Introduction
Kidney tumors may be benign or malignant. Benign
tumors are incidentally findings at autopsy and are rarely of
clinical significance with the exception of oncocytoma. On the
other hand, malignant tumors arise from renal epithelial cells and
are of great clinical importance. They are the third most common
malignancy of the urinary tract after prostate and bladder cancer
(1). The renal cell carcinomas
(RCC) can be pathologically classified into subtypes: the clear
cell type, which constitutes 80% of all cases, the papillary type,
at around 15% and the remaining 5% of other histological types
(chromophobe, collecting duct and unclassified RCC). The subtype
chromophobe RCC cases have a better prognosis compared to those of
clear cell RCC (ccRCC) due to the high incidence of cell invasion
in the ccRCC subtype. Early stage RCC is relatively asymptomatic,
and the classical triad of flank pain, hematuria and of renal mass
only manifests very late in the course of the disease. The
diagnosis is confirmed with imaging studies, and many cases are now
accidentally discovered during routine imaging. Moreover, kidney
biopsy is an invasive technique that may result in complications
and will not be able to provide accurate diagnosis in certain
situations (2). Therefore, there is
a pressing need for non-invasive methods to diagnose carcinoma of
the kidney as well as for follow-up surveillance.
Matrix metalloproteinases (MMPs) form a family of
>25 endopeptidases known to degrade extracellular matrix and
basal membrane. MMPs depend on a Zn2+ ion to degrade
components of the extracellular matrix such as fibrillar and
non-fibrillar collagen, proteoglycans, glycoproteins and denatured
collagen. Furthermore, MMPs are involved in direct and indirect
release of growth factors enhancing tumor growth and
tumorigenicity. In particular, the ability to degrade type IV
collagen, the major component of the basement membrane, is unique
to MMP-2 and MMP-9 also known as gelatinase A (72 kDa) and
gelatinase B (92 kDa), respectively. These two MMPs are most often
linked to the malignant phenotype of tumor cells, and are commonly
used as markers of malignant cancer (3–5).
In the present study, we determined MMP-2 and MMP-9
activity levels in sera and urine from patients with oncocytoma and
clear renal cell carcinoma using gelatin zymography in order to
analyze the pattern of gelatinolityc activities and to verify
whether they may have potential as non-invasive biomarker in
providing useful clinical information in kidney cancer.
Materials and methods
Patients
Peripheral venous blood samples and first morning
urine were collected from patients before surgical or other
therapeutic intervention.
Specimens were obtained from patients who underwent
surgical procedure. Diagnosis of tumors was made by usual clinical
laboratory criteria and confirmed postoperatively by
histopathological findings. The age of the patients was between 40
and 73 years (mean ± SD, 59.2±9.7) and there were 11 males and 19
females. The tumors were classified for grade and stage according
to the pTNM classification (6). All
patients provided written informed consent. The study was approved
by the local ethics committee. Sixteen healthy volunteers with no
concomitant illnesses were used as controls. The age of the healthy
volunteers was between 30 and 70 years (mean ± SD, 57±11) and there
were 9 males and 7 females. Healthy volunteers gave their
permission verbally. The subjects in the controls had no sign of
infections, gastrointestinal hepatic or renal disease, tumors or
immunologic disease. The values of the basic laboratory parameters
of these participants were within the reference limits.
Serum
Native serum was prepared using plastic tubes
without coagulation accelerators, to prevent the release of
gelatinase during platelet activation. Tubes were centrifuged at
1600 × g for 10 min, 30 min after blood collection. For each
sample, determination of protein concentration was performed using
the Bradford method (7). Sera were
aliquoted and stored at −20°C until used. Each aliquot was used
only once in order to prevent enzyme activation due to
freeze-thawing processes.
Urine sample preparation
Prior to analysis, urine samples were tested using
Multistix Combur test (Roche Diagnostic GmbH, Mannheim). Urine
samples positive for leukocytes were excluded because of
confounding leukocytic gelatinases. Microscopic hematuria present
in most cancer samples was not quantified but grossly hematuric
samples were excluded. Samples were frozen immediately after
collection and stored frozen (−20°C) until assay. The samples were
thawed and an aliquot of each sample (15 ml) were centrifuged at
1000 × g for 10 min at 4°C.
An aliquot of the supernatant of each sample (2 ml)
was concentrated by ultrafiltration using Vivaspin 2 spin column
membrane molecular weight cut-off (MWCO): Mr 30000 according to
manufacturer's instructions (Sartorius Stedim Biotech GmbH,
Goettingen, Germany). An aliquot (12 μl) of concentrate urine was
used to determine MMP-2 and MMP-9 by gelatin zymography.
