Introduction
Renal cell carcinoma (RCC) accounted for 90–95% of
neoplasms arising from the kidney and ~3.8% of adult malignancy in
2010 (1). Unfortunately, ~25–30% of
patients have metastatic disease at first diagnosis, and >95% of
these have multiple metastases (2).
An aberrant activation of numerous signal pathways, including EGFR
signal, has been recognized as a hallmark of cancer cell survival
and progression (3). The studies
also have revealed that overexpression of EGFR has been linked to
RCC progression (4–6).
Traditional systematic therapies for metastatic RCC
tumors include immunotherapy, chemotherapy, and targeted therapy
(7,8). However, low response rates have
significantly retarded the efforts to improve the prognosis. There
are still no effective methods for the treatment of metastatic RCC
(9). Thus, searching for novel
therapeutic methods or agents is needed for improving the efficacy
against metastatic RCC.
Acumulated evidence shows that silibinin, a natural
flavonoid antioxidant, isolated from milk thistle (Silybum
marianum), exerted pleiotropic anticancer capabilities in different
human malignant tumors including prostate, bladder, breast, colon,
and oral cancer (10–13). Due to the high efficacy of this drug
against cancer as well as its non-toxic characteristics, the use of
this drug provide a strong rationale for cancer prevention and
adjuvant therapy compare with traditional chemical treatment.
In our previous study, we found that silibinin
inhibited cell proliferation in human RCC Caki-1 cells through
decreasing EGFR signaling activity (3). In this study on metastasis, we
selected three human RCC cell lines that have various levels of
EGFR expression as the cell models to investigate the potential
role of EGFR signaling cascade in RCC progression and possible
inhibitory effect of silibinin on this process. Our results
demonstrated that silibinin suppressed RCC cell progression via
inhibiting the EGFR signal cascade, and resultant EGFR signal
dependent MMP-9 activation in human RCC ACHN, OS-RC-2 and SW839
cell lines, suggesting that silibinin might be a new anti-EGFR drug
for metastatic RCC treatment.
Materials and methods
Cell lines and chemicals
Human RCC cell lines ACHN, OS-RC-2 and SW839
purchased from American Type Culture Collection (Manassas, VA) were
maintained in DMEM medium, supplemented with 10% fetal bovine serum
(Gibco, NY), 1% glycine (Gibco) and 1% penicillin-streptomycin in
humidified 5% CO2 incubator (Thermon, USA). Silibinin
was purchased from Sigma (S0417-1G). Stock solution (50 mM in DMSO)
was added to the media to achieve the indicated concentration, and
then incubated for 24 h for various tests. We obtained EGF from
Sigma (E4127-1MG); EGFR antagonist PD168393 (cat-513033) and MMP-9
inhibitor (cat-444278) from Calbiochem; MMP-2 inhibitor from Santa
Cruz Biotechnology (sc-204092); and U0126 from Cell Signaling
Technology (cat-9903S).
Immunohistochemistry (IHC)
Ten sets of surgical specimens (primary tumor,
adjacent normal renal tissue, and metastatic lymph node) were
stored in the Department of Urology (Xi’an Jiaotong University,
Xi’an, China). Immunohistochemical analyses of paraffin sections
were performed with anti-EGFR Ab (sc-03, diluted 1:100, Cell
Signaling Technology). Briefly, tissues were deparaffinized,
rehydrated, then subjected to 30 min of antigen retrieval in a
microwave oven at 96°C, followed by 15 min of endogenous enzyme
block with 3% hydrogen peroxide solution, incubated with primary
antibody at 4°C overnight, and 30 min of DakoCytomation
EnVision-HRP reagent incubation for rabbit antibodies. Signals were
detected by diaminobenzidine (DAB) buffer followed by hematoxylin
counterstaining. In the negative control, sections were incubated
with N-universal negative control antibody under identical
conditions. Protein expression was quantified with Image-Pro Plus
5.0 (Media Cybernetics Inc., USA) in 10 random microscopic (400x)
fields in each slice, and data are presented as the average
staining intensity of different groups.
