Introduction
Metastasis is a sequential multi-step process that
includes cell migration and cell invasion, important participants
in the progression of the cancerous process. In general, metastasis
involves four major steps: adhesion of tumor cells to the
extracellular matrix (ECM), the ability of tumor cells to degrade
the ECM and intravasate into surrounding blood vessels, survival
against the natural host defenses and settling at the preferred
organ site, and extravasation into the organ and formation of new
tumors (1,2). Therefore, inhibition of metastasis
including tumor cell migration and invasion is an important
mechanism in the anti-metastatic properties of anti-cancer
drugs.
Cell adhesion between neighboring epithelial cells
involving tight junctions (TJs), gap junctions, and adherens
junctions is a crucial and tightly controlled process. TJs
represent one mode of cell-to-cell adhesion in epithelial or
endothelial cells. In addition, TJs are the first barrier,
providing a physical barrier to paracellular transport of solutes
across cells and playing a critical role in establishing and
maintaining epithelial cell polarity. In the cancerous condition
and cancerous lesions of epithelium origin, TJ strands become
disorganized or lost altogether, and TJs become ‘leaky’, as
indicated by decreased resistance to electrical current
(transepithelial electrical resistance; TER) and the increased
paracellular permeability of markers (3,4).
Claudins are key integral membrane proteins, the most important
components of the TJs, and can form homodimers or heterodimers to
produce paired strands between adjacent cells and act as a barrier
to paracellular flux of water, solutions, and transmigration of
other cells, thereby determining the characteristic permeability
properties of different epithelial tissues. Claudins have four
transmembrane domains, with the N-terminus and the C-terminus in
the cytoplasm (5,6). Recently, researchers have reported
overexpression of claudins in metastatic gastric cancer and in
other cancers (6–10) and the inhibition of claudin
expression reduced the cancer invasive potential in ovarian cancer
cells (11–13). Although the exact functional
importance of the overexpressed claudins in cancers remains
unclear, these observations indicate that claudins have key
cellular functions during the metastasis process.
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent endopeptidases that process a broad spectrum of cell
surface molecules and function in several important biological
processes. MMPs are also collectively capable of cleaving virtually
all ECM substrates, and play an important role in cancer
progression, invasion, and metastasis (14,15).
MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B) are included in
proteolytic degradation of the basement membrane by degrading type
IV collagen. In addition, it has been reported that MMP-2 and -9
are overexpressed in gastric cancer areas compared to normal areas,
and high expression is a negative prognostic factor in gastric
cancer (16,17).
Various models have been studied showning epigenetic
regulation is essential for cancer progression. Among them,
decitabine (5-Aza-2′-deoxycytidine), a DNA methyltransferase
inhibitor (DNMTI), is critical for epigenetic regulation through
DNA hypermethylation and hypomethylation in cancer (1,18).
Aberrant methylation is one of the more frequent molecular changes
observed in tumor cells and typically involves the reversal of
normal methylation patterns (19,20).
Common changes involve genome-wide hypomethylation, which impinges
on the expression of oncogenes, loss of imprinting, and
hypermethylation of tumor suppressor genes (21,22).
Several recent studies have indicated that decitabine also inhibits
cancer cell growth and induces apoptotic cell death (23,24).
However, the precise biochemical mechanisms underlying
decitabine-induced anti-metastasis have not yet been clarified.
Therefore, the present study attempted to elucidate the
anti-metastatic potential of decitabine and underlying
intracellular signal transduction pathways involved in inhibiting
metastasis of human gastric carcinoma AGS cells.
Materials and methods
Cell culture and MTT assay
The human gastric carcinoma AGS cell line was
purchased from the American Type Culture Collection (Rockville,
MD). AGS cells were cultured in RPMI-1640 medium (Gibco/BRL,
Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS)
and 1% penicillin-streptomycin in a 37°C incubator with 5%
CO2. Decitabine was purchased from Sigma Chemical Co.
(St. Louis, MO), dissolved in 100% dimethyl sulfoxide (DMSO) to a
stock concentration 10 mM, and stored at −80°C. For the cell
viability study, AGS cells were seeded onto 6-well plates at a
concentration of 5×104 cells/well, grown to 70%
confluence, and then treated with various concentrations of
decitabine for 48 h. Following treatment, cell viability was
determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT,
Sigma) assay, which is based on the conversion of MTT to
MTT-formazan by mitochondrial enzymes.
Wound healing migration assay
AGS cells were grown to confluence on 30-mm cell
culture dishes coated with rat tail collagen (20 μg/ml, BD
Biosciences, Bedford, MA). A scratch was made in the cell layer
with a pipette tip. After the cells were washed with PBS,
serum-free media (to prevent cell proliferation) containing 20 μM
of decitabine was added. Photographs of the wounded area were taken
immediately after the scratch was made and at 12, 24 and 48 h to
monitor cell movement into the wounded area (25).
In vitro invasiveness assay
Matrigel invasion assays were used to assess the
ability of AGS cells to penetrate the ECM in the presence or
absence of decitabine. Briefly, cells were exposed to 10 and 20 μM
of decitabine for 6 h, and treated cells (50,000) were then plated
onto the apical side of the Matrigel-coated filters in serum-free
medium containing 10 and 20 μM of decitabine. Medium containing 20%
FBS was placed in the basolateral chamber to function as a
chemoattractant. After 48 h, cells on the apical side were wiped
off with a Q-tip. Cells on the bottom of the filter were stained
with hematoxylin and eosin Y (Sigma) and counted (three fields of
each triplicate filter) using an inverted microscope.
Measurement of TER
TER was measured with an EVOM Epithelial Tissue
Voltohmmeter (World Precision Instruments, Sarasota, City, FL),
equipped with a pair of STX-2 chopstick electrodes. Briefly, AGS
cells were seeded into the 8.0 μm pore size insert (upper chamber)
of a Transwell (Corning Costar Corp., Cambridge MA) and allowed to
reach full confluence, after which fresh medium was replaced for
additional experiments. Inserts without cells, inserts with cells
in medium, and inserts with cells with 10 and 20 μM of decitabine
were treated for 48 h. Electrodes were placed at the upper and
lower chambers, and resistance was measured with the voltohmmeter
(26).
RNA extraction and reverse
transcription-polymerase chain reaction
Total RNA was prepared using an RNeasy kit (Qiagen,
La Jolla, CA) and primed with random hexamers to synthesize
complementary DNA using AMV reverse transcriptase (Amersham Co.,
Arlington Heights, IL), according to the manufacturer’s
instructions using DNAse I (1 U/μg RNA) pretreated total mRNA.
Polymerase chain reaction (PCR) was carried out in a Mastercycler
(Eppendorf, Hamburg, Germany) using the desired primers. The
conditions for the PCR reactions were 1 cycle (94°C for 3 min), 35
cycles (94°C for 45 sec; 58°C for 45 sec; and 72°C for 1 min), and
1 cycle (72°C for 10 min). Amplification products obtained with PCR
were electrophoretically separated on 1% agarose gel and visualized
with ethidium bromide (EtBr) staining.