Materials
Gelatinase A and gelatinase B were purchesed from
Hoffmann-La Roche Ltd (Basel, Switzerland). Calcium chloride
(CaCl2) glycerol, gelatin, ethylenediaminetetraacetic
(EDTA), Triton X-100, phenylmethylsulphonyl fluoride (PMSF) were
from Sigma Chemical Co. (St. Louis, MO, USA). Ultra filtration spin
columns were from Sartorius Stedim Biotech GmbH. All other reagents
were available from commercial sources.
Gelatin zymography
Zymography was performed using 7.5 % (w/v)
polyacrylamide gels containing 0.1% (w/v) of gelatine as previously
described (8,9). Briefly, serum samples or concentrated
urine samples were mixed with sample buffer (10 mM Tris-HCl pH 6.8,
12.5% SDS, 5% sucrose, 0.1% bromophenol blue) and applied directely
without prior heating or reduction to the gel. After removal of SDS
from the gel by incubation in 2.5% (v/v) Triton X-100 for 1 h, the
gels were incubated at 37°C for 18 h in 50 mM Tris-HCl pH 7.6
containing 0.2 M NaCl, 5 mM CaCl2 and 0.02% (w/v) Brij
35. Gels were stained for 1 h in 30% methanol, 10% glacial acetic
acid containing 0.5% (w/v) Coomassie Brilliant Blue G-250 and
destained in the same solution without dye for several hours. The
gelatinolytic activity of each collagenase was evident as a clear
band against the blue background of stained gelatin. The molecular
size of bands displaying enzymatic activity were identified by
comparison with prestained standard protein, as well as with
purified gelatinase A or gelatinase B. To normalize the possible
difference between zymograms an internal serum or urine sample from
a patient was incorporated in every gel.
Control gels for MMPs
Control gels contained either of the MMP selective
inhibitors, 20 mM EDTA or 10 mM 1,10 phenanthroline, in the MMP
incubation buffer to confirm that the lysis band was the result of
MMPs. Furthermore, the character of proteolytic bands was analyzed
by incubating the identical zymograms in 0.1 mg/ml of PMSF, a
serine protease inhibitor; or 2 mM Pefabloc, an irreversible serine
protease inhibitor.
Analysis of the gels
Following zymography, the degree of gelatin
digestion was quantified as previously described. Briefly, we used
an image analysis software (ImageQuant TL, Amersham Bioscience,
Chicago, IL, USA) according to the manufacturer's specifications.
The image of the gel was inverted to reveal dark bands on a white
background. The molecular weight, volume and background of each
band were determined. The relative amounts of the different forms
of both serum and urine gelatinases were expressed as the
integrated density ×10−3 (volume) of all the pixels
above the background of each band.
Results
During a 1-year period a total of 20 patients with
kidney disease were evaluated. Of these patients, 4 had oncocytoma
and 16 had clear cell renal carcinoma (ccRCC). All patients
provided venous blood samples; among these the 4 patients with
oncocytoma and 9 patients with ccRCC collected their first morning
urine.