Western blot analysis
After silibinin treatments of RCC cells with of
various concentrations, total cellular lysates were prepared in
lysis buffer (50 mM/l of Tris-HCl/pH 7.4, 150 mM/l of NaCl, 0.1%
SDS, 1 mM/l EDTA, 1 mM/l EGTA, 0.3 mM/l of PMSF, 0.2 mM/l of sodium
orthovanadate, 1% NP40, 10 mg/ml of leupeptin, and 10 mg/ml
aprotinin). Total of 50 μg protein were separated in 10% SDS-PAGE
gel, and then transferred onto the PDVF membranes. The membrane was
blocked with 5% non-fat milk in PBS for 1 h at room temperature and
incubated with primary antibody overnight at 4°C. Anti-EGFR (sc-03,
diluted 1:1000) and anti-GAPDH (sc-32233, diluted 1:1000) antibody
were obained from Santa Cruz Biotechnology (Santa Cruz, CA, USA),
anti-phosphorylation of EGFR (Tyr1068) (cat-3777, diluted 1:1000),
anti-ERK (cat-5013, diluted 1:1000), anti-pERK1/2 (Thr202/Tyr204)
(cat-4370, diluted 1:1000), anti-AKT (cat-9272, diluted 1:1000),
anti-pAKT (Ser473) (cat-9271, diluted 1:1000), anti-Stat3
(cat-9132, diluted 1:1000) and anti-pStat3 (cat-9131, diluted
1:1000) antibodies were obtained from Cell Signaling Technology
(MA, USA). The bands were visualized with the ECL detection system
followed by exposure to X-ray film. The relative photographic
density was quantitated and analyzed using Glyko BandScan software
(Glyko, USA).
Reverse transcription and real-time
PCR
Total RNA was isolated with TRIzol reagents
(Invitrogen, CA) and quantitated by absorbance at 260 nm. Reverse
transcription was performed with 2 μg RNA using Rever-tAid™ First
Strand cDNA synthesis kit (MBI Fermentas, Germany) according to the
manufacturer’s instructions. Expressions of MMP-9 and MMP-2 mRNAs
were measured with quantitative PCR and GAPDH mRNA was used as
internal control. The primer sequences are given below: MMP-9
(forward, 5′-TGTCGCTGTCAAAGTTCGAG-3′; reverse,
5′-TTCATCTTCCAAGGCCAATC-3′); MMP-2 (forward,
5′-GGACAGACGGAAGTTCTTGG-3′; reverse, 5′-CACTTTCCTGGGCAACAAAT-3′);
GAPDH (forward, 5′-CATACCAGGAAATGAGCTTGACAA-3′; reverse,
5′-CTCCTCCACCTTTGAGGCTG-3′) was used as an internal control. The
experiment was performed in 3 individual trials in triplicate.
Cell migration and invasion assay
Cell migration and invasion assays were performed
using 24-well transwell plates (Falcon cell culture inserts, 8-μm
pore size, BD, NJ) according to the manufacturer’s instructions.
Briefly, for the invasion assay, ACHN, OS-RC-2 and SW839 cells
(4×104), pretreated with various concentrations of
silibinin (25, 50 and 75 μM), with or without EGF treatment (1, 10
and 50 nM) for 24 h, were seeded into the upper chamber that had
been precoated for 6 h with 50 μl Matrigel (2 mg/μl, BD) in medium
containing no serum. The lower chamber was filled with 600 μl 10%
FBS medium. After a 48-h incubation, the penetrated cell will be
fixed with 75% ethanol, stained with 1% crystal violet solution
(Fisher Scientific, PA, USA), cells number were counted under
microscope. For the migration assay, pretreated cells
(2×104) were seeded into the uncoated transwell upper
chamber, followed by a 24-h incubation, and then the cells in the
membrane were fixed and stained similar to the invasion assay. All
the invasion and migration assays were performed at least 3
individual experiments in triplicate.
Cell viability assay
Cell viability test was performed with a tetrazolium
based assay (MTT). Cells (1×103) were seeded in a
96-well plate with 50 μl media in triplicate, then treated with
indicated doses of silibinin (0, 25, 50 and 75 mM) for 48 h. MTT
solution (20 μl) (5 mg/ml, MTT, Sigma, USA) was added to each well
and incubated for 2.5 h, and then developed with 200 μl DMSO/well.
The absorbance (OD) was detected at the wavelength of 570 nm with
microplate autoreader (Bio-Tek Instruments, VT).