Protein extraction and western blot
analysis
Total cell lysates from decitabine-treated cells
were prepared in an extraction buffer [25 mM Tris-Cl (pH 7.5), 250
mM NaCl, 5 mM ethylendiaminetetra acetic acid, 1% Nonidet P-40, 0.1
mM sodium orthovanadate, 2 μg/ml leupeptin, and 100 μg/ml
phenylmethylsulfonyl fluoride]. Protein concentration was
determined using a Bio-Rad protein assay kit (Bio-Rad,
Laboratories, Hercules, CA). For western blot analysis, proteins
(50 μg) were separated with 8–13% sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis and then
electrotransferred to a nitrocellulose membrane (Schleicher &
Schuell, Keene, NH). Membranes were blocked with 5% skim milk for 1
h, and then subjected to immunoblot analysis using the desired
antibodies. Proteins were then visualized with the enhanced
chemiluminescence (ECL) method, according to the recommended
procedure (Amersham Co.). Primary antibodies were purchased from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA) and Calbiochem
(Cambridge, MA). Peroxidase-labeled donkey anti-rabbit
immunoglobulin and peroxidase-labeled sheep anti-mouse
immunoglobulin were purchased from Amersham Co.
Gelatin zymographic analysis of secreted
MMPs
Following incubation with various concentrations of
decitabine for 48 h, cell culture supernatants were collected and
centrifuged at 400 × g for 5 min. Cell-free supernatant was mixed
with 2× sample buffer (Invitrogen, Grand Island, NY), and
zymography was performed using precast gels (10% polyacrylamide and
0.1% gelatin). Following electrophoresis, the gels were washed
twice at room temperature for 30 min in 2.5% Triton X-100,
subsequently washed in buffer containing 50 mM Tris-HCl, 150 mM
NaCl, 5 mM CaCl2, 1 μM ZnCl2, and 0.02%
NaN3 at pH 7.5, and incubated in this buffer at 37°C for
24 h. Thereafter, the gels were stained with 0.5% (w/v) Coomassie
Brilliant Blue G250 (Bio-Rad) for 1 h and then lightly destained in
methanol:acetic acid:water (3:1:6). Clear bands appear on the
Coomassie stained blue background in areas of gelatinolytic
activity. The gels were scanned, and the images were processed by
extracting the blue channel signal, converting it to black and
white, and inverting it to quantify the gelatinolytic activities
from the integrated optical density (27).
Statistical analysis
All data are presented as mean ± SD. Significant
differences among the groups were determined using the unpaired
Student’s t-test. A value of P<0.05 was accepted as an
indication of statistical significance. All of the figures shown in
this article were obtained from at least three independent
experiments.
Results
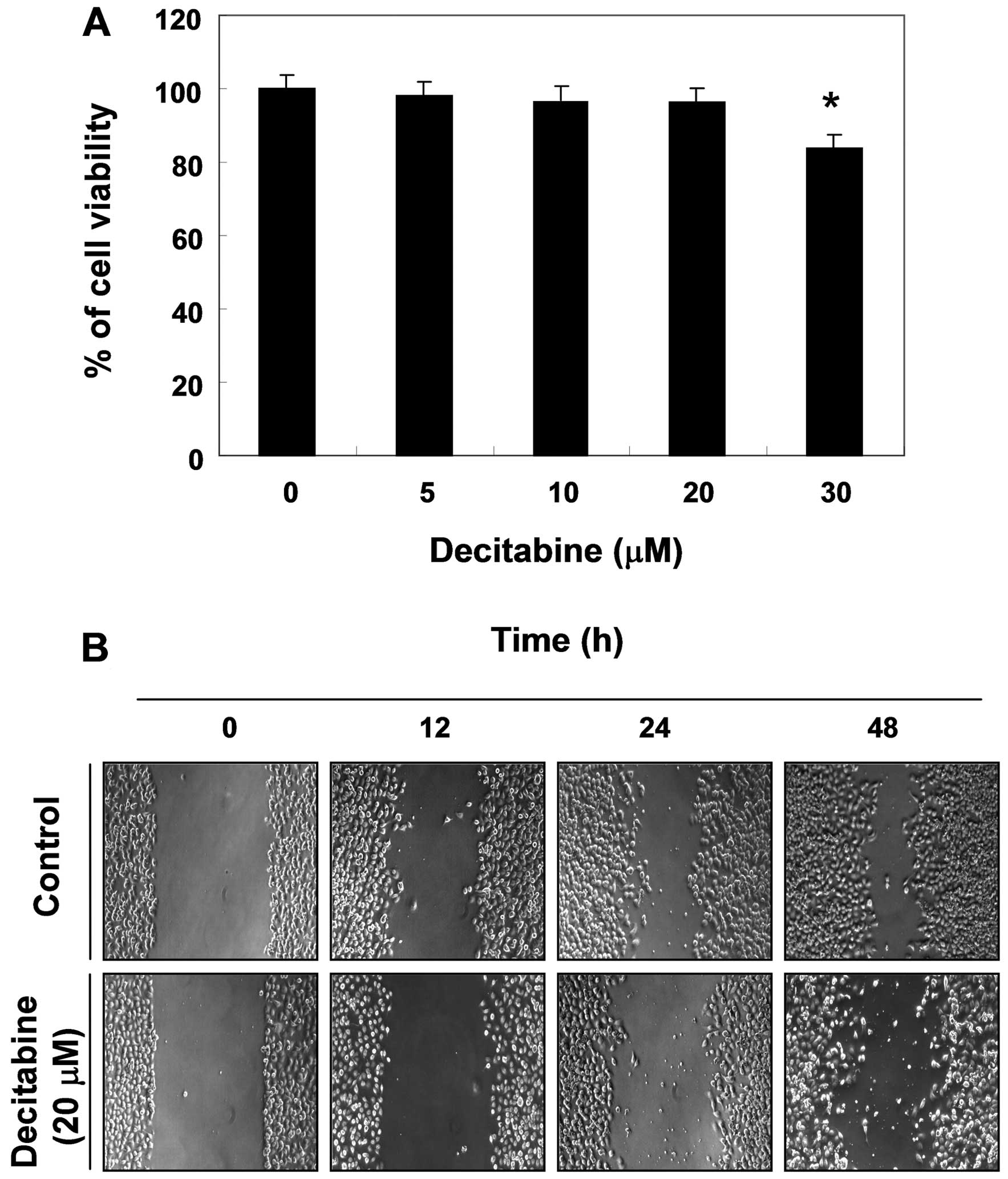
Effects of decitabine on cell viability
and cell migration in AGS cells
To determine the effect of decitabine on cell
viability in AGS cells, an MTT assay was performed at 48 h after
treatment with the indicated concentrations (5–30 μM) of
decitabine. Decitabine alone in the range of 5–20 M mg/ml did not
have any cytotoxic effect on AGS cells, whereas a high
concentration of decitabine (30 μM) significantly caused a decrease
in cell viability. When compared with the control, treatment with
30 μM of decitabine caused approximately 15% inhibition of cell
growth (Fig. 1A). Therefore, we
applied 20 μM decitabine for the optimal treatment concentration to
investigate whether decitabine decreases cell migration in AGS
cells. The wound healing assay results demonstrated that treatment
with 20 μM decitabine time-dependently delayed cell motility, when
compared with controls (Fig.
1B).
