Introduction
27-hydroxycholesterol (27OHC), an abundant
circulating cholesterol metabolite, is a potent regulator of some
mammalian tissues. Few years ago, combined studies by the research
groups of Mangelsdorf and MacDonnell demonstrated that 27OHC,
locally produced from cholesterol by atheroma-infiltrating
macrophages, damaged the bordering blood vessels (1). The effects of 27OHC were exhibited
only by cells containing estrogen receptors (ER), therefore the
concept of 27OHC as a novel selective estrogen receptor modulator
(SERM) was suggested (2). The same
groups reported later on the detrimental activity of 27OHC on bone
homeostasis (3,4). The proliferation of various
ER-positive mammary cancer cell lines is also affected by 27OHC, as
demonstrated by DuSell et al(2) and by us (5). Remarkably, the proliferation rate of
non-tumorigenic mammary MCF10 cells are not affected by the
exposure to micromolar concentrations of 27OHC (5). Recently, we have demonstrated that the
exposure of ER-positive epithelial mammary tumor cells to 2 μM
27OHC for longer than 48 h triggers their transition into a
mesenchymal phenotype (6).
In diverse cell types, the epithelial-mesenchymal
transition (EMT) has been associated with an increase in the
production of reactive oxygen species (ROS) (7). In animals, mitochondrion is the main
source of ROS and in the organelle ≤3% of the transported electrons
can be diverted to the production of superoxide instead of water
(8). The regulation of
mitochondrial ROS production is not completely understood, although
it must be related with the complex modulation of intracellular
metabolic processes (9–11). As mentioned above, reactive oxygen
species are stimulatory molecules, crucial for the generation of
EMT (12). The increased production
of ROS in NB4 cells stimulates the translocation of ERK2 into the
nucleus, triggering phosphorylation of p53 (13); a consequence of p53 activation is an
augmented expression of mitochondrial superoxide dismutase II
(MnSOD, also called SOD type II), enzyme with a major role in the
protection from oxidative stress (14).
Several studies have demonstrated the presence of
ERβ in various tissues (15,16).
Most of the evidence for the presence of this receptor in tissues
and cells has been obtained using specific antibody-based assays
that, in addition, demonstrate its predominantly mitochondrial
localization (17–21). It had been shown that ERβ regulates
the production of energy by mitochondria by controlling the
expression of components from the respiratory chain [(MRC)
(22,23)]. Estrogen-filled receptor promotes
the expression of MnSOD, expanding the survival times of these
target cells (18). Increased
levels of MnSOD protect the cells against oxidative stress and
genotoxic injuries by preventing the damage of the mitochondrial
(mt) genome and by maintaining the activity of mtDNA polymerase.
Ultimately, the enhanced expression of MnSOD defends the cells from
genotoxics damaging effects (24).
The expression of ERs in breast cancer cells seems
to be related to the function of Forkhead transcription factor
FoxM1 (25). Whereas, the
expression of FOXM1 protein and mRNA in breast carcinoma cell lines
appears to be regulated by estrogen-filled ERα (26). Recent studies indicate that the
expression of the FoxM1 transcription factor is regulated by EGFR2
and it was found in ERα-positive breast cancer cell lines that the
expression of this protein kinase-receptor is correlated with the
expression of FoxM1 (27). FOXM1
regulates the expression of genes controlling the cell cycle at the
level of DNA replication and mitosis; an elevated expression of
FOXM1 has been reported in numerous ER-positive tumor cells and
tissues (28,29).
In the present study we compare the responses of
MCF7 cells to 27OHC and estradiol, emphasizing the analysis of the
protein expression of three estrogen-sensitive polypeptides: MnSOD,
FoxM1 and ERβ. Stimulation periods of 48 and 72 h were chosen,
including thus the initial phases of EMT in cells exposed to 27OHC
and comparing the responses with those from either non-treated- or
estradiol-treated cells.
Materials and methods
Tissue culture material was obtained from NalgeNunc
(Rochester, NY, USA). 27-hydroxycholesterol (C6570-000) was
purchased from Steraloids Inc. (Newport, RI, USA). Dulbecco’s
phosphate buffered saline (DPBS) was from Gibco-Invitrogen Corp.
(Carlsbad, CA, USA). Most of the chemicals used here were purchased
from Sigma-Aldrich Inc. (St. Louis, MO, USA) or E. Merck KGaA
(Darmstadt, Germany).
Antibodies and special probes for cell
functionality
Mouse anti-actin (sc-8432), anti-ERα (1D5)
(sc-56833), anti-EGFR2 (sc-08) and anti-β catenin (sc-65482)
monoclonal antibodies were obtained from Santa Cruz Biotechnology
Inc. (Santa Cruz, CA, USA). Rabbit anti-estrogen receptor β (no.
06-629) polyclonal antibody and mouse anti-E-cadherin (MAB3199Z)
monoclonal antibody were from Upstate-Chemicon-Millipore (Temecula,
CA, USA). Rabbit anti-MnSOD (ab-13533) polyclonal antibody was
obtained from Abcam (Cambridge, UK). Mouse anti-FoxM1
(H00002305-M02) monoclonal antibody was from Abnova Inc. (Taipei,
Taiwan). FITC-conjugated goat anti-mouse IgG (F0257),
peroxidase-conjugated goat anti-rabbit IgG (A6667) and
peroxidase-conjugated goat anti-mouse IgG (A9917) were purchased
from Sigma-Aldrich. Alexa Fluor-conjugated donkey anti-rabbit IgG
(A10040) and MitoTracker® Red CMXRos (M7512) were
obtained from Invitrogen/Molecular Probes (Grand Island, NY, USA).
The FITC-Annexin flow kit was purchased from BD Biosciences
(Bedford, MA, USA). Gold-decorated Fab2’ fragment (10
nm) of goat anti-mouse IgG (810.188) and Fab2’ fragment
of goat anti-rabbit IgG (810.166) were obtained from Aurion,
Wageningen, The Netherlands.
Cells
Estradiol-sensitive MCF7 epithelial cells from human
metastatic breast cancer tissue (HTB 22; ATCC, USA), were cultured
in DMEM/F12 containing 10% fetal bovine serum, 100 U/ml penicillin
and 100 μg/ml streptomycin. In the proliferation studies, the cells
were transferred 24 h after seeding to DMEM/F12 containing ITS
(insulin, transferrin, selenium), 1% charcoal/dextran-twice-treated
serum (CDTS), 3% hydroxyethylated starch (HAES), 50 U/ml penicillin
and 50 μg/ml streptomycin. In each of the experiments, the cells
were cultured at 37°C in a humidified incubator, in a 5%
CO2 atmosphere.