Zymography assay
The activities of MMP-2 and MMP-9 were detected by
gelatin zymography protease assays. After treatment with EGF (1,10
and 50 nM), ACHN, OS-RC-2, and SW839 cells (5×104) were
seeded in the 6-well cell culture plate, treated with various
concentrations of silibinin (0, 25, 50 and 75 μM), EGFR-antagonist,
ERK-antagonist, MMP-2 inhibitor or MMP-9 inhibitor, for 24 h. The
conditioned media were collected and loaded into 10% SDS-PAGE
containing 1 mg/ml gelatin. After electrophoresis at 4°C, the
SDS-PAGE gel was washed with 2.5% Triton X-100 washing buffer (2.5%
Triton X-100, 40 mM Tris-HCl/pH 8.0, 10 mM CaCl2, 1 mM
MgCl2) for 1 h, and then the gel was incubated in
reaction buffer (0.02% Brij35), 40 mM Tris-HCl/pH 8.0, 10 mM
CaCl2, 1 mM MgCl2) for 24 h at 37°C and
stained with Coomassie brilliant blue R-250 (Sigma, St. Louis, MO).
The brightness of clear bands, where MMPs were located and gelatin
was degraded, were analyzed by densitometry. The experiments were
performed in triplicate.
Statistical analysis
All statistical analyses were carried out with SPSS
15.0 (SPSS Inc., Chicago, IL). Quantitative data are presented as
mean ± SE, statistical significance among control group and various
treated groups were accomplished by ANOVA, p<0.05 was considered
as statistically significant. The data are representative of three
independent experiments.
Results
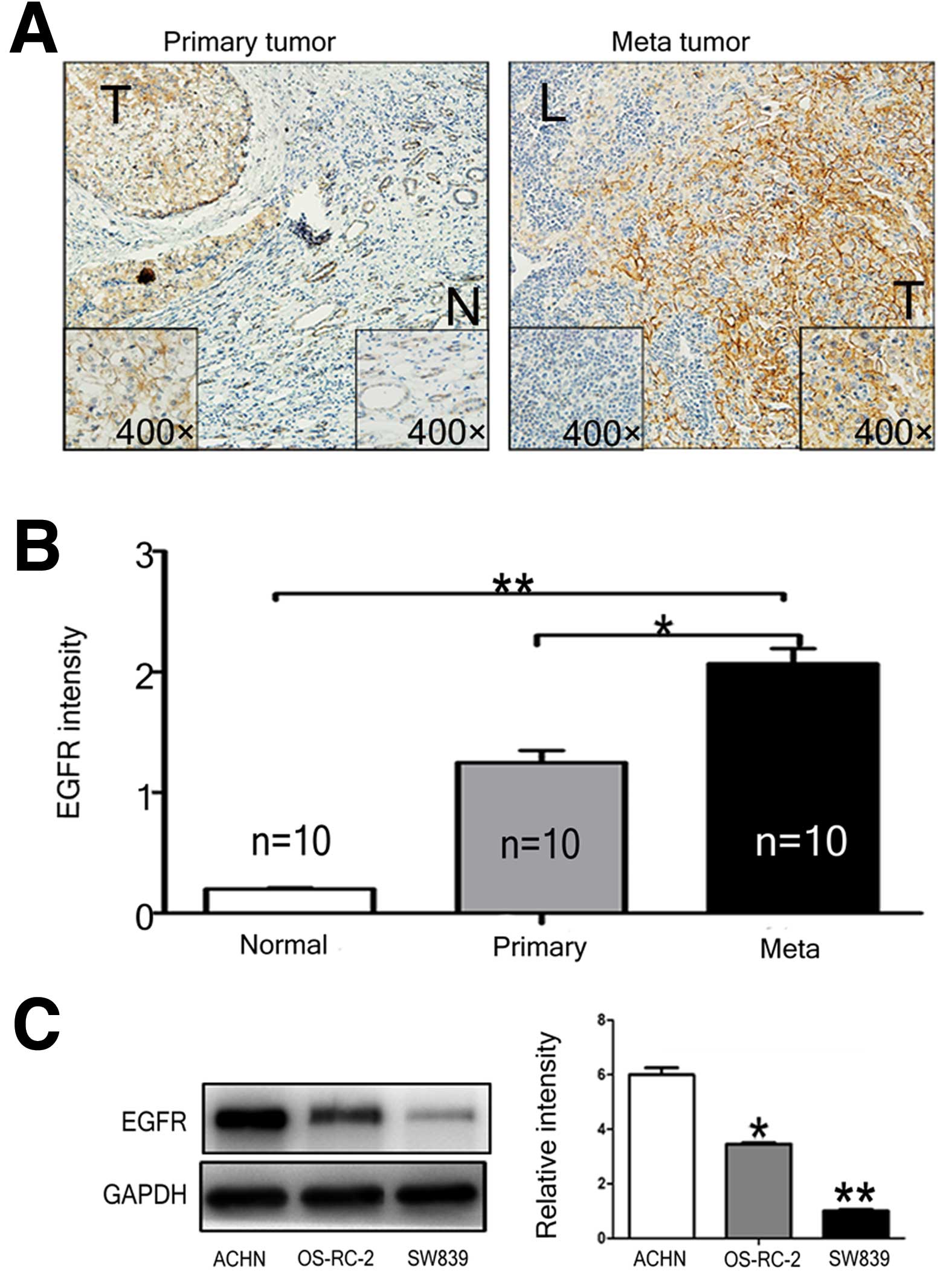
Overexpression of EGFR in RCC cells
To evaluate the role of EGFR signaling in RCC
progression, EGFR expression in RCC surgical tissue samples (ten
