD sequence during thrombin-induced
integrin activation in endothelial cells. The thrombin RGD sequence
is highly conserved in almost all species during evolution
(19), implying that this sequence
may be important for the function of thrombin. However, thrombin
crystal structures show that the RGD sequence is buried within the
catalytic domain. Therefore, the exposure of RGD implies a
conformational change of thrombin (19–21).
Tsopanoglou et al(22)
reported that the catalytic site of thrombin is required for
thrombin-mediated upregulation of integrin
ανβ3 in human endothelial cells. However, the
catalytic site of thrombin is not essential in the immobilized
thrombin-mediated adhesion and migration of endothelial cells which
is dependent on the interaction between thrombin and integrin
ανβ3(22). In
addition, immobilized thrombin, or a synthetic thrombin peptide
which lacks the catalytic site of thrombin but contains the
thrombin RGD sequence can promote the attachment and migration of
endothelial cells via integrin ανβ3(23), indicating that the catalytic site of
thrombin is not essential for integrin activation. Another study
regarding smooth muscle cells has provided evidence that the
interaction of thrombin with integrin ανβ3 in
solution is inhibited by RGD mimetics. This raises the possibility
that exposure of the RGD sequence may also occur independently on
thrombin matrix attachment (24).
In 2005, Papaconstantinou et al directly demonstrated that
surface-absorbed thrombin promotes the attachment and migration of
endothelial cells via an interaction with
ανβ3 and α5β1
integrins. The RGD sequence of thrombin is exposed in crystal
structures of free thrombin grown in a high salt concentration,
independently of denaturation or thrombin proteolysis (25). These findings indicate that, via the
RGD sequence, thrombin can directly interact with integrins in
endothelial cells. However, the effect of immobilized thrombin on
tumor cells remains unknown. Considering the inconsistent results
observed in endothelial cells and lack of knowledge regarding the
effect of immobilized thrombin on tumor cells, it is necessary to
further investigate the interaction between thrombin, especially
immobilized thrombin, and integrins in tumor cells. Moreover, the
roles of the catalytic domain and the RGD sequence in
thrombin-mediated integrin activation have not yet been revealed in
tumor cells.
Though the roles of PAR1 and integrins in
thrombin-mediated tumor invasion have been well-characterized, it
is still not known how thrombin can simultaneously activate PAR1
and integrins on the cell surface. Additionally, it is intriguing
to investigate whether these receptors can cooperate in
thrombin-mediated tumor cell function. In this study, we used human
lung cancer cells to evaluate and compare the effect of native and
immobilized thrombin on PAR1 and integrin
ανβ5 in tumor cells.
Materials and methods
Reagents
Bovine thrombin, bovine serum albumin (BSA), and the
polypeptides GRGDS and SDGRG were purchased from Sigma (St. Louis,
MO, USA). The rabbit anti-human PAR1, mouse anti-p-Erk and rabbit
anti-Erk antibodies were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA) and the mouse anti-human integrin
ανβ5 antibody was obtained from R&D
Systems (Minneapolis, MN, USA). Horseradish peroxidase
(HRP)-labelled goat anti-mouse IgG and goat anti-rabbit IgG were
purchased from Jackson ImmunoResearch (West Grove, PA, USA). HV was
produced in our laboratory (purity >95%, with a specific
activity of 10,000 ATU/mg).
Cell culture
The human pulmonary adenocarcinoma cell line Glc-82
was obtained from the Kunming Cell Bank of the Chinese Academy of
Sciences (Kunming, China). The cells were cultured in RPMI-1640
medium containing 10% foetal bovine serum, in saturated humidity at
37°C with 5% CO2.
Preparation of rat tail collagen
The tails of Wistar rats were soaked in 75% ethanol
for 30 min, the skin was prepared and the tail was cut into 3-cm
fragments. The tail tendons were drawn out, cut into small
fragments, soaked in 0.1% acetic acid at 4°C for 48 h and collagen
was obtained by centrifugation at 200 × g for 30 min. The collagen
concentration was determined using the Lowry method. All animal
studies were approved by the Chinese Animal Research Ethics Board
and all animals received care in compliance with the Chinese
Convention on Animal Care.
Cell adhesion assay
For the native thrombin adhesion assay, Glc-82 cells
were grown for 16 h in serum-free medium, digested with 0.25%
trypsin, washed with PBS, resuspended in serum-free medium and 200
μl aliquots of 5×104 cells were transferred to a 96-well
plate coated with rat tail collagen (2 μg/well). For the antagonism
studies, the anti-PAR1, anti-integrin ανβ5
antibodies (10 μg/ml) or GRGDS (400 μM) were incubated with Glc-82
cells at 37°C for 15 min, and after removal of the antagonists,
thrombin (8 IU/ml) was added to the cells. HV (10 μg/ml) were
pre-incubated with thrombin at 37°C for 15 min, and then the
mixture was added to the Glc-82 cells.
For the immobilized thrombin adhesion assay, the
96-well plates were coated with thrombin (4 IU/ml, 40 μl/well)
instead of rat tail collagen, washed three times with PBS, then
blocked with 3% BSA for 1 h. The Glc-82 cells were serum-starved,
resuspended in serum-free medium and transferred to the
thrombin-coated 96-well plates as previously described. For the HV
and thrombin co-coating experiments, HV (0, 10 or 100 μg/ml) was
incubated with thrombin for 15 min, the 96-well plates were coated
with the mixture, and the cells were added to the plate. For the
experiments where HV was added to the media, 100 μg/ml HV was added
to the thrombin-coated plate for 15 min, then the cells were added.
For the other antagonists, the cells were pre-incubated with the
anti-PAR1 or anti-integrin ανβ5 antibodies,
GRGDS or the negative control SDGRG for 15 min. After removal of
the antagonists, the cells were added to the thrombin-coated
plate.
After a 1 h incubation period, non-adherent cells
were removed by washing with PBS. Adherent cells were fixed in 4%
paraformaldehyde for 30 min, washed in 0.1 M borate buffer (pH
8.5), stained with 1% methylene blue for 10 min, washed four times
in borate buffer, incubated with 0.1 M hydrochloric acid for 40 min
and absorbance was measured at 600 nm on a Multiskan Spectrum Plate
Reader (Thermo Electron Corporation, Waltham, MA, USA).
Cell migration assay
Glc-82 cells were grown in serum-free medium for 16
h prior to the migration assay. For the native thrombin-induced
cell migration assay, 1×106/ml Glc-82 cells were treated
with the indicated agonists or antagonists as described in the cell
adhesion assay section. Then 200 μl of the treated cells were added
to the upper wells of the Transwell migration chambers (0.8 μm,
Corning, NY, USA). For the immobilized thrombin induced cell
migration assay, the bottom of the Transwell migration chamber was
coated with thrombin (4 IU/ml) before the cell migration assay was
performed. Glc-82 cells were treated with the indicated agonists or
antagonists as described in the cell adhesion assay section.
The cells were allowed to migrate for 19 h, and the
chambers were then fixed with 4% paraformaldehyde for 30 min and
stained with 0.05% crystal violet for 10 min. After washing with
PBS, cells which had not migrated were wiped off and the chambers
were observed using an inverted Olympus IX70 microscope (Olympus,
Tokyo, Japan). The number of cells was counted in five fields of
view.