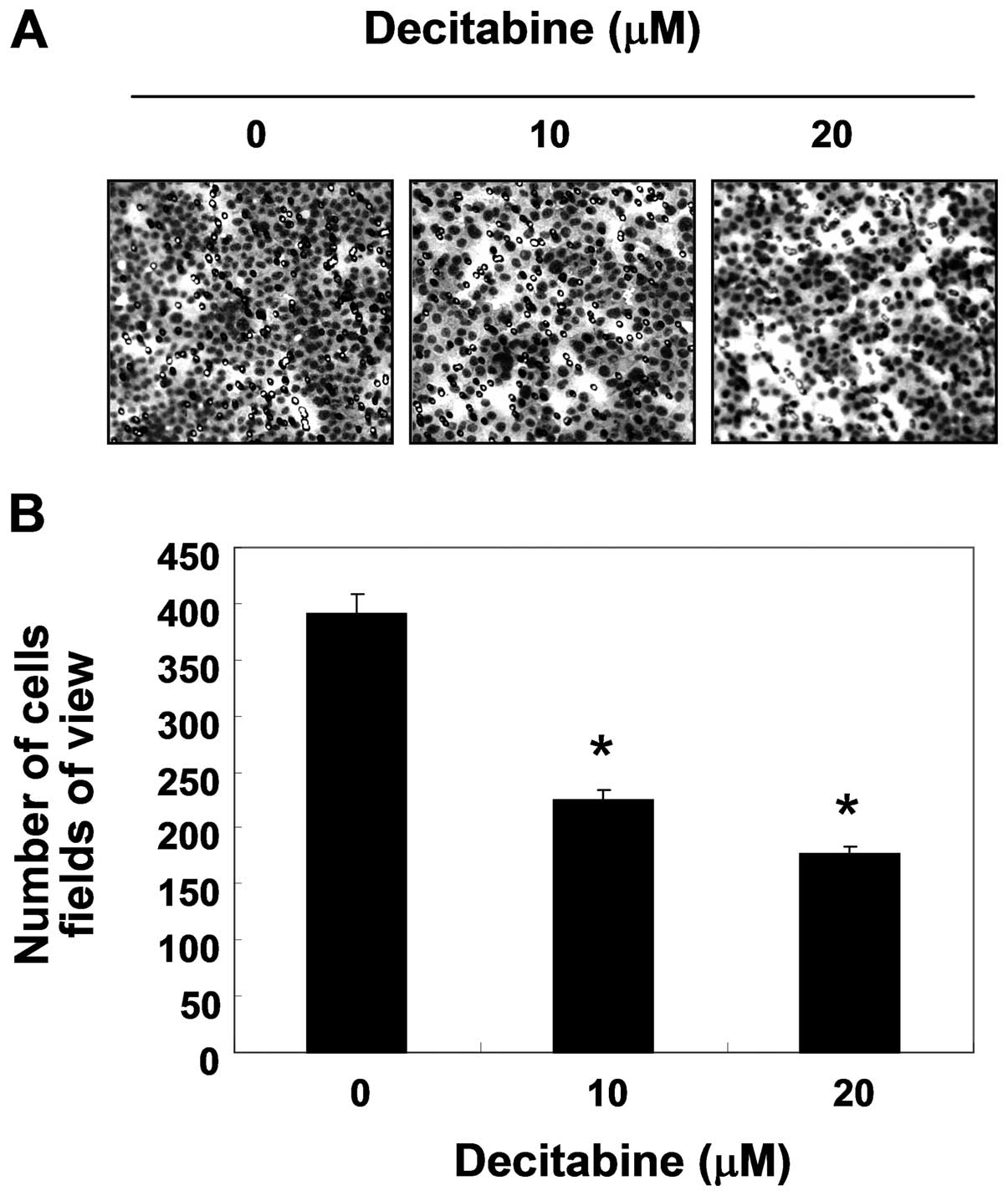
Inhibition of cell invasion by decitabine
in AGS cells
Because cancer invasion is the initial step in
metastasis in destroying the basement membrane, we then used a
Boyden chamber invasion assay to determine whether the inhibitory
effects of decitabine were connected to the decreased activity of
cell invasion. As shown in Fig. 2,
decitabine treatment resulted in markedly reduced cell invasion
through the Matrigel chamber in a concentration-dependent manner,
suggesting the inhibitory effects of cell migration were associated
with inhibition of invasive activity in AGS cells.
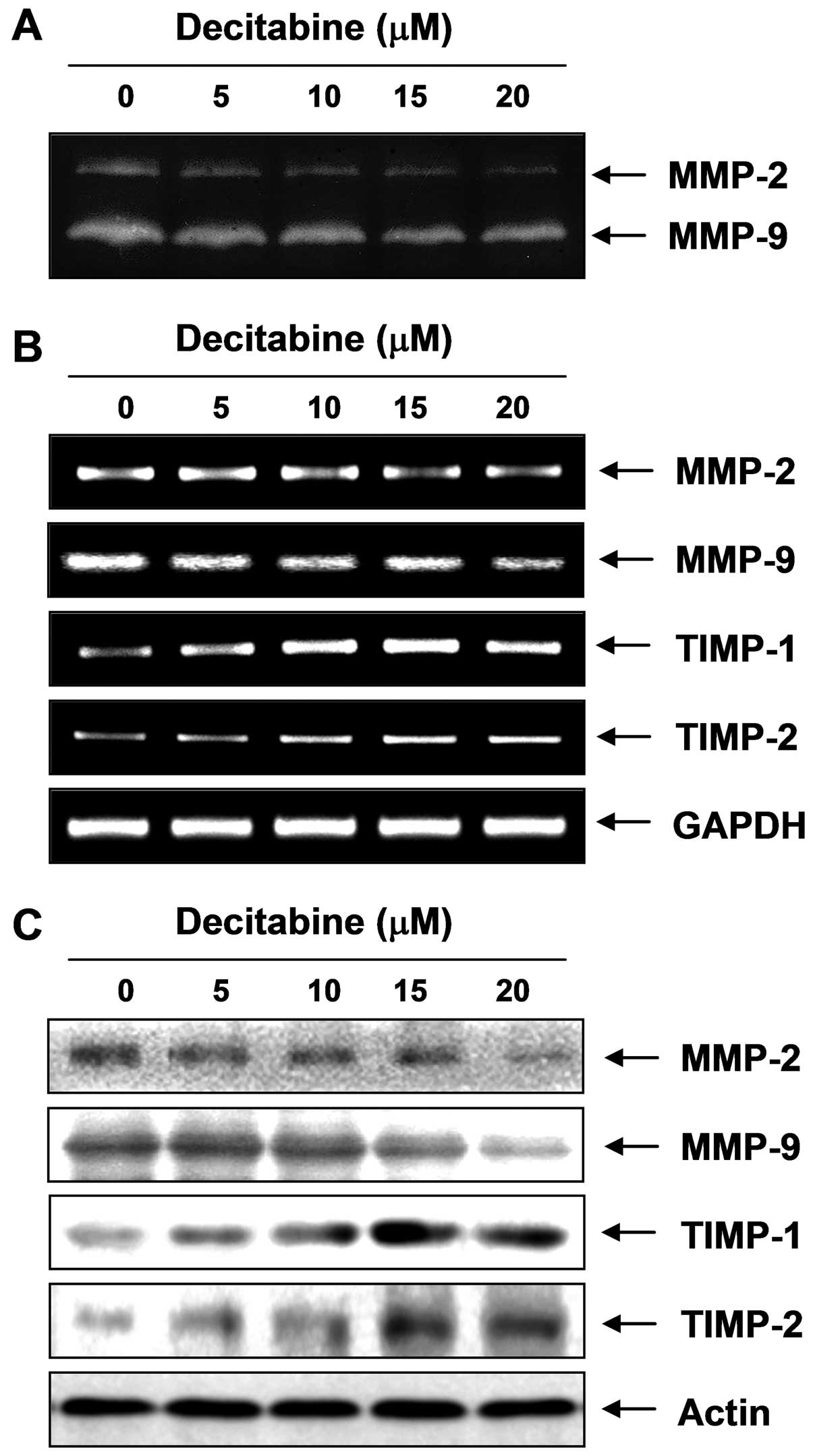
Inhibition of the activity and expression
of MMP-2 and MMP-9 by decitabine in AGS cells
Because migration influences metastasis and invasion
of the basement membrane is primarily mediated by gelatinase MMPs,
we tested the effects of decitabine on the activation and
expression of MMPs using gelatin zymography, RT-PCR and western
blot analyses. Data indicated MMP-2 and MMP-9 activity in AGS cells
was decreased by decitabine treatment, which was connected to
concurrent downregulation of the mRNA and protein levels (Fig. 3). In addition to the MMP roles,
tissue inhibitors of metalloproteinases (TIMPs) are naturally
occurring inhibitors of MMPs, which inhibit MMP catalytic activity
through binding to activated MMPs and controlling the breakdown of
the ECM. Therefore, we tested the effects of decitabine on TIMP-1
and TIMP-2 expression levels. The RT-PCR results showed decitabine
induced a concentration-dependent increase in TIMP-1 and TIMP-2
mRNA levels, which was connected to concurrent upregulation of
their protein levels, as determined with western blotting (Fig. 3), suggesting increasing TIMP
proteins with decitabine could inhibit MMP activity.