sets of primary tumor, metastatic lymph nodes, and adjacent normal
kidney tissue) were examined by IHC staining. Intensive positive
signal was detected in the membrane and cytoplasm of primary (T)
and metastatic tumor tissues (L), whereas weak signals were
detected in the normal (N) kidney tissue (Fig. 1A). Significant higher expression of
EGFR was observed in metastatic tumors compared to the primary
tumors (p<0.05) and normal tissues (p<0.01). We further
investigated EGFR expressions in several selected RCC cell lines
and found that the ACHN cells derived from metastatic RCC displayed
higher EGFR expression than the other two RCC cells (Fig. 1C), OS-RC-2 and SW839, which were
derived from the primary RCC. These three cell lines were used in
experiments in further in vitro studies.
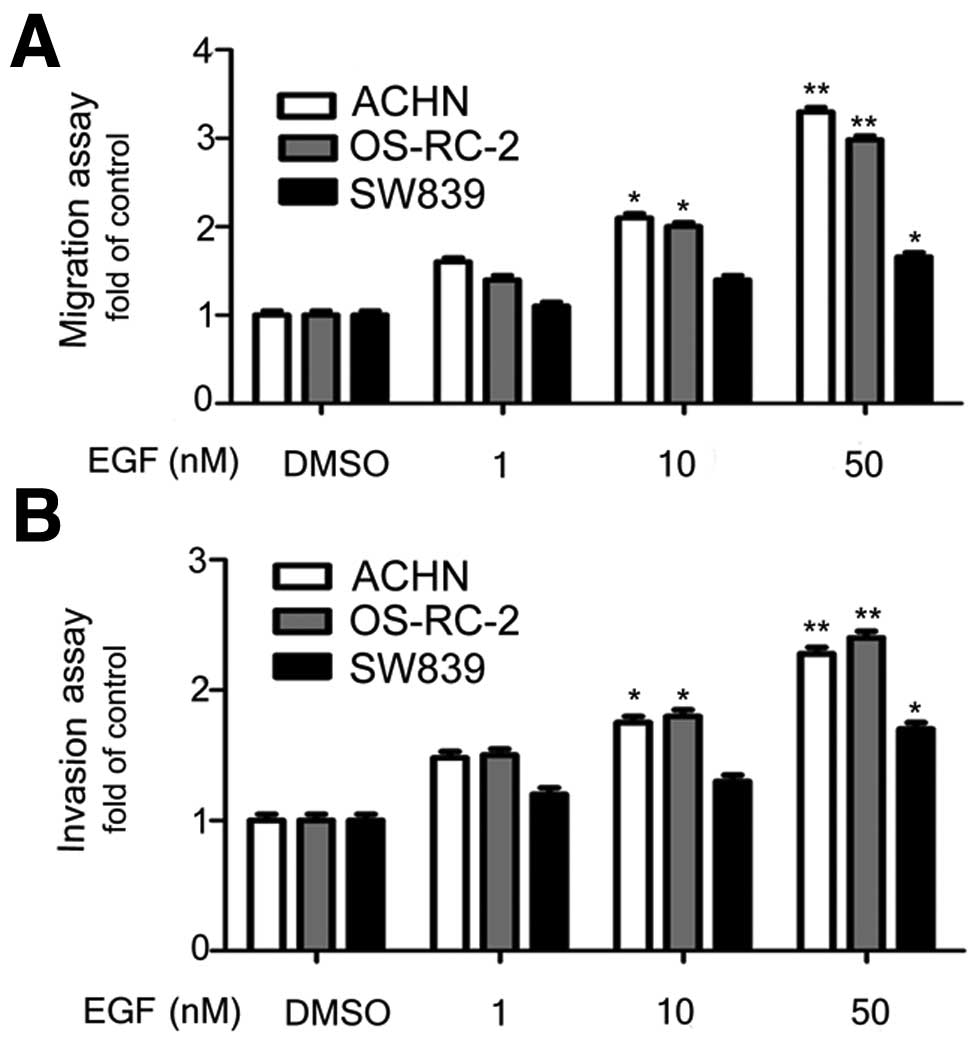
EGFR signal promoted migration and
invasion of ACHN, OS-RC-2, and SW839 cells
We investigated whether the EGFR signal can promote
in vitro migration and invasion of RCC cells. We performed
migration assays in transwell plates with addition of different
doses of EGF. It was shown that the EGFR signal enhanced migration
ability of ACHN and OS-RC-2 cells significantly and the highest
effect was obtained at 10 and 50 nM EGF (Fig. 2A). Similar result was obtained in
the invasion assay (Fig. 2B). We
observed EGF effect on enhancing migration and invasion abilities
of SW839 cells at 50 nM, but not at lower concentration, suggesting
that the EGFR signal promote migration and invasion of RCC
cells.