Immunofluorescence studies
MCF7 in medium with 10% fetal bovine serum (~5,000
cells/cm2), were seeded on sterile cover glasses hold in
P24 plates incubated for 24 h to allow attachment; subsequently,
non-adherent cells and media were removed. The cells were washed
twice and incubated for 24 h with low-serum culture medium; then
the medium was then adjusted to either 2 μM 27OHC or 2 nM
E2 and the cells further incubated for up to 72 h, the
time required by the cells exposed to 2 μM 27OHC to frankly display
EMT, as checked by immunofluorescent studies using antibodies to
E-cadherin, β-catenin and EGFR2, as described in our previous study
(6). At the end of the incubation,
the cells were fixed (absolute methanol at −20°C), rinsed with DPBS
and blocked for 30 min with 2% BSA in DPBS. The samples were then
incubated with the primary antibodies for 1 h at RT. After
extensive washes (DPBS containing 2% BSA), samples were incubated
with the appropriate secondary antibody for 1 h and washed. The
samples were mounted with Biomeda Gel/Mount (Foster City, CA, USA)
and inspected with a Zeiss Axiophot epifluorescence microscope
fitted with a color CCD camera (Kappa GmbH, Goettingen, Germany).
In each experiment, the images were obtained under fixed settings
of illumination, exposure times and camera gain.
Annexin V/PI labeling
The analyses were carried out using the FITC-Annexin
flow kit from BD Biosciences, following the instructions of the
manufacturer, as described previously (6).
NBT staining
MCF7 cells on cover glasses were incubated for 1 h
at 37°C with a filtered solution of 0.3 mg/ml NBT in DPBS. After
washing with DPBS, the cells were fixed for 5 min with absolute
methanol and rinsed with water. Finally, the coverslips were
mounted with Biomeda Gel/Mount and viewed with differential
interference contrast (DIC) with a Zeiss microscope; the images
were digitalized with a CCD digital camera.
Western blot analyses
MCF7 cells were grown at 37°C and 5% CO2
in phenol red-free media, containing 10% fetal bovine serum. For
the exposure experiments, the cells were either left untreated
(control) or stimulated with 2 nM 17β-estradiol (E2) or
2 μM 27OHC in D-Mem/F12 containing 1% charcoal-treated calf serum.
At different times of treatment, cells were lysed to prepare
extracts for western blot analyses of the ERα, ERβ, MnSOD, actin,
EGFR2, E-cadherin and β-catenin expression.
Mitotracker CMXRos uptake
The uptake of MitoTracker CMXRos was analyzed
following the manufacturer instructions. In brief, cells on the
coverslips were incubated for 15 min with 50 nM of probe, fixed in
3.7% paraformaldehyde, rinsed with prewarmed DPBS, mounted with
Biomeda Gel/Mount, viewed with a Zeiss Axiophot fluorescence
microscope and the images documented with a CCD digital camera.
Indirect immunogold labeling
The cells were processed for immunogold labeling
according to Sierralta et al(30). In brief, cells exposed to the
different treatments, were fixed for 1 h at 20°C with 2% freshly
depolymerized paraformaldehyde/0.05% glutaraldehyde in 0.1 M
phosphate buffer, pH 7.3. After fixation, the cells were
cooled-down, carefully scrapped and thoroughly washed with ice-cold
buffer, dehydrated in a graded ethanol series and infiltrated at
4°C with LR Gold (two changes for 1 h each and then overnight). The
samples were transferred to size-0 gelatine capsules filled with LR
Gold containing 0.8% benzoyl peroxide, placed in a pre-cooled
aluminium block and allowed to polymerize for 30 min at 4°C in a
desiccator evacuated to 500 mm Hg. The blocks were cured for 1–2
days af room temperature and then sectioned with a Reichert
ultramicrotome using diamond knives. The 70–90-nm thin sections
were mounted on pioloform-coated gold grids and immediately
incubated. For this purpose, the grids were floated section
side-down for 15 min each at room temperature on droplets of 0.1 M
glycin in 20 mM HEPES-buffered saline, pH 7.4 (HBS) and 1%
ovalbumin in HBS, then transferred to droplets of the primary
antibody solutions appropriately diluted and left for 2 h at room
temperature followed by 14 h at 4°C. After exhaustive washes with
HBS to remove any free antibody, the sections were incubated for 1
h with the appropriated gold-labelled Fab2’s of the
secondary antibodies diluted 1:30 with 1% ovalbumin in HBS. The
specimens were ‘jet-washed’ with microfiltered buffer and distilled
water, mildly postfixed, slightly contrasted with osmium tetroxide
and uranyl acetate and viewed with a Philips CM 100 operating at 80
kV. Appropriated controls were run to assess the specificity of the
localization procedures; with either of these controls, a
background of <1 gold particle in 200 μm2 of the
sections was observed.
Statistical analyses
Student’s t-test was used to evaluate differences
between samples and the respective controls. P<0.05 was
considered significant. Data were analyzed with Statistica for
Windows Software, release 6, Statsoft Inc., USA.
Results
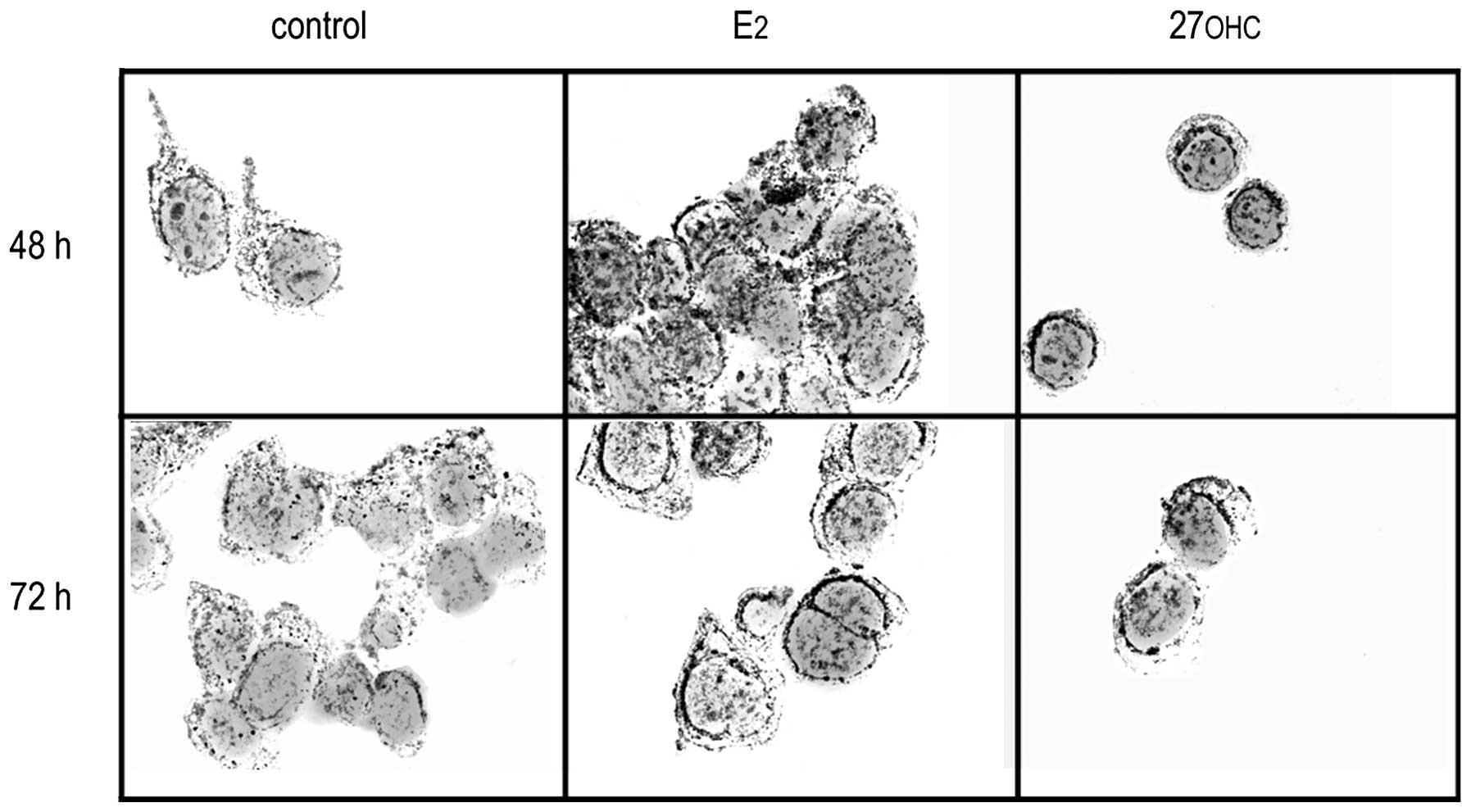
Non-stimulated MCF7 cells exhibited a basal capacity
to build peroxides in the cytoplasm, as detected using the
nitroblue tetrazolium test (NBT). Those MCF7 cells exposed to 2 nM
E2 or 2 μM 27OHC displayed increased precipitation of
insoluble formazan as compared with the non-stimulated, control
cells; the NBT precipitate was associated with particulate elements
of the cytoplasm (Fig. 1).