Western blotting
To investigate the effect of native thrombin on Erk
phosphorylation, Glc-82 cells were serum-starved for 24 h before
the addition of agonists or antagonists, as described in the cell
adhesion assay section. To investigate the effect of immobilized
thrombin on Erk phosphorylation, Glc-82 cells were plated on the
thrombin-coated culture flasks and treated as described in the cell
adhesion assay section.
After 1 h, the cells were washed twice with ice-cold
PBS, lysed in RIPA buffer and subjected to SDS-PAGE
electrophoresis. The proteins were transferred to PVDF membranes
(Millipore, MA, USA), and the membranes were washed and blocked
with 10% non-fat milk in TBS containing 0.1% Tween (TBS-T). The
blots were incubated with primary antibodies to Erk or p-Erk in 10%
non-fat milk/TBS-T. After washing with TBS-T, the membranes were
incubated with HRP-conjugated secondary antibodies for 45 min at
room temperature, washed with TBS-T and the bands were visualized
using the Pro-light HRP Chemiluminescence kit (Tiangen Biotech,
Beijing, China).
Rat tail collagen contraction assay
Serum-starved Glc-82 cells (250
μl/1×106/ml) were treated with antagonists mixed with
500 μl 2.4 mg/ml rat tail collagen and carefully plated in 6-well
plates containing 3 ml serum-free medium/well. The mixture was
allowed to polymerize at room temperature for 1 h, and was then
incubated at 37°C for 2–3 weeks and the diameter of the collagen
was measured.
Immunofluorescence
Glc-82 cells were seeded on sterile 22-mm glass
coverslips in 6-well plates, allowed to attach overnight, cultured
in serum-free medium for 24 h, treated with 1 IU/ml thrombin for 1
h at 37°C and then fixed in ice-cold methanol for 10 min. After
washing with PBS for 3 times, the cells were permeabilized in 0.1%
Triton X-100 for 15 min and blocked in 3% BSA for 30 min at room
temperature. The cells were incubated overnight with an
anti-integrin ανβ5 (1:100) and anti-PAR1
(1:50) primary antibodies, washed in PBS, then incubated for 60 min
with FITC-conjugated goat anti-mouse (1:100) and PE-conjugated goat
anti-rabbit (1:50) secondary antibodies, and cell nuclei were
stained using 4,6-diamidino-2-phenylindole (DAPI).
Immunofluorescence staining was viewed and analyzed
using a LSM 510 META confocal microscope (Carl Zeiss, Inc.,
Oberkochen, Germany).
For the immunofluorescence analysis of immobilized
thrombin-treated cells, the cells were plated on thrombin-coated
glass coverslips and allowed to attach for 24 h in serum-free
medium before immunofluorescent staining.
Statistical analysis
Numerical data are presented as mean ± SE. Each
experiment was repeated at least two times. Data were analyzed
using the Student’s t-test and one-way analysis of variance.
P<0.05 was considered significant and all statistical tests were
two-sided.
Results
Role of PAR1 and integrin
ανβ5 in thrombin-mediated Glc-82
adhesion
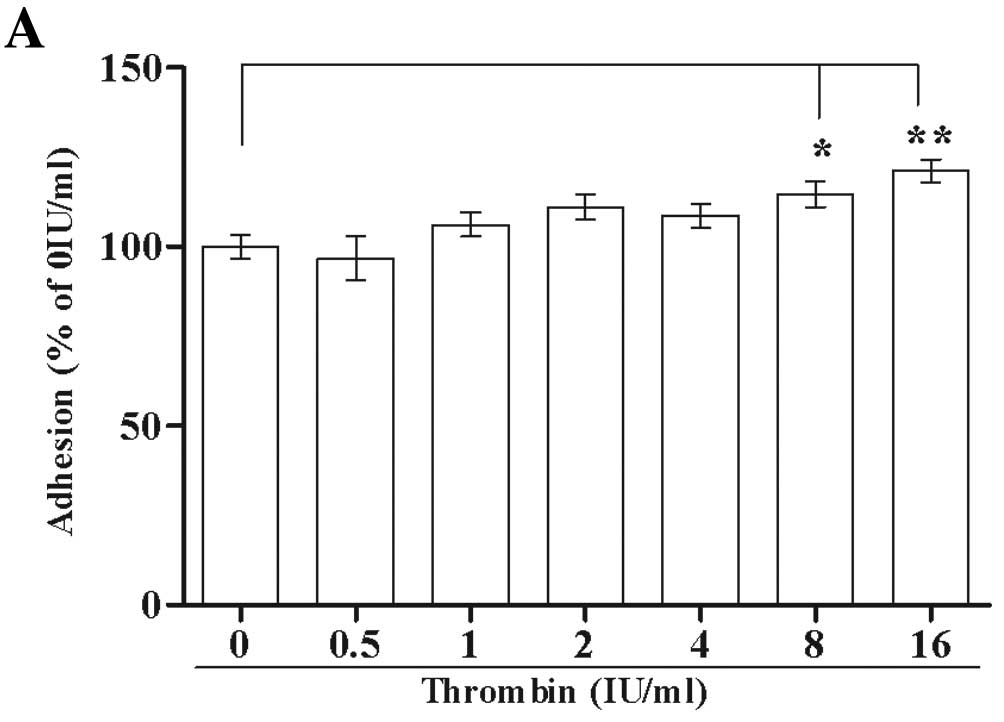
Different thrombin concentrations were used to
analyze the effect of native thrombin on the adhesion of Glc-82
cells to rat tail collagen. Native thrombin at high concentrations
(8 IU/ml, P<0.05, 16 IU/ml, P<0.01) significantly promoted
the adhesion of Glc-82 cells to rat tail collagen (Fig. 1A).
Immobilized thrombin could significantly stimulate
Glc-82 cell adhesion at low concentrations (1 or 2 IU/ml,
P<0.05), with the maximal effect observed at high concentrations
(4, 8 or 16 IU/ml, P<0.01; Fig.
1B).
To evaluate the roles of PAR1 and integrin
ανβ5 in the effects of native thrombin or
immobilized thrombin-induced Glc-82 cell adhesion, PAR1 antagonists
(HV and anti-PAR1 antibody) and integrin ανβ5
antagonists (GRGDS and anti-integrin ανβ5
antibody) were used. As shown in Fig.
2A, the ability of native thrombin to stimulate Glc-82 cell
adhesion was significantly attenuated by all of the PAR1 and
ανβ5 antagonists.