To investigate the gelatinolytic activity present in
the serum and in concentrate urine, substrate gel zymography was
performed. This method allows the detection of the
metalloproteinases that exhibit gelatinolytic activity (gelatinase
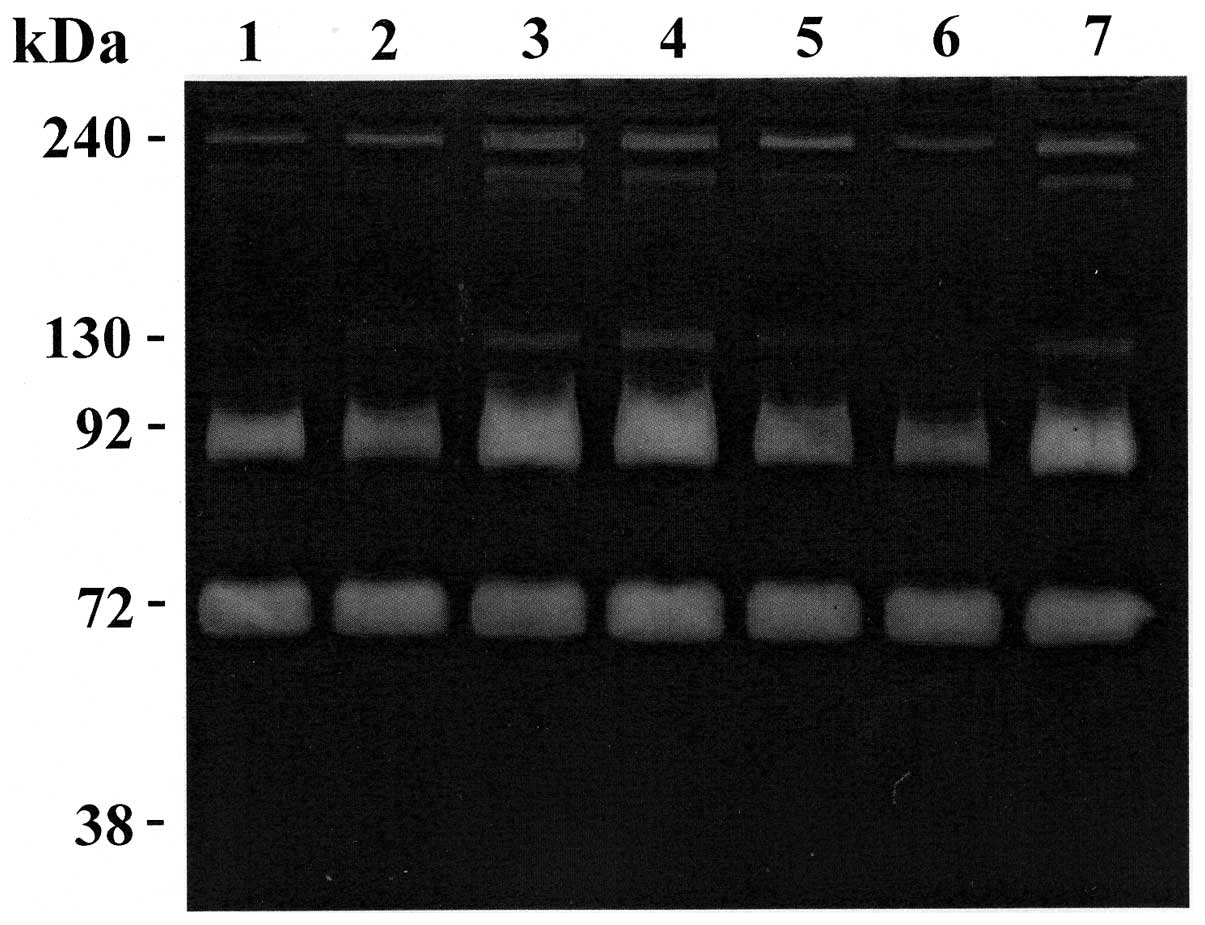
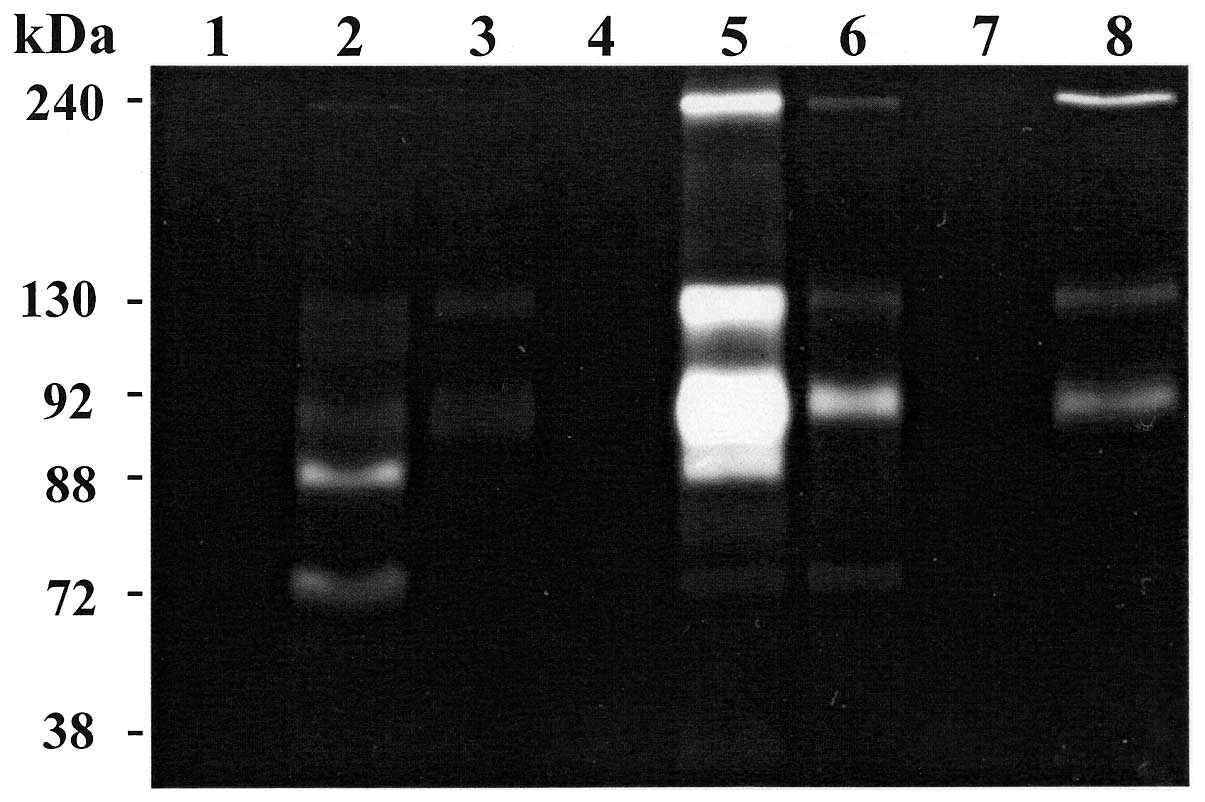
A and B). Representative zymography results are shown in Figs. 1 and 2. Polyacrylamide gels were evaluated for
the presence of clear zone representing degradation of gelatine by
proteolysis. The nature of lytic bands was confirmed by inhibition
assay with a selective inhibitor of serine proteases (Fig. 1, lane 1) and with selective
inhibitors of MMPs (Fig. 2, lane
4). In the sera of all patients, the gels revealed the existence of
four clear zones representing degradation of gelatin by proteolysis
migrating at approximately 240, 130, 92 kDa (MMP-9) and 72 kDa
(MMP-2), respectively. Comparison of these gelatinolytic bands with
prestained standard protein and purified gelatinase A (MMP-2) and
gelatinase B (MMP-9) clearly identified the MMP-constituting bands