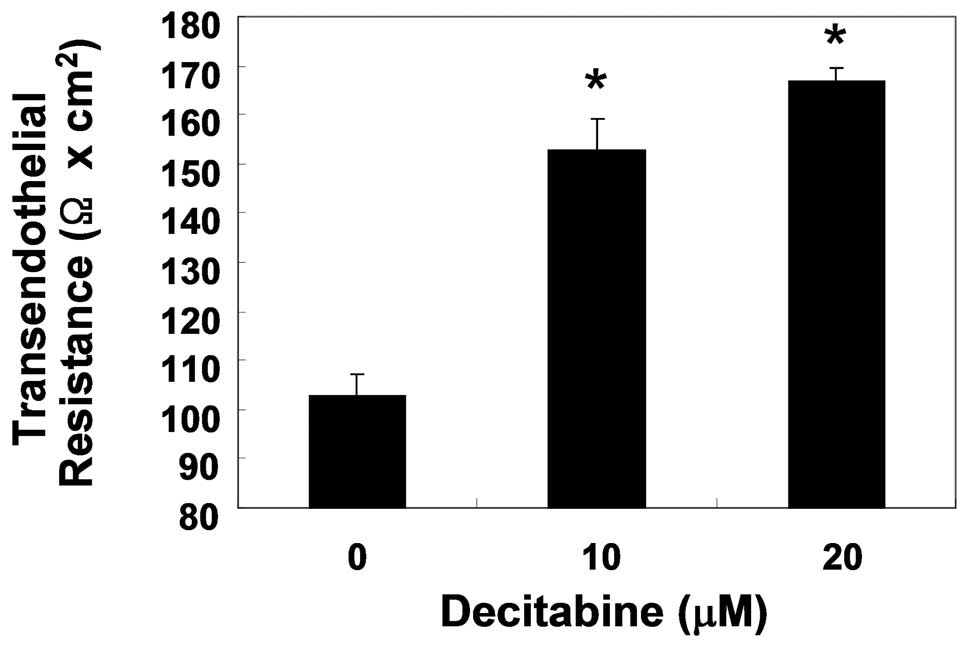
Enhancement of TJs tightening by
decitabine in AGS cells
To examine the relationship between TJs tightening
and the invasive activity of AGS cells treated with decitabine, TER
(a measure of tight junction formation) values were measured using
an EVOM epithelial tissue voltohmmeter. As shown in Fig. 4, incubation of cells with decitabine
resulted in a substantial concentration-dependent increase in their
TER values. The data indicated that decitabine induced an increase
in TJs function in AGS cells, associated with inhibition of cell
invasion.
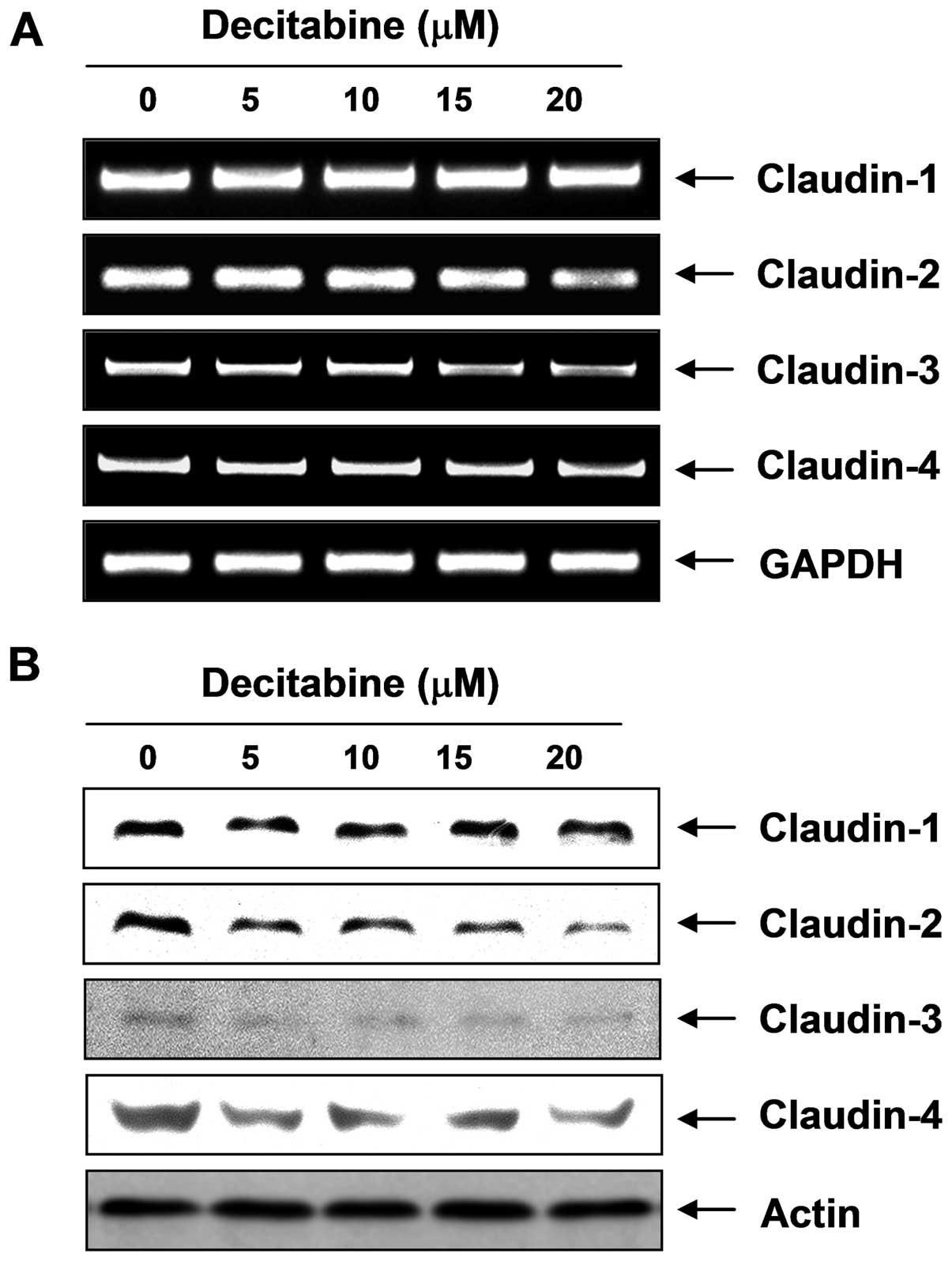
Modulation of TJ-related factors by
decitabine in AGS cells
To elucidate the mechanism by which decitabine
enhances TJs activity and reduces invasive activity in AGS cells,
we determined the levels of claudins, TJ components, using RT-PCR
and western blot analysis. As shown in Fig. 5, the transcriptional and
translational levels of claudins (claudin-2, -3 and -4), the most
important components of TJs, but not claudin-1, were markedly
downregulated in decitabine-treated cells in a dose-dependent
manner, suggesting this modulation contributed to TJs
tightening.