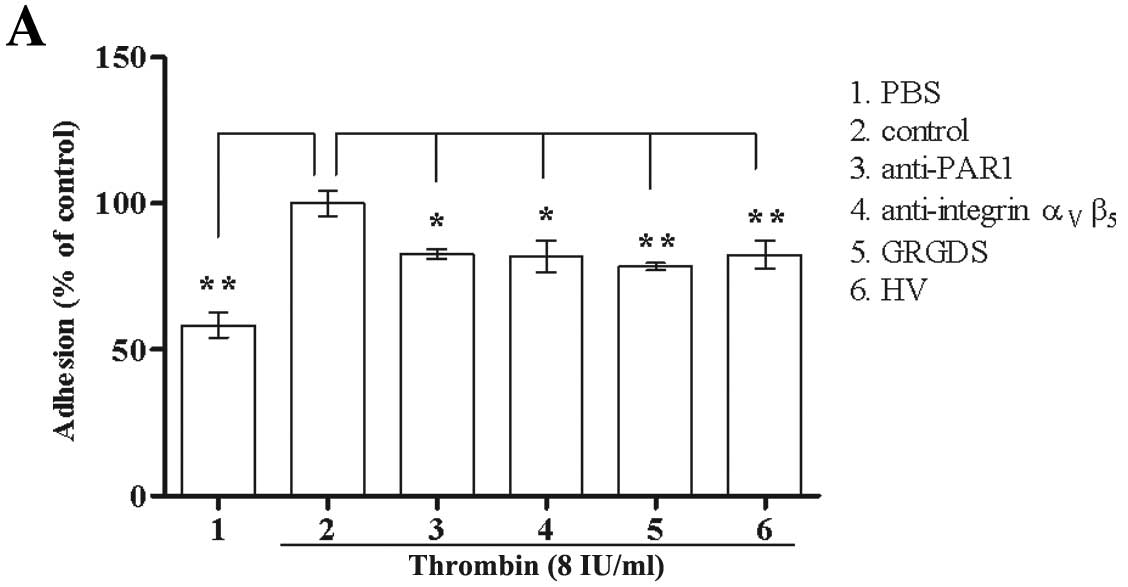 | Figure 2The effect of PAR1 and integrin
ανβ5 antagonists on thrombin-mediated Glc-82
cell adhesion. (A) The roles of PAR1 and integrin
ανβ5 in native thrombin-mediated Glc-82 cell
adhesion. Anti-PAR1, anti-integrin ανβ5
antibodies (10 μg/ml) or GRGDS (400 μM) were pre-incubated with
Glc-82 cells, then the antibodies were removed and 8 IU/ml thrombin
was added to the cells. HV (10 μg/ml) were pre-incubated with
thrombin, and the mixture was added to Glc-82 cells. The treated
cells from all groups were allowed to attach for 1 h. (B) The role
of HV in immobilized thrombin-mediated Glc-82 cell adhesion. HV (0,
10 or 100 μg/ml) was incubated with thrombin, the mixture was
coated on the plate or HV (100 μg/ml) in the media was added to the
thrombin-coated plate, and then the cells were added and allowed to
attach for 1 h. (C) The roles of PAR1 and integrin
ανβ5 in immobilized thrombin-mediated Glc-82
cell adhesion. The cells were pre-incubated with the anti-PAR1
antibody, anti-integrin ανβ5 antibody or
GRGDS, and then added to the thrombin-coated plate and allowed to
attach for 1 h. (D) The role of GRGDS in immobilized
thrombin-mediated Glc-82 cell adhesion. The cells were pretreated
with different concentrations of GRGDS or the negative control
SDGRG for 15 min, and then added to the thrombin-coated plate and
allowed to attach for 1 h (*P<0.05,
**P<0.01). |
HV in the culture media, and the anti-integrin
ανβ5 and anti-PAR1 antibodies did not affect
the ability of immobilized thrombin to induce Glc-82 cell adhesion
(Fig. 2B and 2C). When thrombin was
pre-incubated with HV and this mixture was coated on the plate, the
ability of immobilized thrombin to promote Glc-82 cell adhesion was
significantly attenuated (Fig. 2B).
The integrin ανβ5 antagonist GRGDS also
markedly inhibited the ability of immobilized thrombin to induce
Glc-82 cell adhesion, in a dose-dependent and specific manner
(Fig. 2C and D).
Role of PAR1 and integrin
ανβ5 in thrombin-mediated Glc-82
migration
Both native thrombin (0.5 IU/ml) and immobilized
thrombin (4 IU/ml) significantly promoted Glc-82 cell migration
(P<0.01, Fig. 3). All of the
PAR1 and integrin ανβ5 antagonists
significantly inhibited the ability of native thrombin to induce
cell migration (P<0.01, Fig.
3A). The anti-integrin ανβ5 antibody (10
μg/ml) and GRGDS (400 μM) significantly inhibited the ability of
immobilized thrombin to induce cell migration (P<0.01). The
anti-PAR1 antibody (10 μg/ml) and HV in the culture media had no
effect on the ability of immobilized thrombin to induce cell
migration; however, when HV was co-coated with thrombin on the
plate, it significantly decreased the ability of immobilized
thrombin to induce Glc-82 cell migration (P<0.01, Fig. 3B).
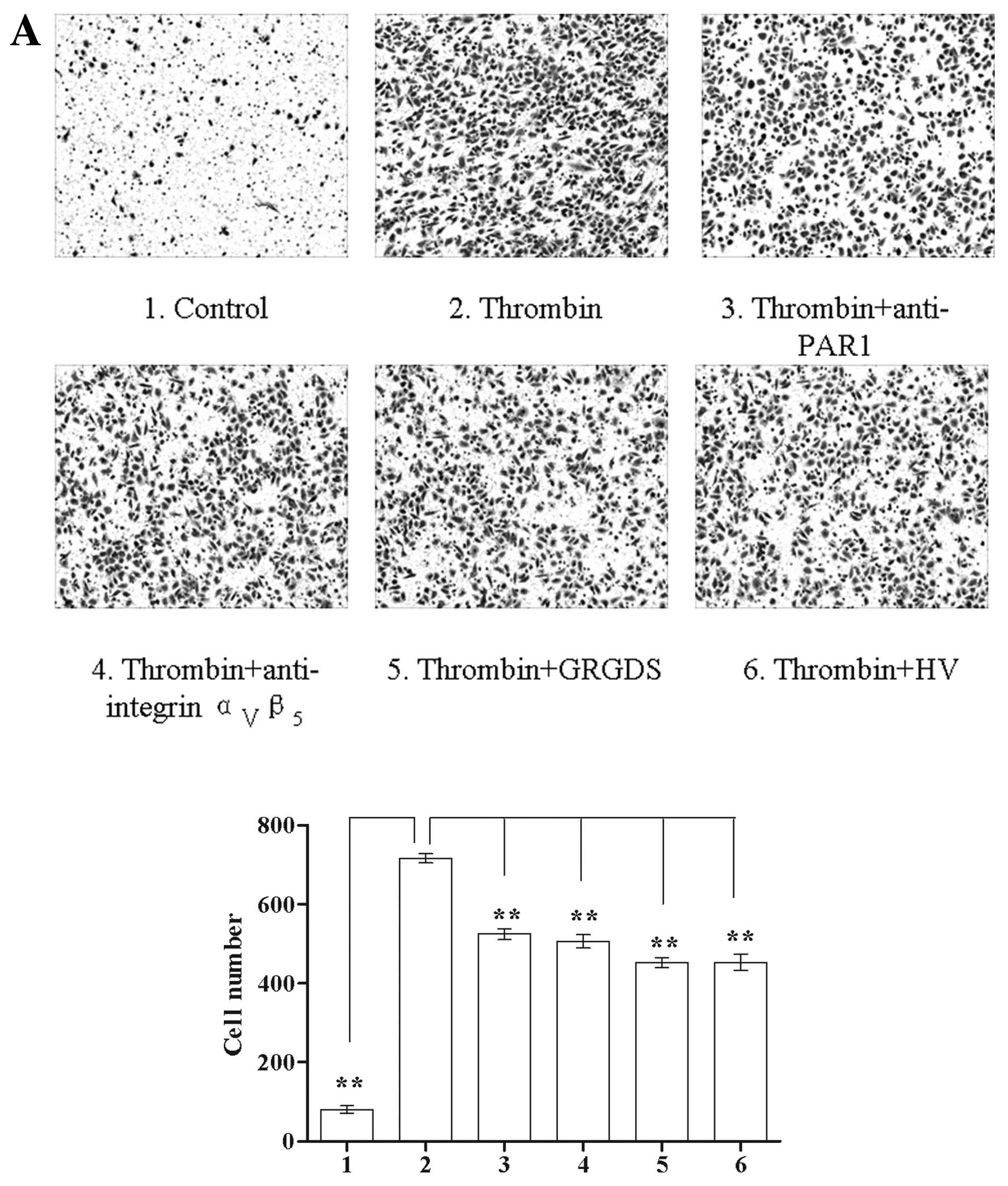 | Figure 3The effect of PAR1 and integrin
ανβ5 antagonists on thrombin-mediated Glc-82
cell migration. (A) The roles of PAR1 and integrin
ανβ5 in native thrombin-mediated Glc-82 cell
migration: 1, PBS control; 2, 0.5 IU/ml thrombin; 3–5, Glc-82 cells
were pre-incubated with 10μg/ml anti-PAR1, anti-integrin
ανβ5 antibody or 400 μM GRGDS, the antibodies