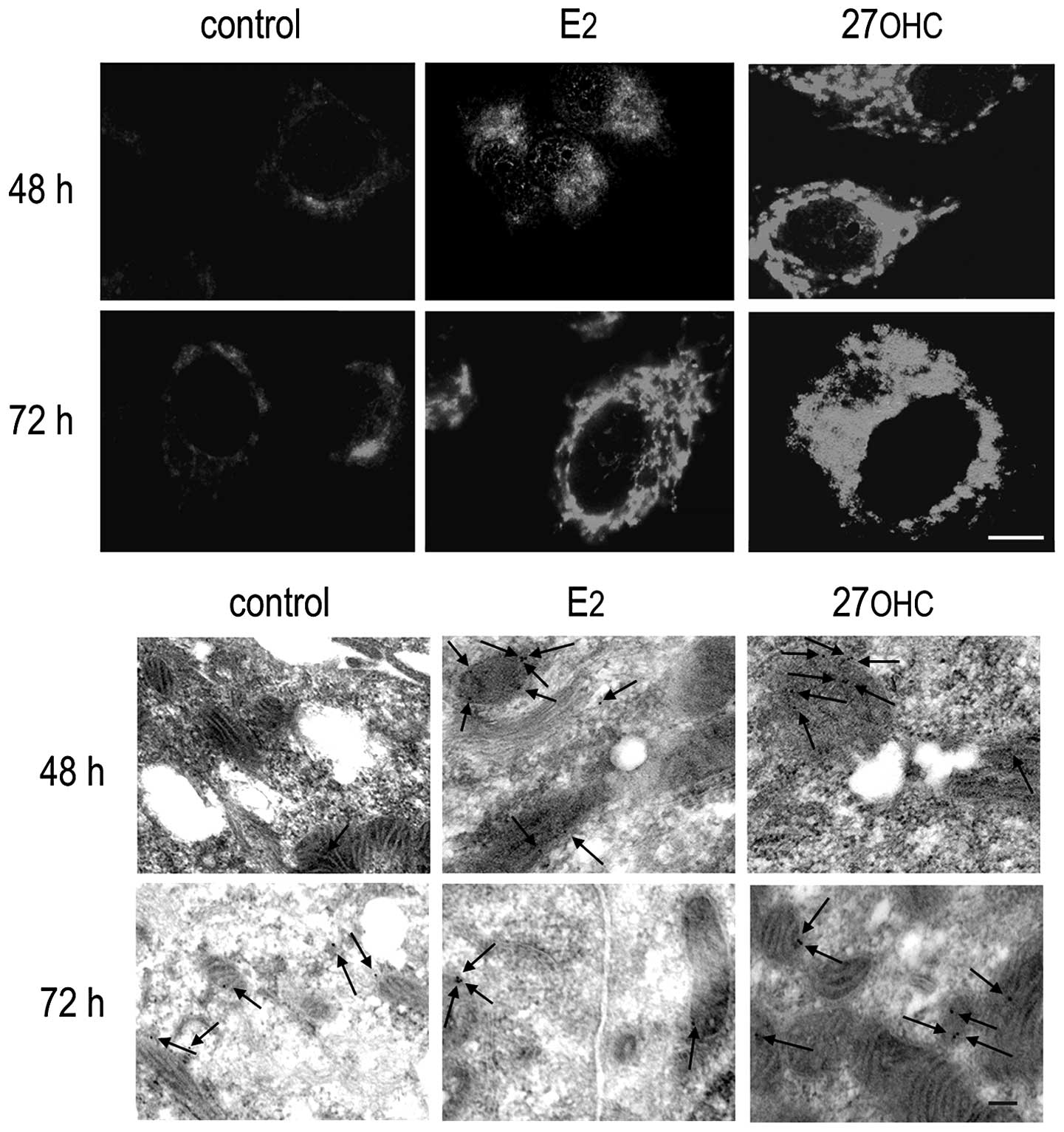
We analyzed for the possible relationship between
the augmented production of insoluble formazan and the expression
levels of MnSOD in MCF7 cells. Using the specific antibody,
immunoreactivity of this dismutase was observed in the mitochondria
of MCF7 cells (upper panel, Fig.
2). Following stimulation with either E2 or 27OHC,
increases were observed in the expression of MnSOD when compared to
non-stimulated cells. The assignation of MnSOD to mitochondria was
confirmed by high-resolution, electron microscopy immunogold
labeling. The lower panel shown in Fig.