as gelatinase A (MMP-2; 72 kDa) and gelatinase B (MMP-9; 92 kDa).
The clear zones with molecular weight >92 kDa might represent
complexes of MMPs that are not dissociated in zymography. In fact,
MMP-9 can be associated with a 25-kDa protein (lipocalin) giving a
band at ~125 kDa (10,11) and can form a complex with its
endogenous inhibitors TIMP-1 giving a band at ~140 kDa (12). Furthermore, MMP-9 can form dimer or
multidimer giving lytic bands at approximately 215 and 240 kDa
(13). Also, several MMPs together
can form complexes of high molecular weight (HMW) gelatinase
species that can only be identified with specific antibodies in
western blot analysis. However, because zymography is much more
sensitive than western blot analysis, it has been difficult to find
antibodies that were sensitive enough to detect small amounts of
MMPs.
Following gelatin zymography, the proteolytic bands
were subjected to densitometric analysis and the data, normalized
to an internal serum standard, were expressed as the integrated
density of all the pixels of each band (volume ×10−3). A
summary of expression patterns of each proteinase is shown in the
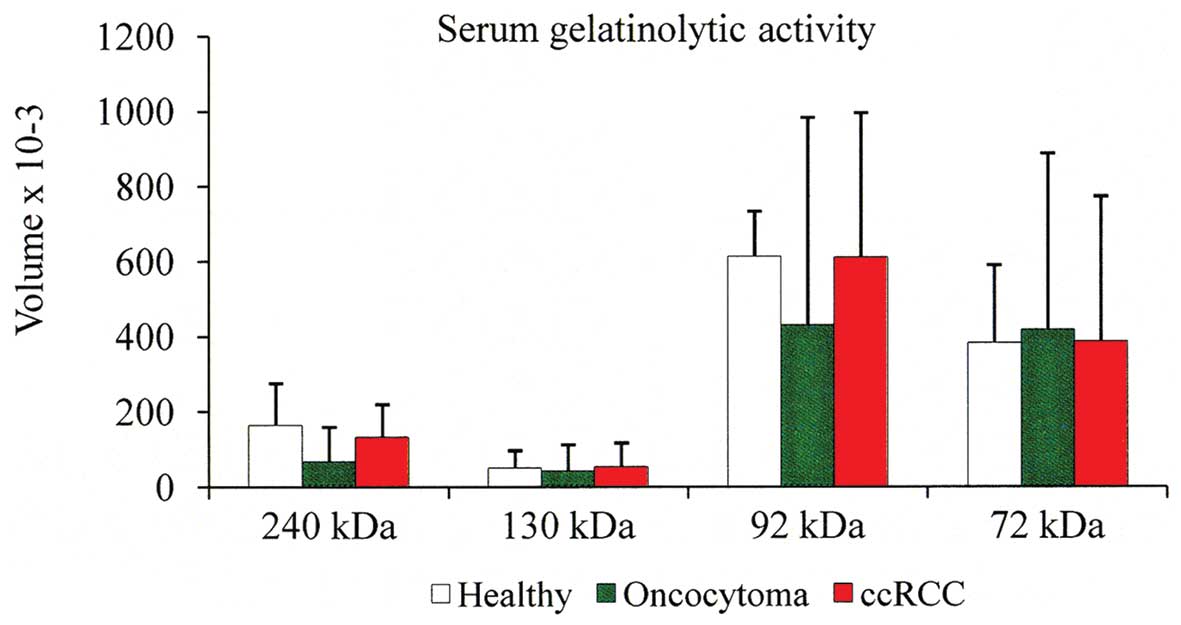
tables. Considering the volume average of each individual band, we
observed that the 92 kDa band is slightly higher in the sera from
ccRCC patients compared with that of oncocytoma patients. The
second point is that the serum 72 kDa band is similar both in ccRCC
and oncocytoma patients as well as in normal individuals (Fig. 3).
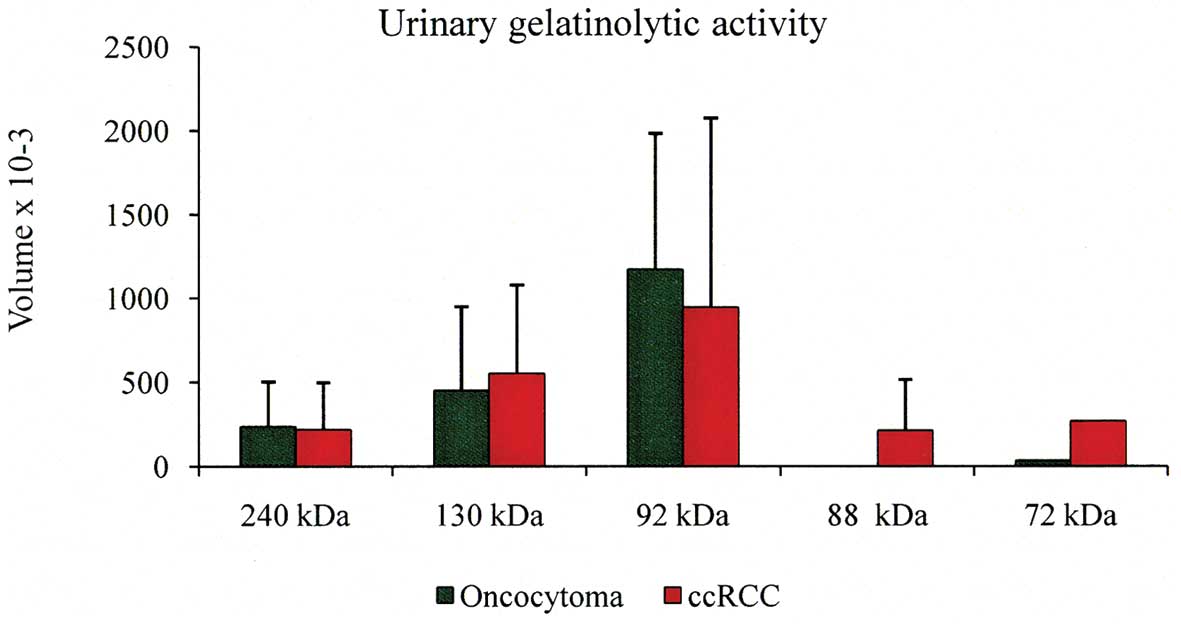
As it concerns urine specimens, gelatin zymography
identified that MMPs were present in the concentrate urine of 2
oncocytoma patients and in 3 out of 9 (33%) of ccRCC patients
(Tables IV and V), whereas MMP could not be detected in
the concentrate urine of healthy subjects with no evidence of
disease (data not shown). In the oncocytoma group, one sample
(patient P1) showed faint small lytic bands at 240, 130 and 72 kDa
and a more strong lytic band at 92 kDa (Fig. 2, lane 6 and Table IV). Another sample (patient P3,
T2N0M0, G1) showed a more intense lytic activity at 240, 130 and 92
kDa with a value of 425, 805 and 1749, respectively and no lytic
band at 72 kDa (Table IV).