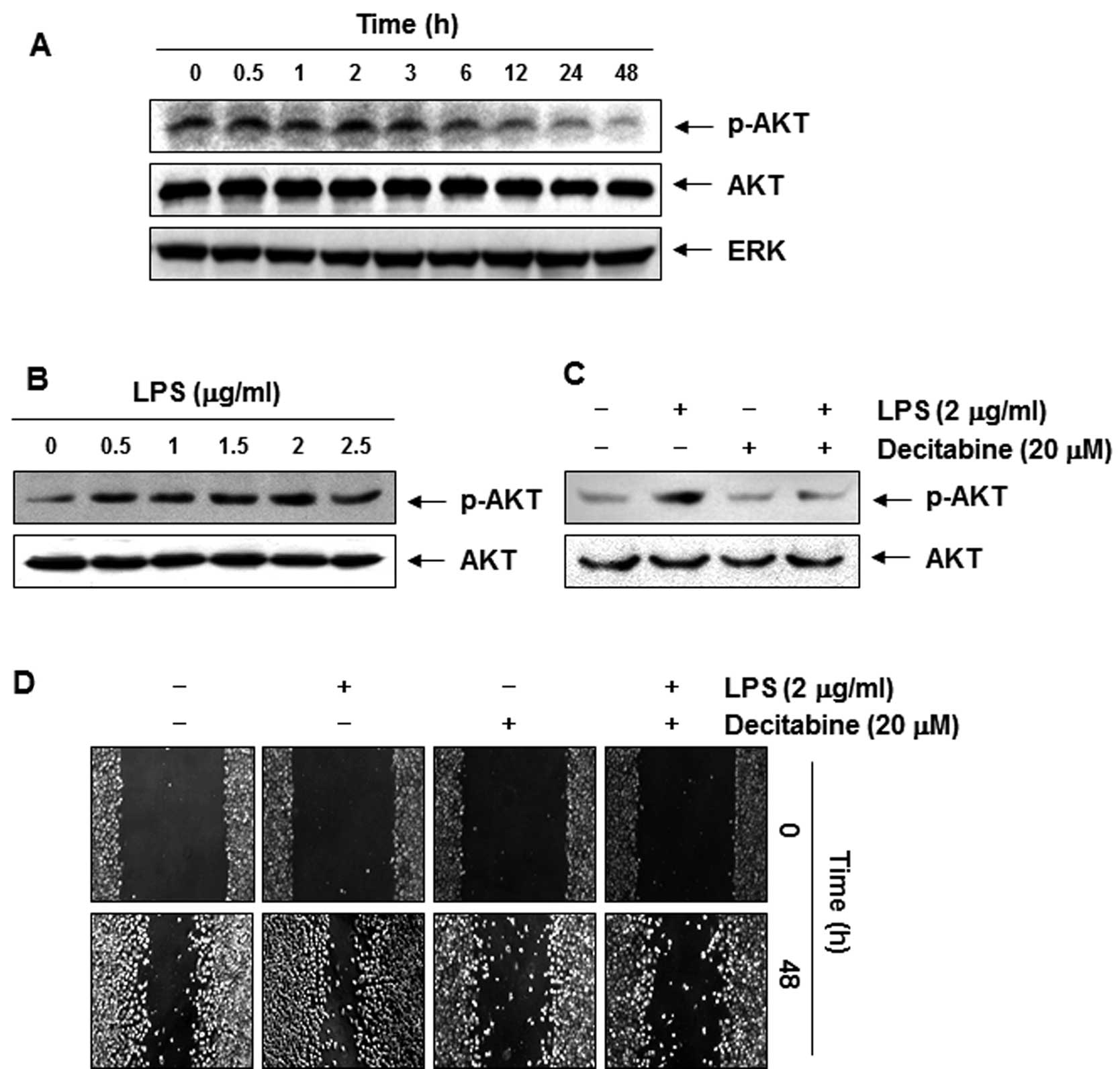
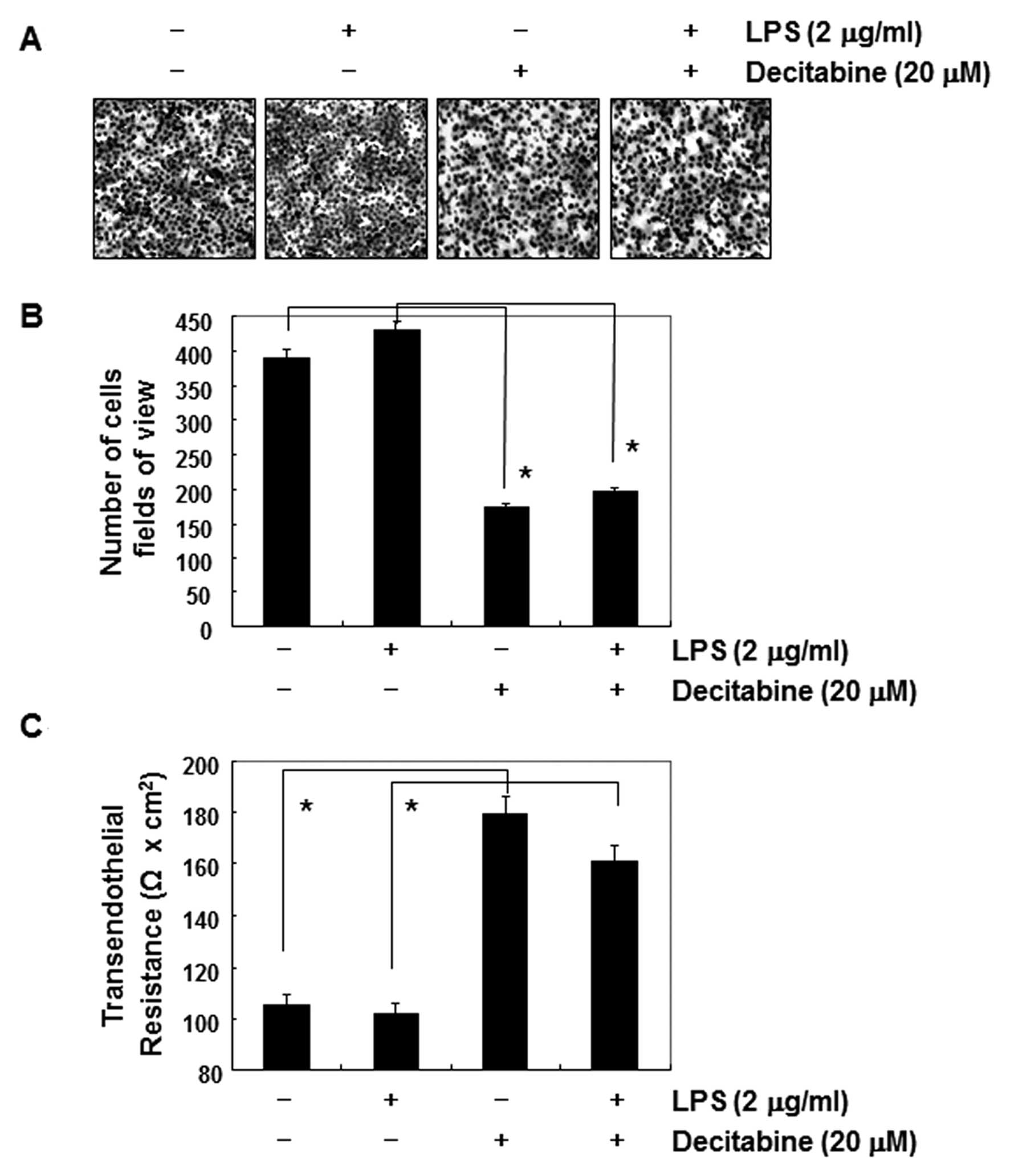
Decitabine-induced anti-invasive activity
is associated with dephosphorylation of AKT
Recent studies have implicated phosphoinositide
3-kinase (PI3K)/Akt in regulating MMPs and TJs, which are involved
in a number of cellular functions, including cell survival,
adhesion, and metastasis (28,29).