2 depicts representative images of labelled mitochondria in
ultrathin sections from non-stimulated and stimulated MCF7 cells
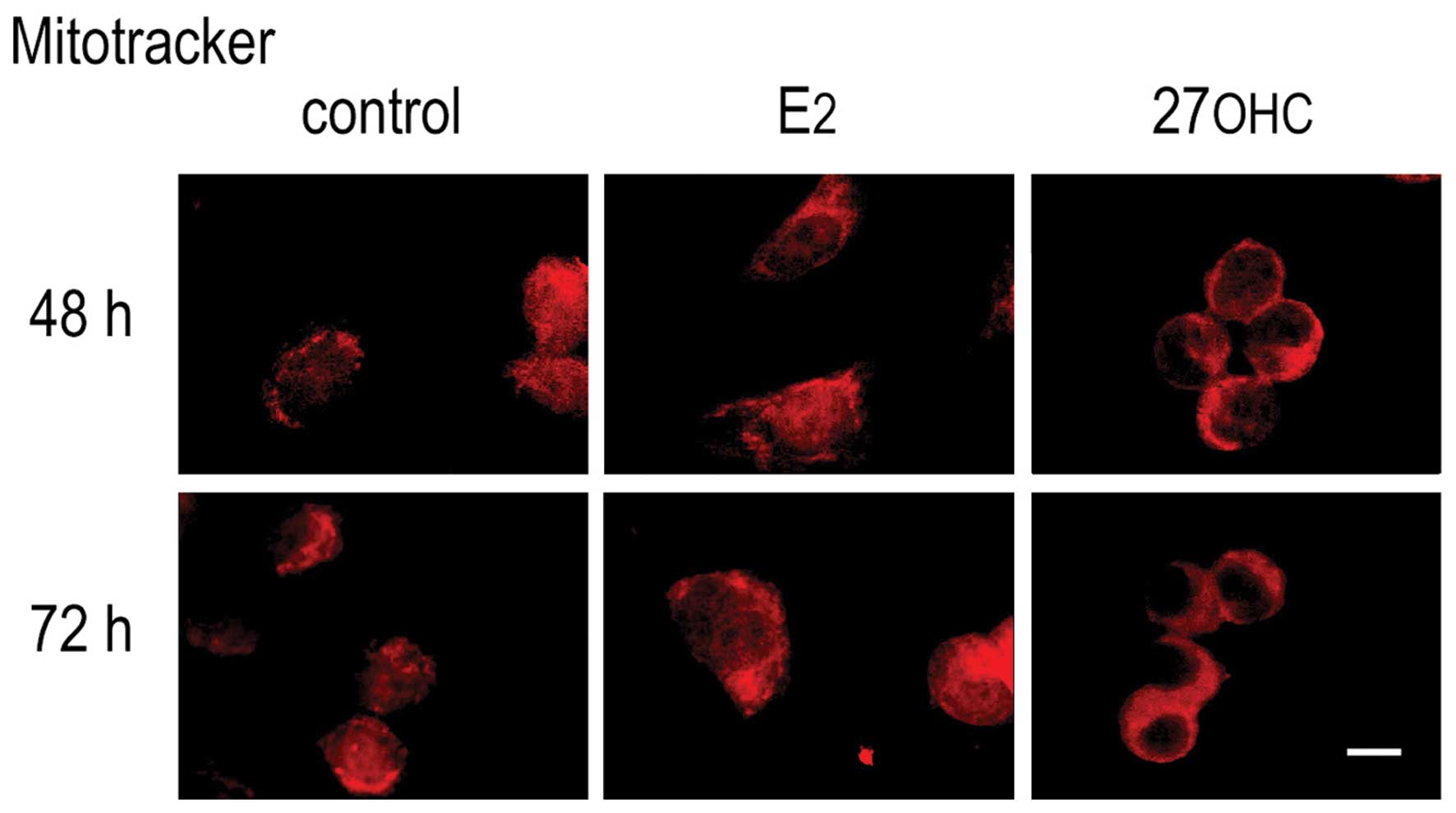
embedded in LR-Gold resin. The fluorescence images of MnSOD
presence shown in Fig. 2 were
analogous to those obtained from the in vivo uptake of
Mitotracker CMXRos (Fig. 3).
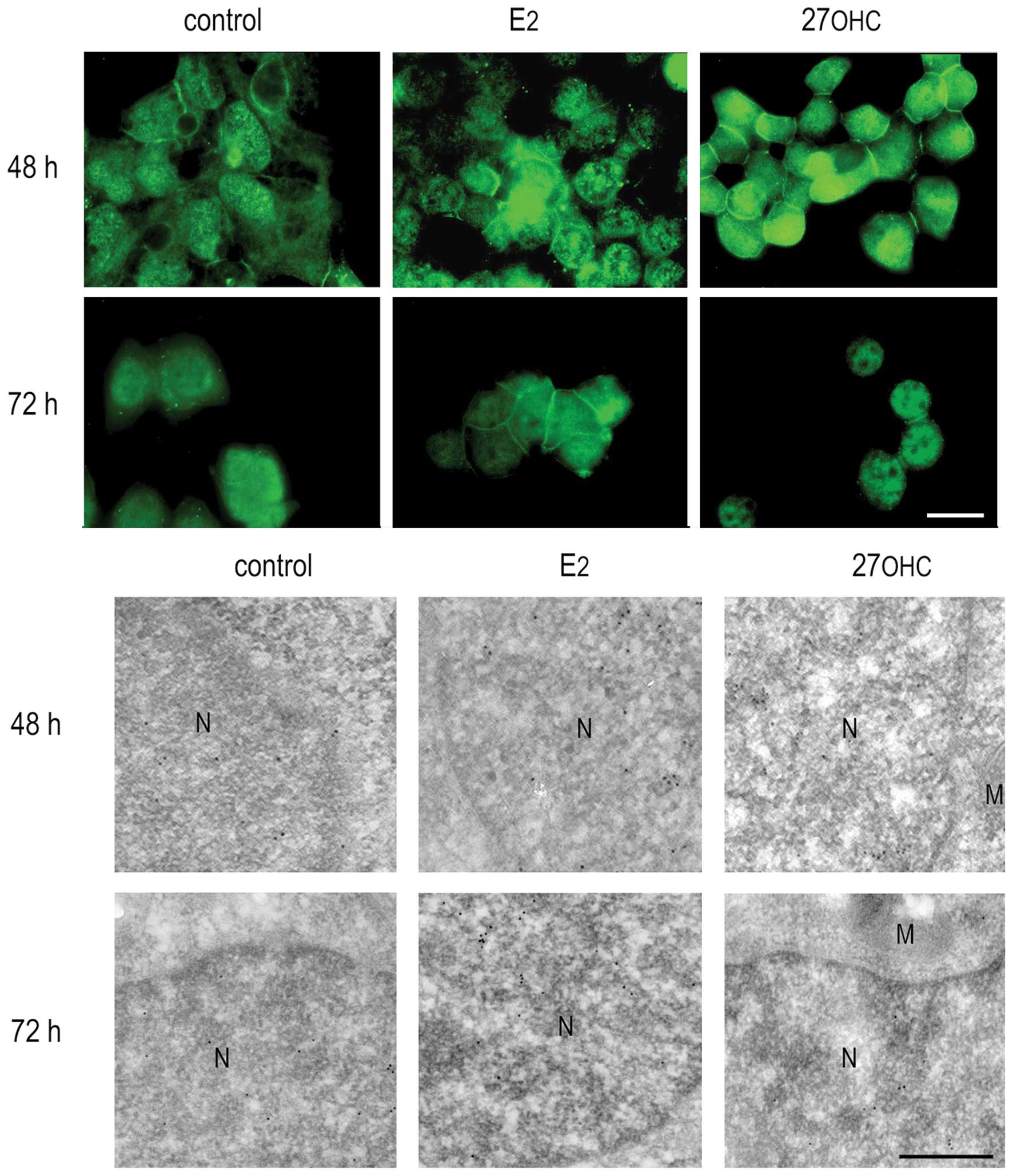
The immunoreactivity of ERβ showed essentially a
mitochondrial localization in stimulated and non-stimulated cells.