EGFR-dependent inhibitory effect of
silibinin on RCC cell migration and invasion
We next tested the effect of silibinin on migration
and invasion of ACHN, OS-RC-2 and SW839 cell lines. The MTT assay
result indicated that silibinin did not result in cytotoxicity of
cells at 25–75 μM range (Fig. 3A).
Therefore, we used this concentration range for further
experiments. We found that the silibinin treatment (from 25 to 75
μM) significantly reduced migration and invasion of OS-RC-2 cell
(20–60% for migration and 30–60% for invasion), and ACHN cell
(30–60% for migration and 40–65% of invasion). However, no
significant inhibitory was demonstrated in SW839 cells, which
express low level of EGFR, even at the high concentration of
silibinin (Fig. 3B and C). These
results indicated that the dose-dependent inhibitory effects of
silibinin on EGFR signal-induced cell migration and invasion occurs
only in the RCC cells that express high level of EGFR. Therefore,
we can conclude that the inhibitory effect of silibinin on
migration and invasion abilities of RCC cells was via suppression
of EGFR signal.
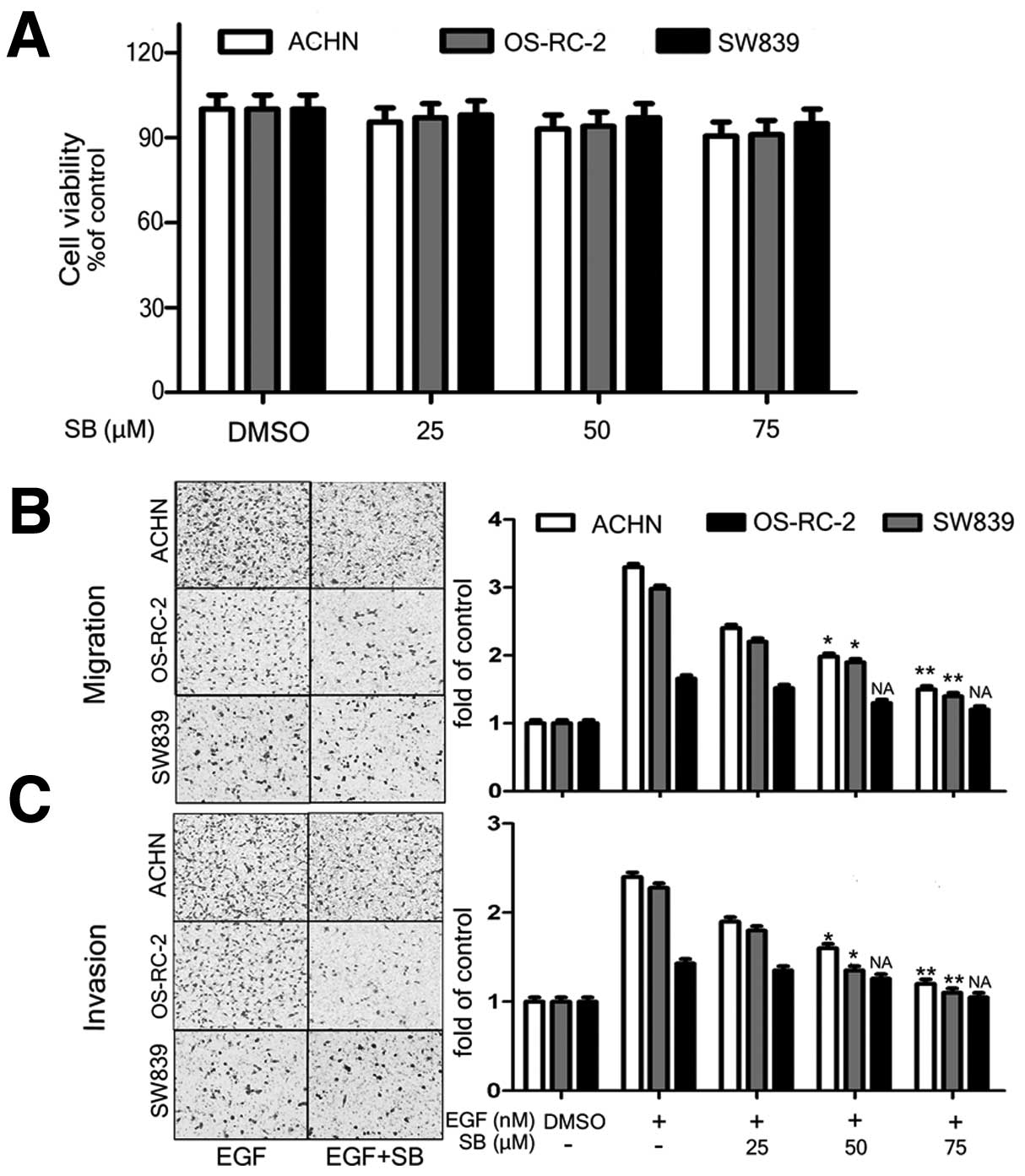 | Figure 3EGFR-dependent inhibitory effect of
silibinin on RCC cell migration and invasion. ACHN, OS-RC-2, and
SW839 cells were treated with varying concentrations of silibinin
(25, 50 and 75 μM) for 24 h, followed by MTT cell viability assay
(A). DMSO treatment was used as control. EGF-pretreated ACHN,