were removed and 0.5 IU/ml thrombin was added; 6, thrombin was
pre-incubated with 10 μg/ml HV and the mixture was added to Glc-82
cells; magnification, ×100. (B) The roles of PAR1 and integrin
ανβ5 in immobilized thrombin-mediated Glc-82
cell migration: 1, Glc-82 cells were added to a control BSA-coated
Transwell migration chamber; 2, 4 IU/ml thrombin-coated Transwell
migration chamber; 3–5, the cells were pre-treated with 10 μg/ml
anti-PAR1, anti-integrin ανβ5 antibody or 400
μM GRGDS and then added to a thrombin-coated Transwell migration
chamber; 6, 4 IU/ml thrombin and 10 μg/ml HV were co-coated on the
Transwell migration chamber before Glc-82 cells were added; 7, 10
μg/ml HV was pre-incubated in the thrombin-coated migration chamber
before Glc-82 cells were added. The cells were allowed to migrate
for 19 h, then stained with crystal violet and observed using a
inverted microscope; magnification, ×100
(**P<0.01). |
Role of PAR1 and integrin
ανβ5 in thrombin-mediated Erk phosphorylation
in Glc-82 cells
Native thrombin markedly stimulated Erk
phosphorylation in Glc-82 cells. Both the anti-integrin
ανβ5 antibody (10 μg/ml) and GRGDS (400 μM)
attenuated the ability of native thrombin to induce Erk
phosphorylation. The anti-PAR1 antibody and HV had no significant
effect on the ability of native thrombin to induce Erk
phosphorylation (Fig. 4A).
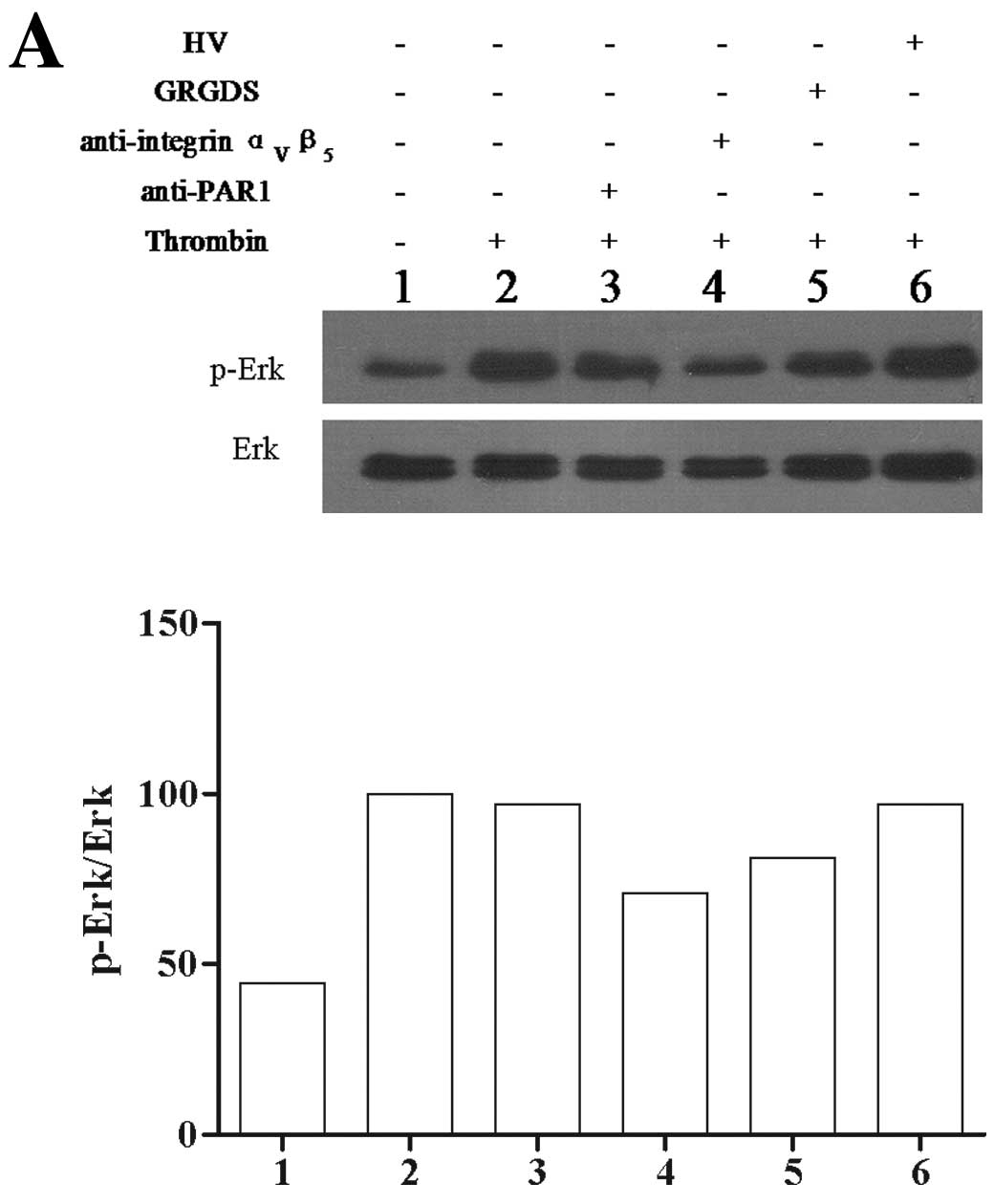 | Figure 4The effect of PAR1 and integrin
ανβ5 antagonists on thrombin-mediated Erk
phosphorylation in Glc-82 cells. (A) The effect of PAR1 and
integrin ανβ5 on native thrombin-mediated Erk
phosphorylation. Western blot and quantification of the Erk
phosphorylation/total Erk ratio in lane 1, Glc-82 cells treated
with PBS; lane 2, Glc-82 cells treated with 0.5 IU/ml thrombin;
lanes 3–5, Glc-82 cells pre-incubated with 10 μg/ml anti-PAR1,
anti-integrin ανβ5 antibody or 400 μM GRGDS,
then treated with 0.5 IU/ml thrombin; lane 6, Glc-82 cells treated
with thrombin which was pre-incubated with 10 μg/ml HV. (B) The
effect of PAR1 and integrin ανβ5 on
immobilized thrombin-mediated Erk phosphorylation. Western blot and
quantification of the Erk phosphorylation/total Erk ratio in Glc-82
cells treated with lane 1, BSA; lane 2, 4 IU/ml thrombin or lanes
3–5, incubated with 10 μg/ml anti-PAR1 or anti-integrin
ανβ5 antibody or 400 μM GRGDS and then plated
into 6-well plates coated with thrombin; lane 6, Glc-82 cells were
plated into wells co-coated with 4 IU/ml thrombin and 10 μ/ml HV;
or lane 7 10 μg/ml HV was pre-incubated in the thrombin-coated
plate before the cells were added to the plate. All data shown are
from a single representative experiment. |
Immobilized thrombin also increased Erk
phosphorylation in Glc-82 cells. The anti-integrin
ανβ5 antibody (10 μg/ml) and GRGDS (400 μM)
significantly inhibited the ability of immobilized thrombin to
induce Erk phosphorylation. The anti-PAR1 antibody and HV in the
culture media had no significant effect on the ability of
immobilized thrombin to induce Erk phosphorylation. When the
culture plates were co-coated with HV and thrombin, HV
significantly inhibited the ability of immobilized thrombin to
induce Erk phosphorylation (Fig.