Higher expression of ERβ was detected after 48 and 72 h estradiol
or 27OHC, as shown in the upper panel of Fig. 4. Immunogold-labeling of ultrathin
sections with the specific anti-ERβ antibody demonstrated a
predominant mitochondrial residence of ERβ after either
sterol-stimulation.
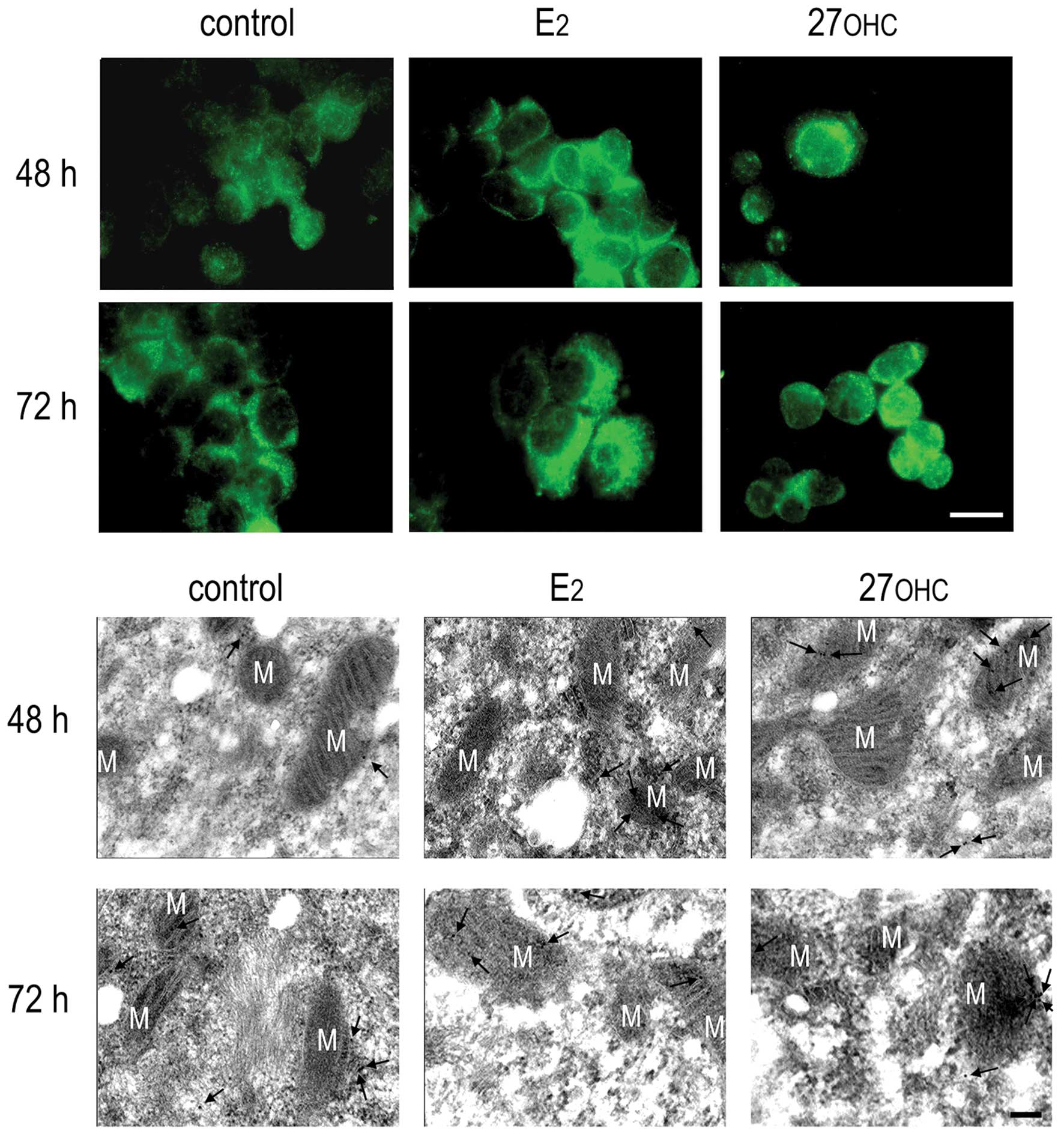
In non-stimulated cells, FoxM1 was customarily
detected both in cytoplasm and nucleus by the specific monoclonal
antibody. The expression of this potent proliferation controller
increased in the nucleus in MCF7 cells exposed for 48 h to 2 nM
estradiol or 2 μM 27OHC; at 72 h the immunoreactivity remained high
in estradiol-treated cells, but declines in the cells treated with
27OHC, as depicted in the upper panel of Fig. 5. This increase in nuclear
immunoreactivity was confirmed at ultrastructural level by
immunogold labelling, as shown in the lower panel of Fig. 5.
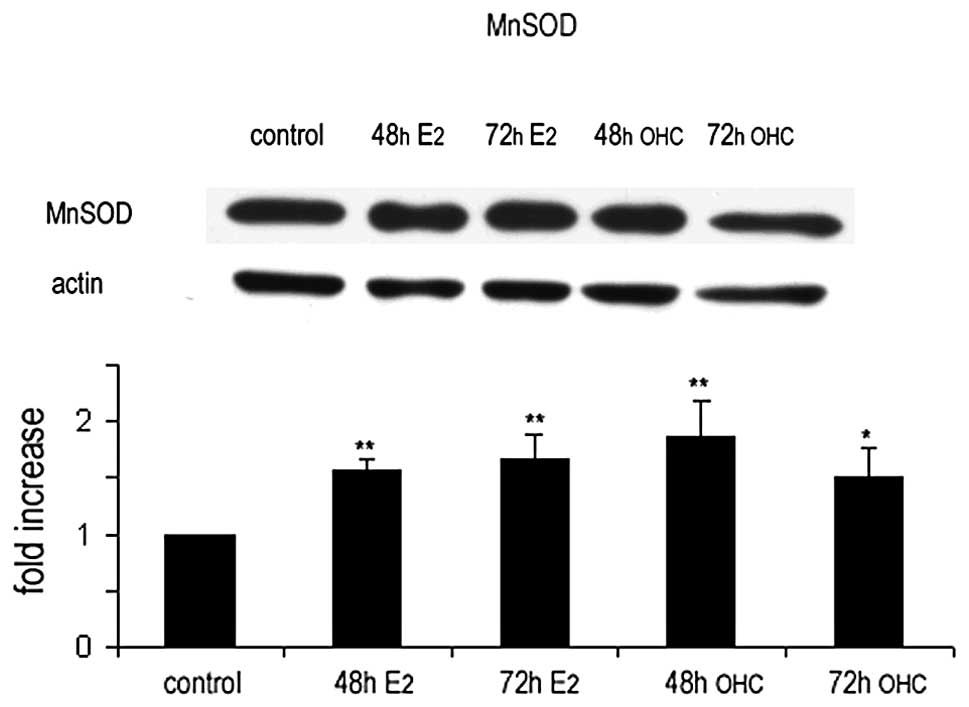
The changes in MnSOD expression in MCF7 cells
exposed to estradiol or 27OHC were analyzed in cell extracts by
western blotting; the results confirmed those obtained by indirect
immunofluorescence and immunogold labelling studies (Fig. 6).