OS-RC-2, and SW839 cells were treated with varying concentrations
of silibinin (25, 50 and 75 μM) for 24 h, followed by cell
migration (B) and invasion (C) assays. DMSO was used as the
negative control, EGF only treatment was the positive control. The
data are presented as mean ± SEM of three independent experiments
(*p<0.05, **p<0.01 compared with
positive control group). |
Decrease in phosphorylation of EGFR and
ERK attribute to the inhibitory effect of silibinin
To further investigate whether the inhibitory effect
of silibinin is via EGFR signal, we tested phosphorylated EGF level
in RCC cells upon silibinin treatment. We treated ACHN, OS-RC-2 and
SW839 cells with EGF (1, 10 and 50 nM) and examined phosphorylation
of EGFR. As shown in Fig, 4A, we
observed EGF-dependent phosphorylation of EGFR. The phophorylation
of EGFR further activated EGFR downstream signals such as ERK1/2,
AKT, and STAT3 (Fig. 4B). So, we
tested whether silibinin can inhibit the phosphorylation of EGFR
and its downstream signals. It was found that silibinin
significantly reduced phosphorylated EGFR and ERK1/2 levels in EGFR
high expressing ACHN and OS-RC-2 cells (p<0.05), but not in EGFR
low expressing SW839 cells (p>0.05), the inhibitory effect of
silibinin on phosphorylated EGFR level was comparable to the effect
of known EGFR signal inhibitor PD168393 (Fig. 4B). However, silibinin did not affect
phosphorylation of STAT3 and AKT in three cell lines tested
(Fig. 4B).