Therefore, the effects of decitabine on the phosphorylated status
of Akt in AGS cells were assessed. Immunoblotting data demonstrated
that decitabine treatment markedly decreased the phosphorylation of
Akt in a concentration-dependent manner (Fig. 6A). To confirm the involvement of the
PI3K/Akt signaling pathway in decitabine-induced anti-invasive
activity of AGS cells, we used lipopolysaccharide (LPS) to activate
Akt and increase the cell invasion. As shown in Fig. 6B, simulation of AGS cells with LPS
led to a concentration-dependent marked increase in the amount of
phosphorylated Akt. Consistent with those results, cell motility
and invasive activity were partly increased by treatment with LPS.
However, the LPS-accentuated increase in Akt phosphorylation, cell
motility, and invasive activity was significantly suppressed by
decitabine (Figs. 6C and D, and
7A and B). On the contrary,
although the TER values in LPS-treated AGS cells were not
significantly different from those of the controls, the values were
markedly increased in response to decitabine (Fig. 7C).
Discussion
Because cancer cell invasion and migration are
critical steps during metastasis, inhibiting tumor cell migration
and invasion are important mechanisms in the anti-metastatic
properties of anticancer drugs. The aim of this study was to
investigate whether decitabine has potent anti-invasion and
anti-metastasis activity in human gastric adenocarcinoma AGS cells.
We found that decitabine markedly inhibited cell motility and
invasive activity through tightening TJs, and decreasing MMP
activity and the Akt signaling pathway.
MMPs are important proteolytic enzymes during organ
development and tissue regeneration; however, they also play
important roles in cancer invasion and metastasis. Above all, MMP-2
and MMP-9 play the most important roles in tumor invasion and
angiogenesis; therefore, tumor metastasis can be inhibited by
blocking MMP synthesis and activity (30,31).
MMP activity is tightly controlled by transcriptional activation,
by a complex proteolytic activation cascade, and by an endogenous
system of TIMPs. TIMPs inhibit MMPs by forming 1:1 stoichiometric
complexes to regulate matrix turnover (32,33).
Because treatment with less than 20 μM of decitabine, which was not
cytotoxic as determined with the MTT assay, markedly inhibited the
cell motility and invasive activity in AGS cells (Figs. 1B and 2), we investigated whether the inhibitory
effects of decitabine were associated with the modulation of TIMPs
and MMPs expression or their activities. Our results indicated that
decitabine induced inhibition of MMP-2 and -9 mRNA and protein
levels as well as their enzymatic activities in a
concentration-dependent manner but not MMP-9 levels. However, the
transcriptional and translational levels of TIMP-1 and TIMP-2 were
concentration-dependently upregulated in response to decitabine
treatment (Fig. 3). The present
data demonstrated that the decitabine-induced inhibition of cell
motility and invasion is related to downregulation of MMP-2 and -9
activities through the elevation of TIMP expression. Therefore, our
results showed that decitabine may increase the TIMPs/MMPs ratio,
as a key factor in regulating the anti-metastatic process, which
subsequently blocks the degradation of the ECM and leads to
inhibition of cell invasion.
Increasing evidence indicates that suppressing the
malignant phenotype of cells in tumorigenesis is an additional and
important function of the TJs (34). Moreover, it is becoming increasingly
clear that the development of human cancer is frequently associated
with the failure of epithelial cells to form TJs and to establish
correct apicobasal polarity (35)
suggesting that changes in permeability properties and loss of cell
polarity are hallmarks of epithelial cell tumorigenesis. Thus, TJs,
the structures critical for maintaining these functions in
epithelial cells, are modulated in a number of epithelial cancers,
including gastric cancer (3,4). These
observations indicated that disruption of TJs and dysregulation of
their composite proteins play critical roles in cancer progression,
invasion, and metastasis. Indeed, early studies have demonstrated
that TJ structures are altered in many epithelial cancers. For
example, Soler et al(3)
first demonstrated that the TER of colon carcinoma tissue was
significantly lower than that of normal colon tissues but showed
higher transepithelial paracellular permeability, which confirmed
the loss of the TJs. Previous studies have shown many anticancer
agents inhibit motility and invasiveness, and act by enhancing
transepithelial paracellular permeability (36–41).
Therefore, we examined the changes in the TER values to investigate
the relationship between the anti-invasive activity and TJs in
response to decitabine treatment. Our results clearly showed that
treatment with decitabine increased the TER values of AGS cells in
a dose-dependent manner, an effect associated with inhibition of
motility and invasiveness. These results indicate that decitabine
may prevent or reverse TJ leakiness.
Since TJ leakiness is associated with cancer
progression and invasion, TJ tightening may have anti-metastatic
activity (42); the anti-invasive
activity of decitabine may be due, in part, to its ability to
enhance TJ activity. The TJ structure represents the conglomerate
of molecules that constitute, associate with, or regulate TJs, and
a number of proteins, as components of TJs, were identified. Among
these, 24 members of the claudin family, transmembrane proteins
with extracellular domains, were identified. Claudins interact with
other claudins to form homodimers or heterodimers to produce paired
strands between cells to regulate paracellular permeability
(6). Many previous studies indicate
that disruption of TJs, with concomitant dysregulation of TJ
proteins, is an early event in cancer cell invasion and metastasis,
and the nature of the dysregulation is highly cancer type specific.
For example, claudin-1 and claudin-7 are downregulated in invasive
ductal carcinomas of the breast, as well as in most established
breast cancer cell lines (43).