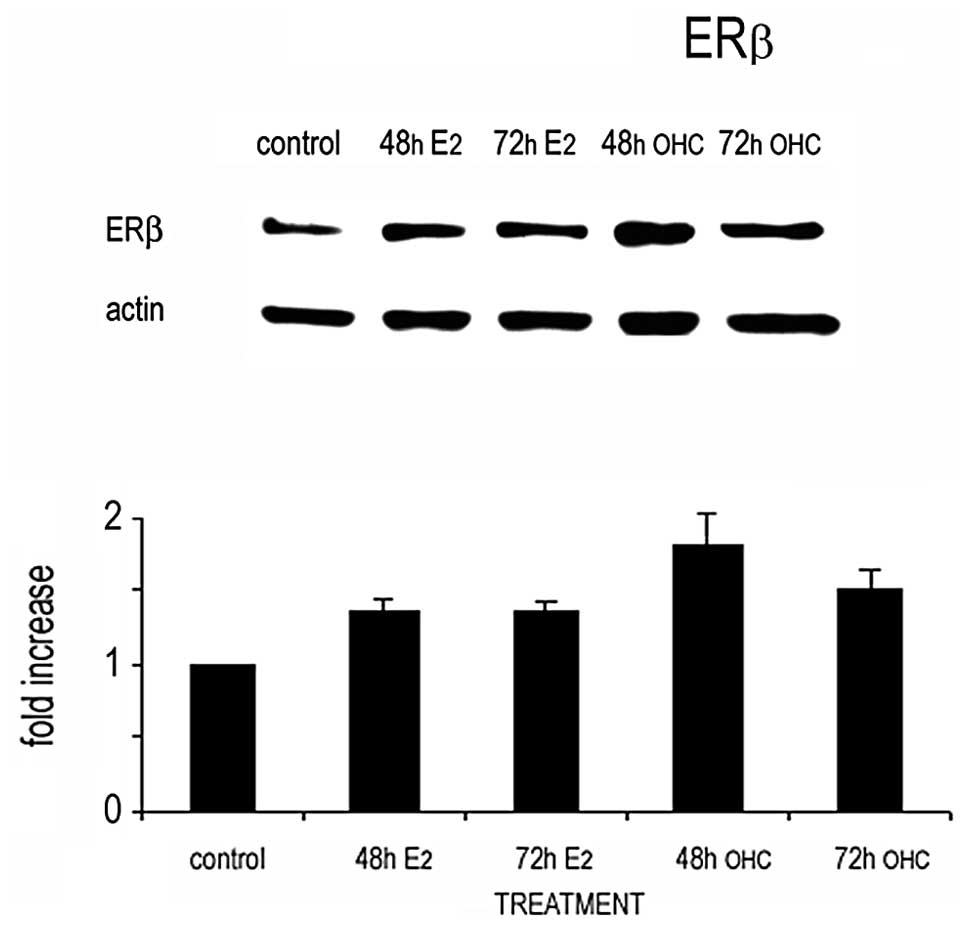
The changes in the expression of ERβ after exposure
to E2 or 27OHC were analyzed in cell extracts by western
blotting. The results obtained were coherent with those obtained by
indirect immunofluorescence of whole cells and immunogold labelling
analyses of cell sections (Fig. 7).
Unfortunately, the specific anti-FoxM1 antibody, used for
immunomicroscopy, was non-satisfactory in our hands to detect the
protein in western blot analyses, independent on the protocol
followed.
Discussion
In active cells, tetrazolium salts are reduced in
vivo to formazan by the superoxide ion (31); therefore, NBT is widely used as an
indicator of mitochondrial metabolism (11,32).
Exposing MCF7 cells to either E2 or 27OHC, we observed
an increased production of superoxide radical in comparison to the
non-treated, control cells. The fluorescence images were comparable
to those obtained from the in vivo uptake of Mitotracker
CMXRos (Fig. 3). Combined, the
results confirm that the treatment with either of these ER-ligands
augment the mitochondrial activity, thus increasing ROS production.
In parallel to an augmented production of superoxide, we detected
the increased expression of MnSOD in stimulated MCF7 cells,
suggesting the potential conformation of a defence machinery,
probably with the goal to reduce the genotoxic effects from ROS
released by the stimulation.
We have demonstrated that an exposure of MCF7 cells
to 2 μM 27OHC for at least 48 h is adequate for the induction of
their epythelial to mesenchymal transition (EMT) (6). The association between an increased
production of mitochondrial ROS and induction of EMT in diverse
cell types has been reported (7).
In the course of EMT, cells acquiring the mesenchymal phenotype
exhibit migration and invasion abilities, with new contractile and
motile properties. As shown by Giannoni et al, ROS activates
the phosphorylation of members from the epidermal growth factor
receptor (EGFR) family; this redox-dependent phosphorylation of
EGFR activates the protein kinases Akt and ERK causing, among
others, the degradation of pro-apoptotic proteins (33). In our earlier report we showed that
MCF7 cells undergoing the 27OHC-triggered EMT displayed a
persistently activated EGFR2 at the plasma membrane with increased
traslocation of pERK into the cell nucleus; interestingly, these
cells did not exhibit variations in cell death rates by Annexin
V/PI labeling (6). In contrast to
the non-genomic mitogenic estradiol effects on MCF7 cells, where
just a transient stimulation of EGFR2 is observed (5), the exposure to 27OHC caused permanent
activation of this membrane receptor. As mentioned in our previous
study, 27OHC-triggered EGFR2 activation is not related to the
secretion of matrix metalloproteinases (MMPs) nor to the trimming
and release of HB-EGF (5). Instead,
the most important visible signals for the ongoing EMT were the
marked evanescence of E-cadherin, the notorious cellular
re-allocation of β-catenin and the permanent activation of EGFR2
(6).
Following the launch of changes in the shape and
function of cells experiencing EMT, increases in the cell death
rate have been observed (11). As
indicated above, we did not detect apoptosis in MCF7 cells
following the 27OHC-induced EMT (6). Probably, the increased MnSOD
expression would represent a protective cellular response,
developed to bypass the toxic effects of the reactive oxygen
species generated upon exposure to 27OHC. An example of 27OHC
toxicity is provided by experiments using hippocampus-containing
rabbit brain slices, where the exposure to 27OHC rapidly induced
ROS and oxidative stress in nerve cells, eliciting anatomical and
physiological alterations comparable to those observed in the brain
of Alzheimer disease patients (34).
The changes induced by estradiol and 27OHC in the
proliferation of MCF7 cells and their subsequent EMT, triggered by
the oxysterol, are accompanied by an augmented expression of ERβ in
mitochondria. It has been reported that the activity of
mitochondrial respiratory complex IV (MRC-IV) in cardiomyocytes
tightly depends on mitochondrial ERβ associated to SERMs; in
addition, the filled-ERβ complex inhibit mitochondrial apoptotic
signaling pathways (22). These
observations indicate that ERβ is closely related with the control
of mitochondrial activity. Conflicting opinions regarding the
expression of ERβ in MCF7 cells are been formulated (35); however, numerous studies have
demonstrated that this type of estrogen receptor is present in MCF7
cells (24,26,36).