4B).
Effect of PAR1 and integrin
ανβ5 on rat tail collagen contraction induced
by thrombin-treated Glc-82 cells
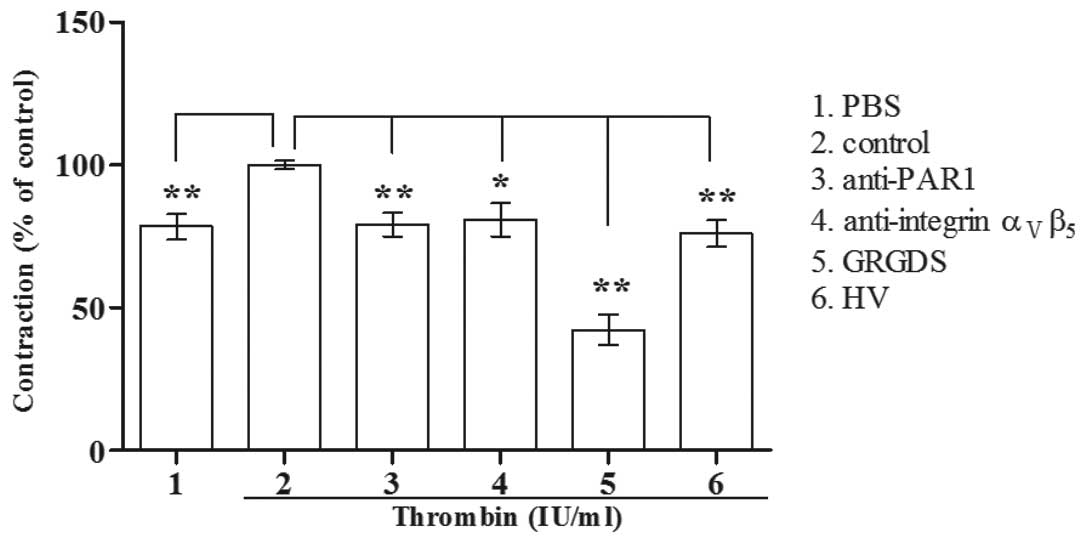
Thrombin-treated (1 IU/ml) Glc-82 cells
significantly promoted the contraction of rat tail collagen. The
PAR1 antagonists (HV and anti-PAR1 antibody) and integrin
ανβ5 antagonists (GRGDS and anti-integrin
ανβ5 antibody) significantly attenuated the
ability of thrombin-treated Glc-82 cells to induce rat tail
collagen contraction (Fig. 5).
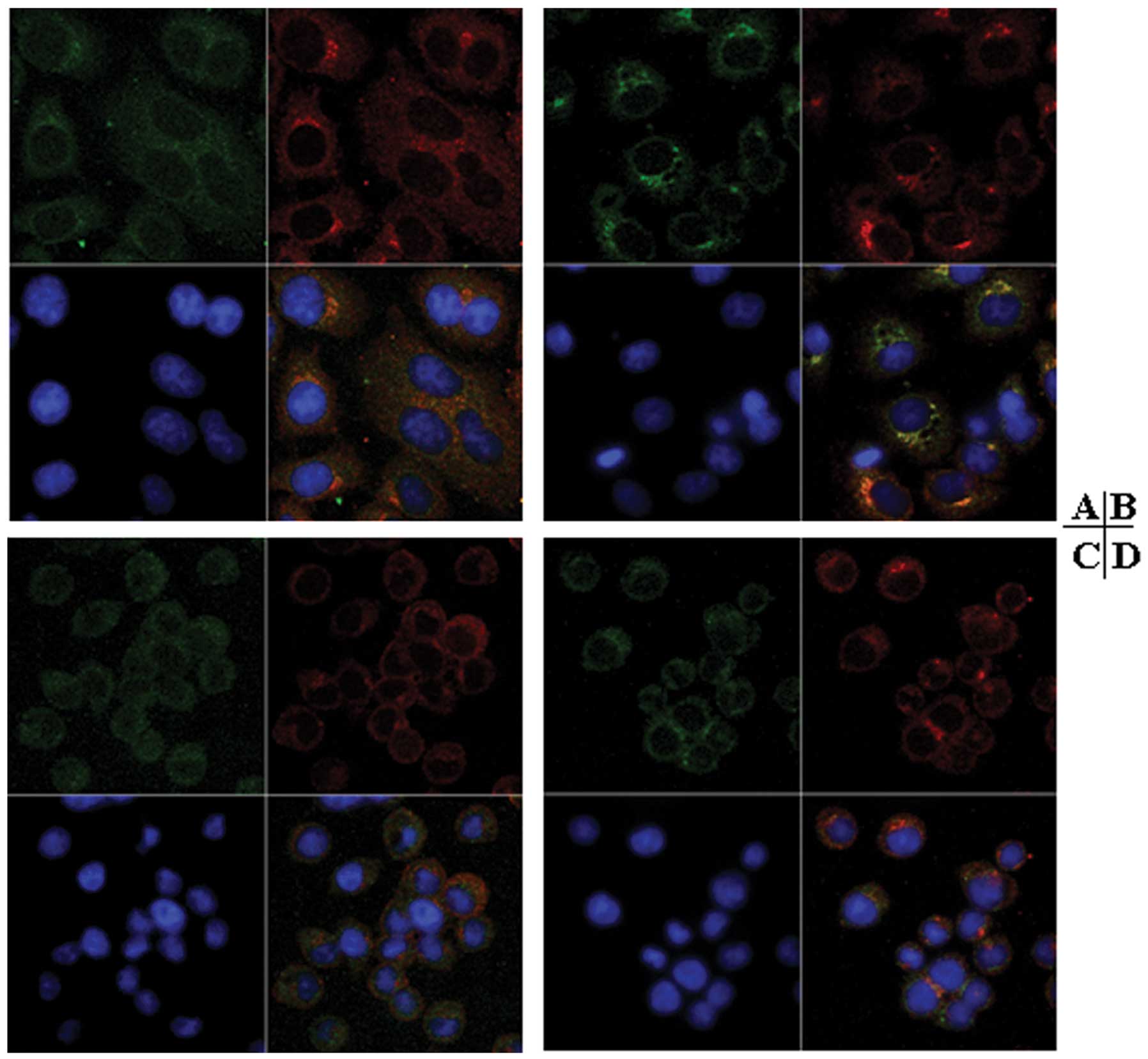
Effect of thrombin on the cell surface
localization of PAR1 and integrin ανβ5 in
Glc-82 cells
Native thrombin treatment affected the cell-surface
distribution of integrin ανβ5 and PAR1 in
Glc-82 cells. After stimulation with native thrombin, both integrin
ανβ5 and PAR1 clustering localized to the
cell surface. Moreover, specific colocalization of PAR1 and
integrin ανβ5 was observed (Fig. 6B). In cells cultured on slides
coated with thrombin, integrin ανβ5 was
diffusely distributed over the cell surface, and PAR1 was clustered
on the cell surface. Colocalization of integrin
ανβ5 and PAR1 was not observed (Fig. 6D).