Regarding ccRCC patients, 2 specimens showed a lytic band at 88 kDa
(active MMP-9) presumably due to an autoactivation during
renaturation period. In particular, case P9 (T1N0M0, G2) (Fig. 2, lane 5) showed very strong lytic
band at 92 kDa (value 2246×10−3). Another specimen (case
P15, T2N0M0, G3) (Fig. 2, lane 2)
showed a very faint lytic band at 240 kDa, a lytic activity at 92
kDa (value 181×10−3) and a more strong lytic band at 88
kDa (value 430×10−3). Furthermore, this patient showed
lytic activity at 72 kDa (value 270×10−3), whereas all
the other specimens showed no activity at 72 kDa (Table V). The volume average of each
individual band of the positive urine specimens is shown in
Fig. 4. It is evident that the most
abundant lytic activity was at 92 kDa band. Finally, specimens P19
(T3N0M1, G3) and P20 (T3bN0M1) had metastasis in the bony skeleton.
We found that the serum lytic activity of these patients was not
increased compared with the serum levels in healthy subjects as
well as with those of cancer patients. Moreover, in the concentrate
urine of these patients we did not find any gelatinolytic activity
(Tables III and V).
 | Table IVUrine MMP content in oncocytoma. |
Table IV
Urine MMP content in oncocytoma.
| | | | | Volume
×10−3 |
|---|
| | | | |
|
|---|
| Case | Age (years) | Gender | Stage | Grade | MMP (240 kDa) | MMP (130 kDa) | MMP (92 kDa) | MMP (72 kDa) |
|---|
| P1 | 42 | F | T1N0M0 | G1 | 48 | 98 | 602 | 34 |
| P2 | 66 | M | T1N0M0 | G1 | 0 | 0 | 0 | 0 |
| P3 | 59 | F | T2N0M0 | G1 | 425 | 805 | 1749 | 0 |
| P4 | 59 | F | T1N0M0 | G1 | 0 | 0 | 0 | 0 |
 | Table VUrine MMP content in clear cell renal
carcinoma. |
Table V
Urine MMP content in clear cell renal
carcinoma.
| | | | | Volume
×10−3 |
|---|
| | | | |
|
|---|
| Case | Age (years) | Gender | Stage | Grade | MMP (240 kDa) | MMP (130 kDa) | MMP (92 kDa) | MMP (88 kDa) | MMP (72 kDa) |
|---|
| P5 | 69 | M | T1N0M0 | G1 | 0 | 0 | 0 | 0 | 0 |
| P6 | 54 | M | T1N0M0 | G1 | 0 | 0 | 0 | 0 | 0 |
| P7 | 53 | M | T1N0M0 | G2 | 91 | 180 | 418 | 0 | 0 |
| P9 | 51 | F | T1N0M0 | G2 | 540 | 927 | 2246 | 26 | 0 |
| P13 | 40 | M | T2N0M0 | G3 | 0 | 0 | 0 | 0 | 0 |
| P14 | 73 | M | T2N0M0 | G3 | 0 | 0 | 0 | 0 | 0 |
| P15 | 70 | M | T2N0M0 | G3 | 23 | 0 | 181 | 430 | 270 |
| P19 | 67 | M | T3N0M1 | G3 | 0 | 0 | 0 | 0 | 0 |
| P20 | 57 | F | T3bN0M1 | G3 | 0 | 0 | 0 | 0 | 0 |
 | Table IIISerum MMP content in clear cell renal
carcinoma. |
Table III
Serum MMP content in clear cell renal
carcinoma.