However, claudin-3 and -4 are overexpressed in breast cancers
(43–45), and other cancers, including gastric
(46), ovarian (7), and pancreatic cancer (47). Conversely, ‘knockdown’ of these two
claudins (claudin-3 and -4) inhibited the invasiveness of cancer
cells (11). We also recently
showed that claudin-1 played a causal role in acquiring invasive
capacity in human liver cells, which was associated with increased
expression of MMP-2. However, small interfering RNA targeting of
claudin-1 in invasive hepatocellular carcinoma cells completely
inhibited cell invasion (13).
These observations indicate that claudins are dysregulated in many
types of cancers, and may prove to be useful biomarkers for
detecting and diagnosing certain cancers. Interestingly, Miyamori
et al(48) reported claudin
promotes activation of pro-MMP-2 mediated by membrane-type MMPs.
Agarwal et al(11)reported
the overexpression of claudin-3 and -4 proteins is associated with
increased MMP-2 activity. Recently, Ip et al(49) also showed downregulation of
claudin-10 reduced MMP activity in human hepatocarcinoma cells.
These reports imply a close relationship between MMP activity,
overexpression of claudin, and metastasis of cancer cells.
Therefore, we investigated the effects of decitabine on the level
of claudin family members, and the data showed that decitabine
significantly inhibited expression of claudin proteins such as
claudin-2, -3, and -4 at the transcriptional and translation levels
(Fig. 5). The data suggested that
the anti-invasive activity of decitabine was associated with the
tightening of TJs through downregulation of claudin family
members.
In addition, many reports have demonstrated that
PI3K/Akt plays an important role in tumor development and
progression through induction of epithelial-mesenchymal transition
and achievement of invasive properties (50–53).
In addition, MMP-2 and -9 expression has been critically associated
with the PI3K/Akt pathway (54).
Therefore, we tested the effect of decitabine on the activities of
the PI3K/Akt signaling pathway. The results demonstrated that
decitabine inhibited phosphorylation of Akt after 12-h treatment
guessing that the anti-invasive effects of decitabine were
associated with downregulation of Akt phosphorylation (Fig. 6A). Furthermore, decitabine treatment
resulted in significant blockage of LPS-induced Akt
phosphorylation, cell motility, and invasion (Figs. 6 and 7). The data indicated that LPS-induced
invasiveness mediated by suppressing the PI3K/Akt pathway, and
anti-invasive effects by decitabine were mediated at least in part
by the inactivation of the PI3K/Akt pathway, associated with TJs
tightening.
In this study, although additional in vivo
studies are needed to establish the role of decitabine as an
anti-metastatic agent for treating cancer, we confirmed that
decitabine inhibits cell motility and invasion of AGS human gastric
cancer cells by increasing TJ activity and downregulation of MMP
activity via inactivation of the PI3K/AKT signaling pathway.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science, and Technology
(2010-0001730), Republic of Korea.
References
|
1
|
Momparler RL: Epigenetic therapy of cancer
with 5-aza-2′-deoxycytidine (decitabine). Semin Oncol. 32:443–451.
2005.
|
|
2
|
Gassmann P, Enns A and Haier J: Role of
tumor cell adhesion and migration in organ-specific metastasis
formation. Onkologie. 27:577–582. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soler AP, Miller RD, Laughlin KV, Carp NZ,
Klurfeld DM and Mullin JM: Increased tight junctional permeability
is associated with the development of colon cancer. Carcinogenesis.
20:1425–1431. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneeberger EE and Lynch RD: The tight
junction: a multifunctional complex. Am J Physiol Cell Physiol.
286:1213–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruffer C and Gerke V: The C-terminal
cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif
is required for apical localization at epithelial and endothelial
tight junctions. Eur J Cell Biol. 83:135–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morin PJ: Claudin proteins in human
cancer: promising new targets for diagnosis and therapy. Cancer
Res. 65:9603–9606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rangel LB, Agarwal R, D’Souza T, Pizer ES,
Alò PL, Lancaster WD, Gregoire L, Schwartz DR, Cho KR and Morin PJ:
Tight junction proteins claudin-3 and claudin-4 are frequently
overexpressed in ovarian cancer but not in ovarian cystadenomas.
Clin Cancer Res. 9:2567–2575. 2003.PubMed/NCBI
|
|
8
|
Mees ST, Mennigen R, Spieker T, Rijcken E,
Senninger N, Haier J and Bruewer M: Expression of tight and
adherens junction proteins in ulcerative colitis associated
colorectal carcinoma: upregulation of claudin-1, claudin-3,
claudin-4 and beta-catenin. Int J Colorectal Dis. 24:361–368. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouban A and Ahmed AA: Claudins in human
cancer: a review. Histol Histopathol. 25:83–90. 2010.
|
|
10
|
Jung H, Jun KH, Jung JH, Chin HM and Park
WB: The expression of claudin-1, claudin-2, claudin-3, and
claudin-4 in gastric cancer tissue. J Surg Res. 167:e185–191. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agarwal R, D’Souza T and Morin PJ:
Claudin-3 and claudin-4 expression in ovarian epithelial cells
enhances invasion and is associated with increased matrix
metalloproteinase-2 activity. Cancer Res. 65:7378–7385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun C, Yi T, Song X, Li S, Qi X, Chen X,
Lin H, He X, Li Z, Wei Y and Zhao X: Efficient inhibition of
ovarian cancer by short hairpin RNA targeting claudin-3. Oncol Rep.
26:193–200. 2011.PubMed/NCBI
|
|
13
|
Yoon CH, Kim MJ, Park MJ, Park IC, Hwang
SG, An S, Choi YH, Yoon G and Lee SJ: Claudin-1 acts through
c-Abl-protein kinase Cdelta (PKCδ) signaling and has a causal role
in the acquisition of invasive capacity in human liver cells. J
Biol Chem. 285:226–233. 2010.PubMed/NCBI
|
|
14
|
Duffy MI, Maguire TM, Hill A, McDermott E
and O’Higgins N: Metalloproteinases: role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vihinen P, Ala-aho R and Kähäri VM: Matrix
metalloproteinases as therapeutic targets in cancer. Curr Cancer
Drug Targets. 5:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwartz GK: Invasion and metastases in
gastric cancer: in vitro and in vivo models with clinical
correlations. Semin Oncol. 23:316–324. 1996.PubMed/NCBI
|
|
17
|
Yasui W, Oue N, Aung PP, Matsumura S,
Shutoh M and Nakayama H: Molecular-pathological prognostic factors
of gastric cancer: a review. Gastric Cancer. 8:86–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patra SK and Bettuzzi S: Epigenetic
DNA-(cytosine-5-carbon) modifications: 5-aza-2′-deoxycytidine and
DNA-demethylation. Biochemistry (Mosc). 74:613–619. 2009.PubMed/NCBI
|
|
19
|
Jubb AM, Bell SM and Quirke P: Methylation
and colorectal cancer. J Pathol. 195:111–134. 2001. View Article : Google Scholar
|
|
20
|
Claus R, Almstedt M and Lübbert M:
Epigenetic treatment of hematopoietic malignancies: in vivo targets
of demethylating agents. Semin Oncol. 32:511–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feinberg AP and Vogelstein B:
Hypomethylation distinguishes genes of some human cancers from
their normal counterparts. Nature. 301:89–92. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goelz SE, Vogelstein B, Hamilton SR and
Feinberg AP: Hypomethylation of DNA from benign and malignant human
colon neoplasms. Science. 228:187–190. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gomyo Y, Sasaki J, Branch C, Roth JA and
Mukhopadhyay T: 5-aza-2′-deoxycytidine upregulates caspase-9
expression cooperating with p53-induced apoptosis in human lung
cancer cells. Oncogene. 23:6779–6787. 2004.
|
|
24
|
Schnekenburger M, Grandjenette C, Ghelfi
J, Karius T, Foliguet B, Dicato M and Diederich M: Sustained
exposure to the DNA demethylating agent, 2′-deoxy-5-azacytidine,
leads to apoptotic cell death in chronic myeloid leukemia by
promoting differentiation, senescence, and autophagy. Biochem
Pharmacol. 81:364–378. 2011.PubMed/NCBI
|
|
25
|
Zhu W, Fu A, Hu J, Wang T, Luo Y, Peng M,
Ma Y, Wei Y and Chen L: 5-Formylhonokiol exerts anti-angiogenesis
activity via inactivating the ERK signaling pathway. Exp Mol Med.