Discussion
Few reports have described the relationship between
PAR1 and integrins in thrombin-mediated tumor cell functional
responses. In human melanoma cells, PAR1 has been reported to
cooperate with integrin ανβ5 via its
cytoplasmic tail, and PAR1 activation affects integrin
ανβ5 distribution on the cell surface without
altering integrin ανβ5 expression levels. The
specific activation of PAR1 by the activation polypeptide TRAP
induces co-precipitation of integrin ανβ5 and
paxillin (16). However, the
specific activation mechanism of PAR1 and integrins by thrombin,
especially immobilized thrombin, and the potential relationship
between PAR1 and integrin in thrombin-induced tumor cell functions
have not been characterized.
In this study, we evaluated the effect of
immobilized thrombin on lung cancer cell invasion. Immobilized
thrombin could promote tumor cell invasion in a similar manner to
native thrombin, and lead to increased cell adhesion and migration,
activation of the Erk signaling pathway and collagen contraction
(Fig. 1–5). These experiments provide a strong
model to elucidate the effects of ECM-immobilized thrombin on tumor
invasion. It is known that there is a high concentration of
thrombin in the stromal tissue surrounding tumor cells or the
metastasis environment. The thrombin stored in the fibrin around
the tumor tissue or bound to subendothelial ECM is protected from
inactivation by circulating thrombin inhibitors (26–29).
This results in a prolonging stimulation of the thrombin receptor
and transduction of tumor-promoting signals, and eventually
facilitates tumor invasion. However, it is not known how
immobilized thrombin promotes tumor cell invasion, or whether
immobilized thrombin can activate PAR1 and the integrins.
Antagonists of PAR1 and integrin
ανβ5 were used to evaluate the role of PAR1
and integrin ανβ5 on immobilized
thrombin-mediated tumor cell invasion. We compared the results with
the effects mediated by native thrombin. In view of the
inconsistent results in previous studies on the activation of PAR1
and integrins by thrombin in endothelial cells or tumor cells, we
presume that the illustration for the roles of PAR1 and integrin
ανβ5 would provide evidence to describe the
activation mechanism of the two receptors by thrombin, and
simultaneously evaluate the function of the thrombin catalytic
domain and RGD motif during PAR1 and integrin
ανβ5 activation by native and immobilized
thrombin.
In the cell adhesion assay, PAR1 antagonists
(anti-PAR1 antibody and HV) and integrin ανβ5
antagonists (anti-integrin ανβ5 antibody and
GRGDS) significantly attenuated the ability of native thrombin to
stimulate Glc-82 cell adhesion (Fig.
2A). These results indicate that PAR1 and integrin
ανβ5, as well as the catalytic domain of
thrombin are required for native thrombin-mediated tumor cell
function. However, HV in the culture media had no effect on the
ability of immobilized thrombin to stimulate Glc-82 cell adhesion,
and intriguingly, when the plates were co-coated with HV and
thrombin, thrombin-mediated cell adhesion was dramatically
attenuated (Fig. 2B). We
hypothesize that the promotion effect of immobilized thrombin on
Glc-82 cell adhesion is not dependent on the thrombin catalytic
domain. The conformation of thrombin changes when it is
immobilized, thus blocking the ability of HV to bind thrombin. The
inhibitory effect of co-coated HV on cell adhesion in response to
immobilized thrombin suggests pre-incubation of HV with thrombin
prevents, at least in part, a conformational change in thrombin,
resulting in a reduced ability to promote cell adhesion. Thus, we
investigated whether the conformation change in immobilized
thrombin exposed the RGD sequence and destroyed the catalytic
domain. An anti-PAR1 antibody had no effect on the ability of
immobilized thrombin to stimulate cell adhesion (Fig. 2C), indicating that immobilized
thrombin-mediated cell adhesion is not dependent on the thrombin
catalytic domain. The anti-integrin ανβ5
antibody also had no effect on immobilized thrombin-mediated cell
adhesion. However, the integrin antagonist GRGDS significantly,
specifically and dose-dependently inhibited immobilized
thrombin-mediated cell adhesion (Fig.
2C and D). Collectively, these results indicate that integrins,
but not integrin ανβ5 participate in
immobilized thrombin-mediated tumor cell adhesion, and that
immobilization of thrombin may induce a conformational change in
the catalytic domain of thrombin and expose the RGD sequence.
In the cell migration assay, both native and
immobilized thrombin promoted Glc-82 cell migration (Fig. 3). In a similar manner to the cell
adhesion assay, all of the PAR1 and integrin
ανβ5 antagonists attenuated the ability of
native thrombin to induce cell migration (Fig. 3A), providing further evidence for a
role of both PAR1 and integrin ανβ5 in native
thrombin-stimulated tumor cell function. PAR1 antagonists
(anti-PAR1 antibody and HV in the culture media) had no effect on
immobilized thrombin-mediated cell migration, suggesting that the
catalytic domain is not required for immobilized thrombin to
stimulate cell migration. Integrin ανβ5
antagonists (anti-integrin ανβ5 antibody and
GRGDS) significantly inhibited the ability of immobilized thrombin
to stimulate cell migration, indicating that integrin
ανβ5 is required for immobilized
thrombin-mediated cell migration. When HV was co-coated with
thrombin, the ability of immobilized thrombin to stimulate cell
migration was attenuated (Fig. 3B),
in a similar manner to cell adhesion. We presume that binding of HV
to thrombin inhibits the catalytic activity, and represses the
conformation change and an attenuated ability to stimulate tumor
cell invasion.
Previous research has indicated that the Erk
signaling pathway plays an important role in thrombin/PAR1-mediated
platelet (30–32) or cancer cell function (33,34).
The Erk signaling pathway is reported to participate in integrin
function (35,36). However, it is not known if Erk
signaling plays a role in thrombin-mediated integrin function. We
evaluated the relationship between Erk signaling and activation of
PAR1 and integrin ανβ5 in thrombin-treated
lung cancer cells. Phosphorylation of Erk occurred independently of
thrombin-induced PAR1 activation (Fig.