| | | | | Volume
×10−3 |
|---|
| | | | |
|
|---|
| Case | Age (years) | Gender | Stage | Grade | MMP (240 kDa) | MMP (130 kDa) | MMP (92 kDa) | MMP (72 kDa) |
|---|
| P5 | 69 | M | T1N0M0 | G1 | 183 | 24 | 1153 | 1017 |
| P6 | 54 | M | T1N0M0 | G1 | 94 | 29 | 463 | 100 |
| P7 | 53 | M | T1N0M0 | G2 | 23 | 7 | 160 | 188 |
| P8 | 60 | F | T1N0M0 | G2 | 135 | 0 | 632 | 791 |
| P9 | 51 | F | T1N0M0 | G2 | 295 | 215 | 1151 | 1070 |
| P10 | 63 | F | T1N0M0 | G2 | 237 | 124 | 1185 | 913 |
| P11 | 63 | M | T2N0M0 | G2 | 125 | 40 | 343 | 113 |
| P12 | 60 | F | T2N0M0 | G2 | 128 | 73 | 634 | 105 |
| P13 | 40 | M | T2N0M0 | G3 | 29 | 15 | 241 | 207 |
| P14 | 73 | M | T2N0M0 | G3 | 87 | 0 | 558 | 107 |
| P15 | 70 | M | T2N0M0 | G3 | 279 | 168 | 1341 | 886 |
| P16 | 61 | M | T2N0M0 | G3 | 117 | 47 | 623 | 114 |
| P17 | 73 | F | T2N0M0 | G3 | 221 | 47 | 432 | 97 |
| P19 | 43 | M | T2N0M0 | G3 | 99 | 45 | 411 | 117 |
| P19 | 67 | M | T3N0M1 | G3 | 34 | 9 | 214 | 186 |
| P20 | 57 | F | T3bN0M1 | G3 | 35 | 13 | 253 | 179 |
Discussion
Due to its asymptomatic clinical course RCC is being
detected incidentally in two-thirds of patients (14). Therefore, the diagnosis of RCC is a
critical issue in the management of the patients. One of the
strategies to improve this situation is the identification of
biomarkers in serum or urine samples whose levels are sensitive to
detect tumor forms and to monitor for disease progression. Several
tumor markers have been tested in the past, but there are no
definitive biomarkers available for such purpose (15). Among protein markers, MMP-2 and
MMP-9 have been investigated with variable results. Tissue MMP-2
and MMP-9 were found to be overexpressed in tumors and more
frequently in non-ccRCC (16,17).
In particular, by immunochemistry Kallakury et al(17) found overexpression of MMP-2 and
MMP-9 in 43% of tumors and this increased expression correlated
with poor prognostic variables. Moreover, by immunostaining
Abdel-Wahed et al found a positive correlation between MMP-2
expression and tumor size, histologic type and high levels of
cellular proliferation (18).
However, although these studies on tissue markers are highly
promising, there are some limitations. In fact, immunochemistry is
semi-quantitative and highly dependent on a range variables such as
choice of antibody, antibody concentration, fixation techniques,
variability in the interpretation and stratification criteria, and
inconsistency in specimens handling and technical procedures. Using
the RT-PCR technique, Kugler et al analyzed MMP-2 and-9 and
their inhibitors in 17 RCC patients and demonstrated a strong
correlation between increased gene expression and tumor stage and
aggressiveness (19). By in
situ zymography, Kamiya et al found that the lytic
activity is higher at the peripheries of tumors in inflammatory
sites (20). As it concerns
peripheral blood, Lein et al measured MMP-2 and -9 and their
inhibitors using ELISA technique in 36 RCC patients and found that
plasma MMP-2 levels were higher in healthy controls, whereas MMP-9
concentrations were significantly higher in RCC patients than in
healthy controls with a sensitivity of only 36% in detecting RCC
and found no correlation with tumor type, grade or stage (21). The results shown here indicate that
MMP-2 (72 kDa lytic band) and MMP-9 (92 kDa lytic band) are present
in the sera of all the patients analyzed. The mean values of MMP-2
are similar in ccRCC and oncocytoma patients, whereas the mean
values of MMP-9 are slightly higher in ccRCC patients compared with
those of oncocytoma patients. However, there was a broad overlap of
the data and we found no correlation to type of carcinoma,
pathological TNM stage or histological grading.
The idea to follow localized tumors or to monitor
drug-based therapy results by simply analyzing tumor-specific
markers in the easily available excretory product of the kidney is
desirable. However, to the best of our knowledge there is only
scant literature on urine markers for RCC. In the urine samples, we
found that only few specimens have lytic activity. Our data are in
keeping with the data of Cannon and Getzenberg (22) but in contrast with those of Sherief
et al(23).
So far, despite the tissue evidence, serum and urine
MMP activities seem to be not an adequate test to identify renal
cancer. Nevertheless, due to the small number of patients included
in the studies, the conclusion may not be transferable to the
general population and therefore merits further evaluation. Future
investigation involving gelatine zymography in larger cohorts of
patients could clear up if MMP-2 and MMP-9 are useful biomarkers
for RCC.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Cohen HT and McGoven FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagase H and Woessner JF: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
4
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cell. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
6
|
Sobin LH and Wittekind CH: International
Union Against Cancer (UICC) TNM classification of malignant tumors.