In the studies presented here, we confirmed the presence of this
receptor subtype inside mitochondria of MCF7 cells and, in
addition, observed changes of its expression following stimulation
with estradiol and 27OHC.
Transcription factor Forkhead box M1 (FOXM1) is a
key regulator of cell proliferation being overexpressed in many
forms of cancers and leading to uncontrolled cell division and
genomic instability (37). An
increased expression of FOXM1 is associated with the augmented
proliferation of many tumor cell lines: FOXM1 regulates the
transcription of genes that participate in the control of the cell
cycle (38). The antagonist SERM
4-hydroxy-tamoxifen inhibits the expression of FOXM1 in ER-positive
but not in ER-negative breast cancer cell lines, supporting the
concept of FOXM1 as a key mediator for the activity of SERMs in
ER-positive breast cancer cells (39). A positive correlation between FOXM1
expression and EGFR2 status in ERα-positive breast cancer cells has
been found (28), but more
information on the interaction between FOXM1 and EGFR2 is required
to discern whether FOXM1 directly activates or not the expression
of EGFR2. In our experiments, the MCF7 cells exposed for 72 h to
27OHC exhibited a slightly lower expression of FOXM1 as compared to
cells exposed to E2 for the same time. Cells treated for
72 h with 27OHC, display a complete EMT, as judged from the
immunolabelling patterns of β-catenin, E-cadherin and EGFR2 and the
absence of proliferation (6). It
has been demonstrated in glioma tumorigenesis, that FOXM1
associates with β-catenin and activate the Wnt pathways (40).
The results presented in this and previous studies
(5,6) suggest that in MCF7 cells, 2 μM 27OHC
initially functions as a regular agonist SERM; an exposure to the
oxysterol exceeding 48 h will initiate a non-reversible
phenotypical change into a mesenchymal cell type, with increased
expression of MnSOD, ERβ and FOXM1. At the level of these proteins,
comparable results were obtained at 48-h exposure between 27OHC and
E2, the most specific ER-ligand. However, we detected
lower nuclear levels of FOXM1 after an exposure of 72 h to 27OHC
and estradiol. The oxisterol-treated cells displayed lower
immunoreactivity of the protein, in parallel with an arrested
proliferation in the MCF7 cells, transformed by an EMT process by
activation of the Wnt signaling pathway.
It is well known that resistance may develop during
the treatment of mammary cancer with anti-estrogens and aromatase
inhibitors. This may be related to a continual operation of
estrogen signaling pathways; a permanently activated path may be
the consequence of an association of ERs with endogenous SERMs,
such as 27OHC. Through metabolism of profusely available
cholesterol, some cells produce and secrete 27OHC in their
neighborhood; the infiltration with macrophages is a negative
prognostic indicator in patients with a mammary gland tumor,
probably because macrophages vigorously produces 27OHC (41). It is quite possible that in
infiltrated tissues, tumor cells are constantly stimulated by local
macrophages transforming cholesterol into 27OHC.
It has been suggested that obesity increases the
risk of breast cancer because the high aromatase levels of adipose
tissue produce elevated local concentrations of estradiol. However,
obesity is associated with hypercholesterolemia and increased
levels of circulating 27OHC, therefore, a stimulatory effect of the
oxysterol will likely be observed in ER-positive cancer cells from
obese patients. Furthermore, the triggering of EMT by 27OHC in MCF7
cells strengthens the warning about the risks from obesity for
breast cancer patients.
Acknowledgements
This study was supported by Fondecyt Chile, grant
no. 1090057.
References
|
1
|
Umetani M, Domoto H, Gormley AK, Yuhanna
IS, Cummins CL, Javitt NB, Korach KS, Shaul PW and Mangelsdorf DJ:
27-Hydroxycholesterol is an endogenous SERM that inhibits the
cardiovascular effects of estrogens. Nat Med. 13:1185–1192. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DuSell CD, Umetani M, Shaul PW,
Mangelsdorf DJ and McDonnell DP: 27-Hydroxycholesterol is an
endogenous selective estrogen receptor modulator. Mol Endocrinol.
22:65–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DuSell CD, Nelson ER, Wang X, Abdo J,
Mödder UI, Umetani M, Gesty-Palmer D, Javitt NB, Khosla S and
McDonnell DP: The endogenous selective estrogen receptor modulator
27-hydroxycholesterol is a negative regulator of bone homeostasis.
Endocrinology. 151:3675–3685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nelson ER, DuSell CD, Wang X, Howe MK,
Evans G, Michalek RD, Umetani M, Rathmell JC, Khosla S,
Gesty-Palmer D and McDonnell DP: The oxysterol,
27-hydroxycholesterol links cholesterol metabolism to bone
homeostasis through its actions on the estrogen and liver X
receptors. Endocrinology. 152:4691–4705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cruz P, Torres C, Ramírez ME, Epuñán MI,
Valladares LE and Sierralta WD: Proliferation of human mammary
cancer cells exposed to 27-hydroxycholesterol. Exp Ther Med.
1:531–536. 2010.PubMed/NCBI
|
|
6
|
Torres CG, Ramírez ME, Cruz P, Epuñan MI,
Valladares LE and Sierralta WD: 27hydroxycholesterol induces the
transition of MCF7 cells into a mesenchymal phenotype. Oncol Rep.
26:389–397. 2011.PubMed/NCBI
|
|
7
|
Zhou G, Dada LA, Wu M, Kelly A, Trejo H,
Zhou Q, Varga J and Sznajder JI: Hypoxia-induced alveolar
epithelial-mesenchymal transition requires mitochondríal ROS and
hypoxia-inducible factor I. Am J Physiol Lung Cell Mol Physiol.
297:L1120–L1130. 2009.PubMed/NCBI
|
|
8
|
Boveris A and Chance B: The mitochondrial
generation of hydrogen peroxide: general properties and effect of
hyperbaric oxygen. Biochem J. 134:707–716. 1973.PubMed/NCBI
|
|
9
|
Nemoto S, Takeda K, Yu Z-X, Ferrans VJ and
Finkel T: Role for mitochondrial oxidants as regulators of cellular
metabolism. Mol Cell Biol. 20:7311–7318. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicholls DG and Budd SU: Mitochondria and
neuronal survival. Physiol Rev. 80:315–360. 2000.PubMed/NCBI
|
|
11
|
Werner E and Werb Z: Integrins engage
mitochondrial function for signal transduction by a mechanism
dependent on Rho GTPases. J Cell Biol. 158:357–368. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giannoni E, Bianchini F, Calorini L and
Chiarugi P: Cancer associated fibroblasts exploit reactive oxygen
species through a proinflammatory signature leading to epithelial
mesenchymal transition and stemness. Antioxid Redox Signal.