4), suggesting that the thrombin catalytic domain is not
necessary for the activation of Erk signaling. The interaction of
both native and immobilized thrombin with integrin
ανβ5 significantly induced Erk
phosphorylation, which could be inhibited by the anti-integrin
ανβ5 antibody, GRGDS and co-coated HV
(Fig. 4). As the anti-PAR1 antibody
and HV in the culture media had no effect on thrombin-induced Erk
phosphorylation, activation of PAR1 and the thrombin catalytic
domain may not be necessary for Erk activation. These results
indicate that activation of the Erk signaling pathway by native or
immobilized thrombin is due to activation of integrin
ανβ5, suggesting that Erk phosphorylation
results from outside-in activation of the integrin
ανβ5 signaling pathway which may involve the
thrombin RGD sequence. These observations are not consistent with
the interactions between thrombin and integrins reported by others,
where the activation of integrins by thrombin is PAR1 dependent on
inside-out signaling (14,16).
Tumor invasion and metastasis depend on the ability
of tumor cells to invade beyond the primary site and establish at
remote sites. Tumor cell invasion is characterized by remodeling of
the tumor microenvironment, and ECM remodeling by tumor cells can
be evaluated using the collagen contraction assay (37,38).
Native thrombin-treated cells significantly enhanced rat tail
collagen contraction, resulting in a smaller diameter of the
collagen mass. PAR1 and integrin ανβ5
antagonists attenuated the ability of native thrombin-treated cells
to mediate collagen contraction (Fig.
5), in agreement with the cell adhesion and migration assays
results, indicating that both PAR1 and integrin
ανβ5 are required for thrombin to induce ECM
remodeling, which may facilitate tumor cell invasion.
Aside from the effects on Erk signaling, our
experiments demonstrate that both PAR1 and integrin
ανβ5 play an important role in the ability of
native thrombin to affect Glc-82 cell function. Therefore, we
assessed if potential interactions exist between PAR1 and integrin
ανβ5 during thrombin-induced tumor cell
invasion using a dual immunofluorescent colocalization assay. After
treatment with native thrombin, PAR1 and integrin
ανβ5 were clustering distributed and
colocalized on the cell surface of Glc-82 cells (Fig. 6B). We assume that the thrombin
exosite I domain combines with PAR1, leading to a conformational
change in thrombin, which exposes the RGD sequence allowing
integrin ανβ5 to interact with thrombin.
Under this situation, thrombin, at least transiently, acts as a
bridge between PAR1 and integrin ανβ5.
Therefore, PAR1 and integrin ανβ5 are
simultaneously required for native thrombin-mediated Glc-82 cell
function. This hypothesis is in compliance with studies in other
cells. Thrombin can simultaneously bind to and activate PAR1 and
PAR4 in platelets, possibly via binding of the thrombin exosite I
to the HV-like motif of cleaved PAR1, allowing the active site of
thrombin free to potentially interact with PAR4. This indicates
that thrombin may remain tethered to the surface of platelets via
its association with cleaved PAR1. PAR1 and PAR4 form a stable
heterodimer which enables thrombin to act as a bivalent functional
agonist (39). Another study has
indicated that transient binding of thrombin to PAR1 prior to
receptor cleavage may serve as an RGD-exposing event, which enables
integrin binding during PAR1 activation (20). Thus, it is possible, at least
theoretically, that the thrombin exosite I domain remains bound to
PAR1 while the thrombin catalytic domain is liberated from PAR1,
followed by a conformation change in thrombin and exposure of the
RGD sequence to allow integrin ανβ5
binding.
In conclusion, we studied the effects of native and
immobilized thrombin on tumor cell invasion, and evaluated the role
and relationship between PAR1 and integrin
ανβ5. The results of this study suggest that
immobilized thrombin can induce tumor cell invasion in a similar
manner to native thrombin. Integrin ανβ5 play
a pivotal role in both immobilized thrombin and native
thrombin-mediated tumor cell invasion. Thus, inhibition of thrombin
or its receptors, especially integrin ανβ5,
may provide an attractive therapeutic target. The ability of PAR1
and integrin ανβ5 to cooperate in native
thrombin-induced cell invasion suggests that targeting of the
PAR1-integrin complex may present an important therapeutic
opportunity to prevent tumor invasion.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30770833).
References
|
1
|
Rickels FR, Levine M and Edwards RL:
Hemostatic alterations in cancer patients. Cancer Metastasis Rev.
11:237–248. 1992. View Article : Google Scholar
|
|
2
|
Heit JA, Silverstein MD, Mohr DN,
Petterson TM, O’Fallon WM and Melton LJ III: Risk factors for deep
vein thrombosis and pulmonary embolism: a population-based
case-control study. Arch Intern Med. 160:809–815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kasthuri R, Taubman M and Mackman N: Role
of tissue factor in cancer. J Clin Oncol. 27:4834–4838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amirkhosravi A, Meyer T, Amaya M, Davila
M, Mousa SA, Robson T and Francis JL: The role of tissue factor
pathway inhibitor in tumor growth and metastasis. Semin Thromb
Hemost. 33:643–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Jiang P, Capkova K, Xue D, Ye L,
Sinha SC, Mackman N, Janda KD and Liu C: Tissue factor-activated
coagulation cascade in the tumor microenvironment is critical for
tumor progression and an effective target for therapy. Cancer Res.
71:6492–6502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hron G, Kollars M, Binder BR, Eichinger S
and Kyrle PA: Identification of patients at low risk for recurrent
venous thromboembolism by measuring thrombin generation. JAMA.
296:397–402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zigler M, Kamiya T, Brantley EC, Villares
GJ and Bar-Eli M: PAR-1 and thrombin: the ties that bind the
microenvironment to melanoma metastasis. Cancer Res. 71:6561–6566.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wojtukiewicz MZ, Zacharski LR, Memoli VA,
Kisiel W, Kudryk BJ, Rousseau SM and Stump DC: Malignant melanoma.
Interaction with coagulation and fibrinolysis pathways in situ. Am
J Clin Pathol. 93:516–521. 1990.PubMed/NCBI
|
|
9
|
Zacharski LR, Memoli VA, Morain WD,
Schlaeppi JM and Rousseau SM: Cellular localization of
enzymatically active thrombin in intact human tissues by hirudin
binding. Thromb Haemost. 73:793–797. 1995.PubMed/NCBI
|
|
10
|
Ornstein DL and Zacharski LR: Treatment of
cancer with anticoagulants: rationale in the treatment of melanoma.
Int J Hematol. 73:157–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Even-Ram S, Uziely B, Cohen P,
Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I and
Bar-Shavit R: Thrombin receptor overexpression in malignant and
physiological invasion processes. Nat Med. 4:909–914. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grisaru-Granovsky S, Salah Z, Maoz M,
Pruss D, Beller U and Bar-Shavit R: Differential expression of
protease activated receptor 1 (Par1) and pY397FAK in benign and
malignant human ovarian tissue samples. Int J Cancer. 113:372–378.
2005. View Article : Google Scholar
|
|
13
|
Henrikson KP, Salazar SL, Fenton JW II and
Pentecost BT: Role of thrombin receptor in breast cancer
invasiveness. Br J Cancer. 79:401–406. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Radjabi AR, Sawada K, Jagadeeswaran S,
Eichbichler A, Kenny HA, Montag A, Bruno K and Lengyel E: Thrombin
induces tumor invasion through the induction and association of
matrix metalloproteinase-9 and beta1-integrin on the cell surface.