6th edition. Wiley-Liss; New York, NY: pp. 193–195. 2002
|
|
7
|
Bradford MM: A rapid and sensitive method
for quantitation of microgram quantities of protein utilizing the
principle of protein-dye binding. Anal Biochem. 72:248–254. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Urinary gelatinase activities (matrix metalloproteinase
2 and 9) in human bladder tumors. Oncol Rep. 15:1321–1326.
2006.PubMed/NCBI
|
|
9
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Matrix metalloproteinase-2 and matrix
metalloproteinase-9 type IV collagenases in serum of patients with
pleural effusions. Int J Oncol. 26:1363–1368. 2005.PubMed/NCBI
|
|
10
|
Triebel S, Bläser J, Reinke H and
Tschesche H: A 25 kDa α2-microglobulin-related protein
is a component of the 125 kDa form of human gelatinase. FEBS Lett.
314:386–388. 1992.
|
|
11
|
Yan L, Borregaard N, Kjeldsen L and Moses
MA: The high molecular weight urinary matrix metalloproteinase
(MMP) activity is a complex of gelatinase B/MMP and neutrophil
gelatinase-associated lipocalin (NGAL). J Biol Chem.
276:37258–37265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roy R, Louis GL, Loughlin KR, Wiederschain
D, Kilroy SM, Lamb CC, Zurakowski D and Moses MA: Tumor-specific
urinary matrix metalloproteinase fingerprinting: identification of
high molecular weight urinary matrix metalloproteinase species.
Clin Cancer Res. 14:6610–6617. 2008. View Article : Google Scholar
|
|
13
|
Goldberg GI, Strongin A, Collier IE,
Genrich LT and Marmer BL: Interaction of 92-kDa type IV collagenase
with the tissue inhibitor of metalloproteinases prevents
dimerization, complex formation with interstitial collagenase, and
activation of proenzyme with stromelysin. J Biol Chem.
267:4583–4591. 1992.
|
|
14
|
Russo P: Localized renal cell carcinoma.
Curr Treat Options Oncol. 2:447–455. 2001. View Article : Google Scholar
|
|
15
|
Kashyap MK, Kumar A, Emelianenko N,
Kaahyap A, Kaushik R, Huang R, Khullar M, Sharma SK, Singh SK,
Bhargave AK and Upadhyava SK: Biochemical and molecular markers in
renal cell carcinoma: an update and future prospects. Biomarkers.
10:258–294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Struckmann K, Mertz K, Steu S, Storz M,
Staller P, Krek W, Schraml P and Moch H: pVHL co-ordinately
regulates CXCR4/CXCL12 and MMP2/MMP9 expression in human clear-cell
renal cell carcinoma. J Pathol. 214:464–471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
18
|
Abdel-Wahed MM, Asaad NY and Aleskandarany
M: Expression of matrix metalloproteinase-2 in renal cell
carcinoma. J Egypt Nat Canc Inst. 16:168–177. 2004.PubMed/NCBI
|
|
19
|
Kugler A, Hemmerlein B, Thelen P,
Kallerhoff M, Radzun HJ and Ringert RH: Expression of
metalloproteinase 2 and 9 and their inhibitors in renal cell
carcinoma. J Urol. 160:1914–1918. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamiya N, Kishimoto T, Suzuki H, Sekita N,
Nagai Y, Oosumi N, Kito H, Tochigi N, Shinbo M, Nemori R, et al:
increased in situ gelatinolytic activity in renal cell tumor
tissues correlates with tumor size, grade and vessel invasion. Int
J Cancer. 106:480–485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lein M, Jung K, Laube C, Hübner T,
Winkelmann B, Stephan C, Hauptmann S, Rudolph B, Schnorr D and
Loening SA: Matrix-metalloproteinases and their inhibitors in
plasma and tumor tissue of patients with renal cell carcinoma. Int
J Cancer. 85:801–804. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cannon GM and Getzenberg RH: urinary
matrix metalloproteinases activity is not significantly altered in
patients with renal cell carcinoma. Urology. 67:848–850. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sherief MH, Low SS, Miura M, Kudo N,
Novick A and Weimbs T: Matrix metalloproteinase activity in urine
of patients with renal cell carcinoma leads to degradation of
extracellular matrix proteins: possible use as a screening assay. J
Urol. 169:1530–1534. 2003. View Article : Google Scholar
|