43:146–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grant-Tschudy KS and Wira CR: Effect of
oestradiol on mouse uterine epithelial cell tumour necrosis
factor-alpha release is mediated through uterine stromal cells.
Immunology. 115:99–107. 2005. View Article : Google Scholar
|
|
27
|
Song HY, Ju SM, Goh AR, Kwon DJ, Choi SY
and Park J: Suppression of TNF-alpha-induced MMP-9 expression by a
cell-permeable superoxide dismutase in keratinocytes. BMB Rep.
44:462–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shukla S, Maclennan GT, Hartman DJ, Fu P,
Resnick MI and Gupta S: Activation of PI3K-Akt signaling pathway
promotes prostate cancer cell invasion. Int J Cancer.
121:1424–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J and Zhang LJ: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar
|
|
30
|
Matrisian LM: The matrix-degrading
metalloproteinases. Bioessays. 14:455–463. 1992. View Article : Google Scholar
|
|
31
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
32
|
Uzui H, Harpf A, Liu M, Doherty TM, Shukla
A and Chai N: Increased expression of membrane type 3-matrix
metalloproteinase in human atherosclerotic plaque: role of
activated macrophages and inflammatory cytokines. Circulation.
106:3024–3030. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lambert E, Dasse E, Haye B and Petitfrere
E: TIMPs as multifacial proteins. Crit Rev Oncol Hematol.
49:187–198. 2004. View Article : Google Scholar
|
|
34
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Latorre IJ, Roh MH, Frese KK, Weiss RS,
Margolis B and Javier RT: Viral oncoprotein-induced mislocalization
of select PDZ proteins disrupts tight junctions and causes polarity
defects in epithelial cells. J Cell Sci. 118:4283–4293. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gitter AH, Bendfeldt K, Schmitz H,
Schulzke JD, Bentzel CJ and Fromm M: Epithelial barrier defects in
HT-29/B6 colonic cell monolayers induced by tumor necrosis factor.
Ann N Y Acad Sci. 915:193–203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Verghese GM, Gutknecht MF and Caughey GH:
Prostasin regulates epithelial monolayer function: cell-specific
Gpld1-mediated secretion and functional role for GPI anchor. Am J
Physiol Cell Physiol. 291:1258–1270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Deun K, Pasmans F, Van Immerseel F,
Ducatelle R and Haesebrouck F: Butyrate protects Caco-2 cells from
Campylobacter jejuni invasion and translocation. Br J Nutr.
100:480–484. 2008.PubMed/NCBI
|
|
39
|
Kim SO, Choi BT, Choi IW, Cheong J, Kim
GY, Kwon TK, Kim ND and Choi YH: Anti-invasive activity of histone
deacetylase inhibitors via the induction of Egr-1 and the
modulation of tight junction-related proteins in human
hepatocarcinoma cells. BMB Rep. 42:655–660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SO, Kwon JI, Jeong YK, Kim GY, Kim ND
and Choi YH: Induction of Egr-1 is associated with anti-metastatic
and anti-invasive ability of β-lapachone in human hepatocarcinoma
cells. Biosci Biotechnol Biochem. 71:2169–2176. 2007.PubMed/NCBI
|
|
41
|
Shin DY, Kim GY, Kim JI, Yoon MK, Kwon TK,
Lee SJ, Choi YW, Kang HS, Yoo YH and Choi YH: Anti-invasive
activity of diallyl disulfide through tightening of tight junctions
and inhibition of matrix metalloproteinase activities in LNCaP
prostate cancer cells. Toxicol In Vitro. 24:1569–1576. 2010.
View Article : Google Scholar
|
|
42
|
Mullin JM, Agostino N, Rendon-Huerta E and
Thornton JJ: Keynote review: epithelial and endothelial barriers in
human disease. Drug Discov Today. 10:395–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kominsky SL, Vali M, Korz D, Gabig TG,
Weitzman SA, Argani P and Sukumar S: Clostridium perfringens
enterotoxin elicits rapid and specific cytolysis of breast
carcinoma cells mediated through tight junction proteins claudin 3
and 4. Am J Pathol. 164:1627–1633. 2004. View Article : Google Scholar
|
|
44
|
Kominsky SL, Argani P, Korz D, Evron E,
Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP and Sukumar S:
Loss of the tight junction protein claudin-7 correlates with
histological grade in both ductal carcinoma in situ and invasive
ductal carcinoma of the breast. Oncogene. 22:2021–2033. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tokés AM, Kulka J, Paku S, Szik A, Páska
C, Novák PK, Szilák L, Kiss A, Bögi K and Schaff Z: Claudin-1, -3
and -4 proteins and mRNA expression in benign and malignant breast
lesions: a research study. Breast Cancer Res. 7:296–305.
2005.PubMed/NCBI
|
|
46
|
Cunningham SC, Kamangar F, Kim MP, Hammoud
S, Haque R, Iacobuzio-Donahue CA, Maitra A, Ashfaq R, Hustinx S,
Heitmiller RE, et al: Claudin-4, mitogen activated protein kinase
kinase 4, and stratifin are markers of gastric adenocarcinoma
precursor lesions. Cancer Epidemiol Biomarkers Prev. 15:281–287.
2004. View Article : Google Scholar
|
|
47
|
Michl P, Barth C, Buchholz M, Lerch MM,
Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Löhr M, et al:
Claudin-4 expression decreases invasiveness and metastatic
potential of pancreatic cancer. Cancer Res. 63:6265–6271.
2003.PubMed/NCBI
|
|
48
|
Miyamori H, Takino T, Kobayashi Y, Tokai
H, Itoh Y, Seiki M and Sato H: Claudin promotes activation of
pro-matrix metalloproteinase-2 mediated by membrane-type matrix
metalloproteinases. J Biol Chem. 276:28204–28211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ip YC, Cheung ST, Lee YT, Ho JC and Fan
ST: Inhibition of hepatocellular carcinoma invasion by suppression
of claudin-10 in HLE cells. Mol Cancer Ther. 6:2858–2867. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Samuels Y and Ericson K: Oncogenic PI3K
and its role in cancer. Curr Opin Oncol. 18:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chakraborti S, Mandal M, Das S, Mandal A
and Chakraborti T: Regulation of matrix metalloproteinases: an
overview. Mol Cell Biochem. 253:269–285. 2003. View Article : Google Scholar : PubMed/NCBI
|