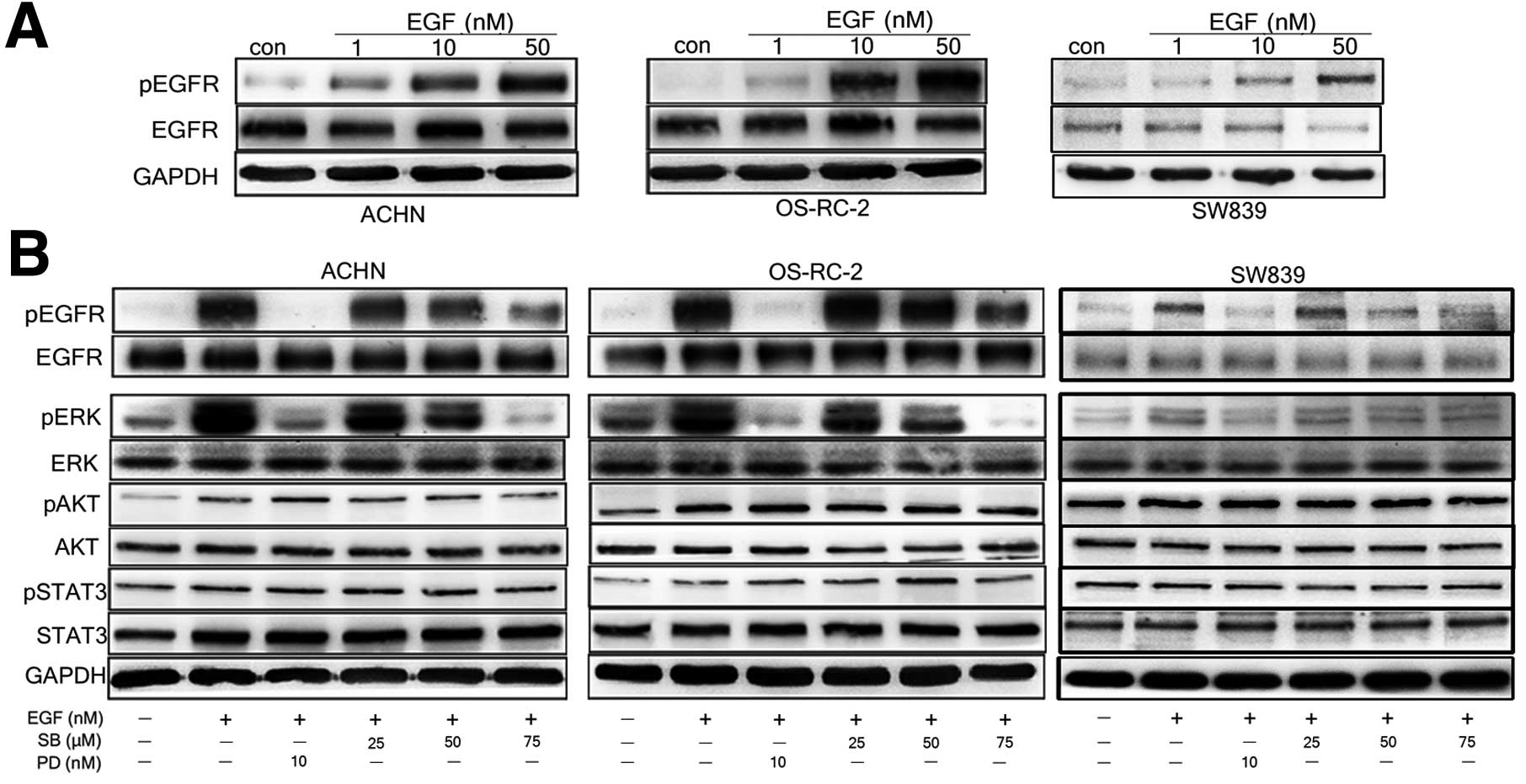 | Figure 4Silibinin significantly inhibits
activation of EGFR/ERK signal pathway in EGFR high expressing RCC
cells. Serum starved ACHN, OS-RC-2, and SW839 cells were treated
with EGF (1, 10 and 50 nM) for 30 min, and then western blot
analysis was performed using anti-phosphorylation and total EGFR
antibodies (A). Before EGF treatment, cells were incubated with
varying concentrations of silibinin (0, 25, 50 and 75 μM), or
combination with 10 nM PD168393, an antagonist of EGFR, then
western blot analysis was peformed using anti-p-ERK/ERK,
anti-pAKT/AKT and anti-pStat3/Stat3 antibody (B). GAPDH served as a
loading control. The data are presented as three independent
experiments. |
Silibinin diminishes EGFR-mediated
increase in MMP-9 expression and activity in EGFR high expressing
RCC cells
Previous studies have demonstrated that MMP-9 and
MMP-2 play important roles in mediating migration and invasion of
variety of cancer cells (14).
Here, we found that the EGFR signal significantly unregulated MMP-9
mRNA expression and activity, but did not affect MMP-2, expression
and activity, in the three cell lines tested (Fig. 5A and B).