14:2361–2371. 2011. View Article : Google Scholar
|
|
13
|
Li Z, Shi K, Guan L, Cao T, Jiang Q, Yang
Y and Xu C: ROS leads to MnSOD upregulation through ERK2
translocation and p53 activation in selenite-induced apoptosis of
NB4 cells. FEBS Lett. 584:2291–2297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bakthavatchalu V, Dey S, Xu Y, Noel T,
Jungsuwadee P, Holley AK, Dhar SK, Batinic-Haberle I and St Clair
DK: Manganese superoxide dismutase is a mitochondrial fidelity
protein that protects Polγ against UV-induced inactivation.
Oncogene. Sep 12–2011.(Epub ahead of print). View Article : Google Scholar
|
|
15
|
Chen M, Ni J, Chang HC, Lin CY, Muyan M
and Yeh S: CCDC62/ERAP75 functions as a coactivator to enhance
estrogen receptor beta-mediated transactivation and target gene
expression in prostate cancer cells. Carcinogenesis. 30:841–850.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monje P and Boland R: Subcellular
distribution of native estrogen receptor alpha and beta isoforms in
rabbit uterus and ovary. J Cell Biochem. 82:467–479. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fox EM, Davis RJ and Shupnik MA: ERβ in
breast cancer -onlooker, passive player or active protector?
Steroids. 73:1039–1051. 2008.
|
|
18
|
Yager ID and Chen JQ: Mitochondrial
estrogen receptors-new insights into specific functions. Trends
Endocrinol Metab. 18:89–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klinge CM: Estrogenic control of
mitochondrial function and biogenesis. J Cell Biochem.
105:1342–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Psarra AM and Sekeris CE: Steroid and
thyroid hormone receptors in mitochondria. IUBMB Life. 60:210–223.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simpkins JW, Yang SH, Sarkar SN and Pearce
V: Estrogen actions on mitochondria: physiological and pathological
implications. Mol Cell Endocrinol. 290:51–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh YC, Yu HP, Suzuki T, Choudhry MA,
Schwacha MG, Bland KI and Chaudry IH: Upregulation of mitochondrial
respiratory complex IV by estrogen receptor-beta is critical for
inhibiting mitochondrial apoptotic signaling and restoring cardiac
functions following trauma-hemorrhage. J Mol Cell Cardiol.
41:511–521. 2006. View Article : Google Scholar
|
|
23
|
Chen JQ, Russo PA, Cooke C, Russo IH and
Russo J: ERbeta shifts from mitochondria to nucleus during
estrogen-induced neoplastic transformation of human breast
epithelial cells and is involved in estrogen-induced synthesis of
mitochondrial respiratory chain proteins. Biochim Biophys Acta.
1773:1732–1746. 2007. View Article : Google Scholar
|
|
24
|
Bakthavatchalu V, Dey S, Xu Y, Noel T,
Jungsuwadee P, Holley AK, Dhar SK, BatinicHaberle I, St Chen JQ,
Delannoy M, Cooke C and Yager ID: Mitochondrial localization of
ERalpha and ERbeta in human MCF7 cells. Am J Physiol Endocrinol
Metab. 286:E1011–E1022. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madureira PA, Varshochi R, Constantinidou
D, Francis RE, Coombes RC, Yao KM and Lam EW: The Forkhead box M1
protein regulates the transcription of the estrogen receptor alpha
in breast cancer cells. J Biol Chem. 281:25167–25176. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Millour J, Constantinidou D, Stavropoulou
AV, Wilson MS, Myatt SS, Kwok JM, Pedram A, Razandi M, Wallace DC
and Levin ER: Functional estrogen receptors in the mitochondria of
breast cancer cells. Mol Biol Cell. 17:2125–2137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Francis RE, Myatt SS, Krol J, Hartman J,
Peck B, McGovern UB, Wang J, Guest SK, Filipovic A, Gojis O,
Palmieri C, Peston D, Shousha S, Yu Q, Sicinski P, Coombes RC and
Lam EW: FoxM1 is a downstream target and marker of HER2
overexpression in breast cancer. Int J Oncol. 35:57–68.
2009.PubMed/NCBI
|
|
28
|
Bektas N, Haaf A, Veeck J, Wild PI,
Lüscher-Firzlaff J, Hartmann A, Knüchel R and Dahl E: Tight
correlation between expression of the Forkhead transcription factor
FOXMi and HER2 in human breast cancer. BMC Cancer. 8:422008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sivanandan K, Coombes RC, Medema RH,
Hartman J, Lykkesfeldt AE and Lam EW: FOXM1 is a transcriptional
target of ERalpha and has a critical role in breast cancer
endocrine sensitivity and resistance. Oncogene. 29:2983–2995. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sierralta WD, Boenig I and Thole HH:
Immunogold labelling of estradiol receptor in MCF 7 cells. Cell Tis
Res. 279:445–452. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Flohé L and Otting F: Superoxide dismutase
assays. Methods Enzymol. 105:93–104. 1984.
|
|
32
|
Powers SK, Lieu FK, Criswell D and Dodd S:
Biochemical verification of quantitative histochemical analysis of
succinate dehydrogenase activity in skeletal muscle fibres.
Histochem J. 25:491–496. 1993. View Article : Google Scholar
|
|
33
|
Giannoni E, Buricchi F, Grimaldi G, Parri
M, Cialdai F, Taddei ML, Raugei G, Ramponi G and Chiarugi P: Redox
regulation of anoikis: reactive oxygen species as essential
mediators of cell survival. Cell Death Differ. 15:867–878. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prasanthi JRP, Larson T, Schommer J and
Ghribi O: Silencing GADD153/CHOP gene expression protects against
Alzheimer’s disease-like pathology induced by 27-hydroxycholesterol
in rabbit hippocampus. PLoS One. 6:e26420 View Article : Google Scholar : 2011.PubMed/NCBI
|
|
35
|
Nilsson S and Gustafsson JA: Estrogen
receptors: therapies targeted to receptor subtypes. Clin Pharmacol
Ther. 89:44–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao C, Dahlman-Wright K and Gustafsson
JA: Estrogen signaling via estrogen receptors. J Biol Chem.
285:39575–39579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kwok JM, Peck B, Monteiro U, Schwenen HD,
Millour J, Coombes RC, Myatt SS and Lam EW: FOXM1 confers acquired
cisplatin resistance in breast cancer cells. Mol Cancer Res.
8:24–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chua PJ, Yip GW and Bay BH: Cell cycle
arrest induced by hydrogen peroxide is associated with modulation
of oxidative stress related genes in breast cancer cells. Exp Biol
Med. 234:1086–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karadedou CT: Regulation of the FOXM1
transcription factor by the estrogen receptor alpha at the protein
level in breast cancer. Hippokratia. 10:128–132. 2006.PubMed/NCBI
|
|
40
|
Zhang N, Wei P, Gong A, Chiu WT, Lee HT,
Colman H, Huang H, Xue J, Liu M, Wang Y, Sawaya R, Xie K, Yung WK,
Medema RH, He X and Huang S: FoxM1 promotes R-catenin nuclear
localization and controls Wnt target-gene expression and glioma
tumorigenesis. Cancer Cell. 20:427–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Steele RJ, Eremin O, Brown M and Hawkins
RA: A high macrophage content in human breast cancer is not
associated with favourable prognostic factors. Br J Surg.
71:456–458. 1984. View Article : Google Scholar : PubMed/NCBI
|