J Biol Chem. 283:2822–2834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang RS, Chiang HS, Tang CH, Yeh CS and
Huang TF: Rhodostomin inhibits thrombin-enhanced adhesion of ROS
17/2.8 cells through the blockade of alphavbeta3 integrin. Toxicon.
46:387–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Even-Ram SC, Maoz M, Pokroy E, Reich R,
Katz BZ, Gutweini P, Altevogti P and Bar-Shavit R: Tumor cell
invasion is promoted by activation of protease activated receptor-1
in cooperation with the alpha v beta 5 integrin. J Biol Chem.
276:10952–10962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiang HS, Yang RS and Huang TF: Thrombin
enhances the adhesion and migration of human colon adenocarcinoma
cells via increased beta 3-integrin expression on the tumor cell
surface and their inhibition by the snake venom peptide,
rhodostomin. Br J Cancer. 73:902–908. 1996. View Article : Google Scholar
|
|
18
|
Chiang HS, Yang RS and Huang TF: The
Arg-Gly-Asp-containing peptide, rhodostomin, inhibits in vitro cell
adhesion to extracellular matrices and platelet aggregation caused
by Saos-2 human osteosarcoma cells. Br J Cancer. 71:265–270. 1995.
View Article : Google Scholar
|
|
19
|
Bode W, Turk D and Karshikov A: The
refined 1.9-A X-ray crystal structure of D-Phe-Pro-Arg
chloromethylketone-inhibited human alpha-thrombin: structure
analysis, overall structure, electrostatic properties, detailed
active-site geometry, and structure-function relationships. Protein
Sci. 1:426–471. 1992. View Article : Google Scholar
|
|
20
|
Bar-Shavit R, Sabbah V, Lampugnani MG,
Marchisio PC, Fenton JW II, Vlodavsky I and Dejana E: An
Arg-Gly-Asp sequence within thrombin promotes endothelial cell
adhesion. J Cell Biol. 112:335–344. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bar-Shavit R, Eskohjido Y, Fenton JW II,
Esko JD and Vlodavsky I: Thrombin adhesive properties: induction by
plasmin and heparan sulfate. J Cell Biol. 123:1279–1287. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsopanoglou NE, Andriopoulou P and
Maragoudakis ME: On the mechanism of thrombin-induced angiogenesis:
involvement of alphavbeta3-integrin. Am J Physiol Cell Physiol.
283:C1501–C1510. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsopanoglou NE, Papaconstantinou ME,
Flordellis CS and Maragoudakis ME: On the mode of action of
thrombin-induced angiogenesis: thrombin peptide, TP508, mediates
effects in endothelial cells via alphavbeta3 integrin. Thromb
Haemost. 92:846–857. 2004.PubMed/NCBI
|
|
24
|
Sajid M, Zhao R, Pathak A, Smyth SS and
Stouffer GA: Alphavbeta3-integrin antagonists inhibit
thrombin-induced proliferation and focal adhesion formation in
smooth muscle cells. Am J Physiol Cell Physiol. 285:C1330–C1338.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Papaconstantinou ME, Carrell CJ, Pineda
AO, Bobofchak KM, Mathews FS, Flordellis CS, Maragoudakis ME,
Tsopanoglou NE and Di Cera E: Thrombin functions through its RGD
sequence in a non-canonical conformation. J Biol Chem.
280:29393–29396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bar-Shavit R, Eldor A and Vlodavsky I:
Binding of thrombin to subendothelial extracellular matrix.
Protection and expression of functional properties. J Clin Invest.
84:1096–1104. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hernández-Rodríguez NA, Correa E, Sotelo
R, Gómez-Ruiz C, Contreras-Paredes A and Green L: Thrombin is
present in the lungs of patients with primary extremity
osteosarcoma and pulmonary metastases. Int J Biol Markers.
17:189–195. 2002.PubMed/NCBI
|
|
28
|
Fenton JW II: Regulation of thrombin
generation and functions. Semin Thromb Hemost. 14:234–240. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weitz JI, Hudoba M, Massel D, Maraganore J
and Hirsh J: Clot-bound thrombin is protected from inhibition by
heparin-antithrombin III but is susceptible to inactivation by
antithrombin III-independent inhibitors. J Clin Invest. 86:385–391.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Begonja AJ, Geiger J, Rukoyatkina N,
Rauchfuss S, Gambaryan S and Walter U: Thrombin stimulation of p38
MAP kinase in human platelets is mediated by ADP and thromboxaneA2
and inhibited by cGMP/cGMP-dependent protein kinase. Blood.
109:616–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malaver E, Romaniuk MA, D’atri LP, Pozner
RG, Negrotto S, Benzadón R and Schattner M: NF-kappaB inhibitors
impair platelet activation responses. J Thromb Haemost.
7:1333–1343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shankar H, Garcia A, Prabhakar J, Kim S
and Kunapull SP: P2Y12 receptor-mediated potentiation of
thrombin-induced thromboxane A2 generation in platelets occurs
through regulation of Erk1/2 activation. J Thromb Haemost.
4:638–647. 2006. View Article : Google Scholar
|
|
33
|
Liu J, Schuff-Werner P and Steiner M:
Thrombin/thrombin receptor (PAR-1)-mediated induction of IL-8 and
VEGF expression in prostate cancer cells. Biochem Biophys Res
Commun. 343:183–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang LH, Chen CH, Huang DY, Pai HC, Pan
SL and Teng CM: Thrombin induces expression of twist and cell
motility via the hypoxia-inducible factor-1α translational pathway
in colorectal cancer cells. J Cell Physiol. 226:1060–1068.
2011.PubMed/NCBI
|
|
35
|
Sil H, Sen T and Chatterjee A:
Fibronectin-integrin (alpha5beta1) modulates migration and invasion
of murine melanoma cell line B16F10 by involving MMP-9. Oncol Res.
19:335–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu CT, Wu JR, Cheng CC, Wang S, Wang HT,
Lee MC, Wang LJ, Pan SM, Chang TY and Wu WS: Reactive oxygen
species-mediated PKC and integrin signaling promotes tumor
progression of human hepatoma HepG2. Clin Exp Metastasis.
28:851–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sood AK, Fletcher MS, Coffin JE, Yang M,
Seftor EA, Gruman LM, Gershenson DM and Hendrix MJC: Functional
role of matrix metalloproteinases in tumor cell plasticity. Am J
Obstet Gynecol. 190:899–909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma N, Seftor RE, Seftor EA, Gruman LM,
Heidger PM Jr, Cohen MB, Lubaroff DM and Hendrix MJ: Prostatic
tumor cell plasticity involves cooperative interactions of distinct
phenotypic subpopulations: role in vasculogenic mimicry. Prostate.
50:189–201. 2002. View Article : Google Scholar
|
|
39
|
Leger AJ, Jacques SL, Badar J, Kaneider
NC, Derian CK, Andrade-Gordon P, Covic L and Kuliopulos A: Blocking
the protease-activated receptor 1–4 heterodimer in
platelet-mediated thrombosis. Circulation. 113:1244–1254. 2006.
|