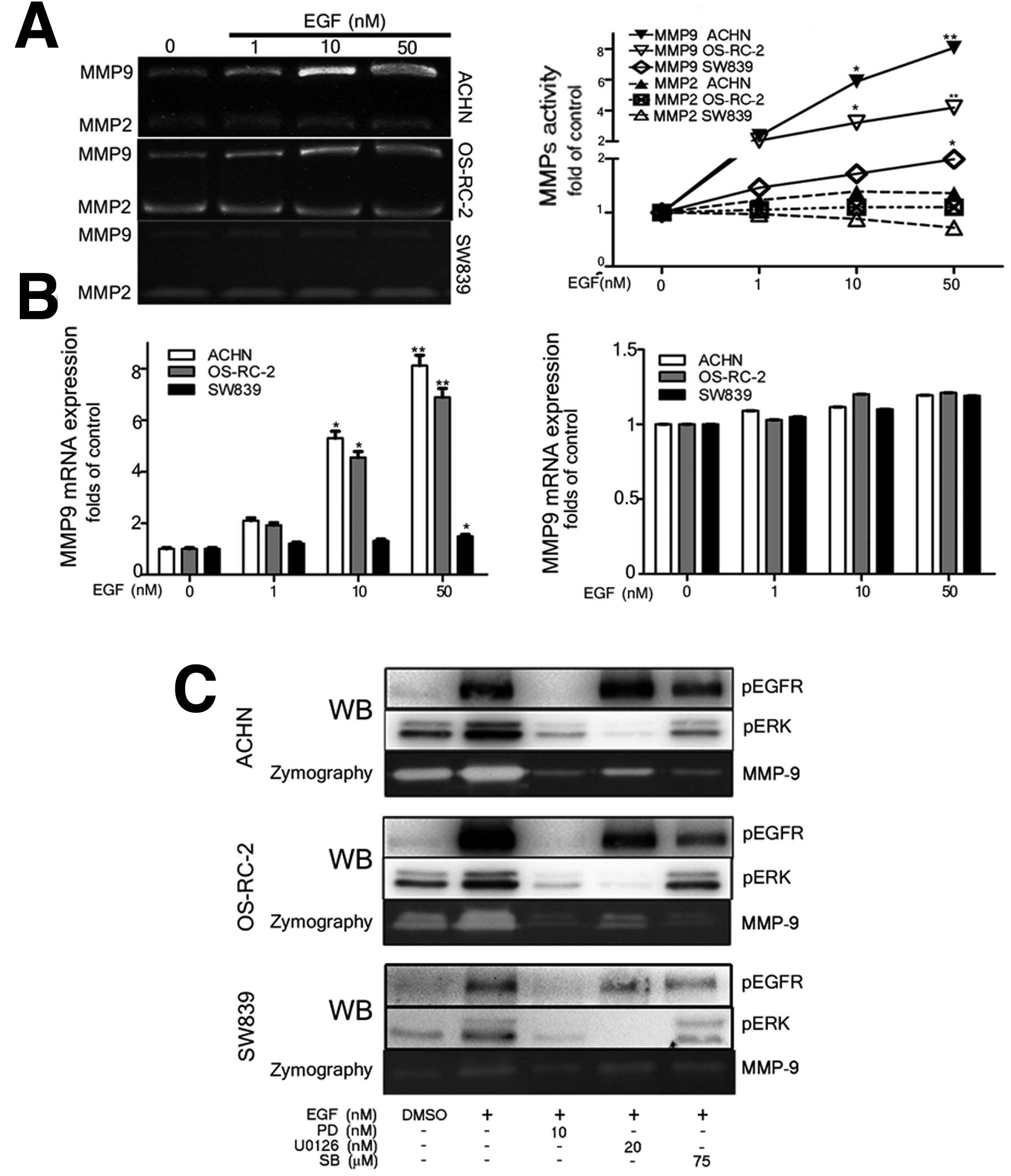 | Figure 5Silibinin diminishes EGFR signal
induced MMP-9 via the blocking the activated EGFR/ERK signal. Serum
starved ACHN, OS-RC-2 and SW839 cells were treated with varying
various concentrations of EGF (1, 10 and 50 nM) for 24 h, and then
MMPs zymography and q-PCR assay for MMP-2 and MMP-9 activity (A)
and mRNA expression (B). EGF-treated ACHN, OS-RC-2, and SW839 cells
(at 50 nM) were incubated with either silibinin, PD168393, or
U0126, for 1 h, followed by western blotting (C) and MMP-9
zymography assay (C). DMSO was used as negative control, EGF only
treated was the positive control. The data are presented as mean ±
SEM of three independent experiments (*p<0.05,
**p<0.01 compared with positive control group). |
Interestingly, our data showed that silibinin
abolished EGFR-induced MMP-9 activity similar to the effect shown
by the EGFR inhibitor, PD168393, and the ERK inhibitor, U0126, in
the EGFR high expressing ACHN and OS-RC-2 cells, not in the EGFR
low expressing SW839 cells (Fig.
5C). These results suggest that silibinin blocked the EGFR and
its downstream ERK signals, which in turn, inhibited expression and
activity of MMP-9, especially in EGFR high expressing cells.
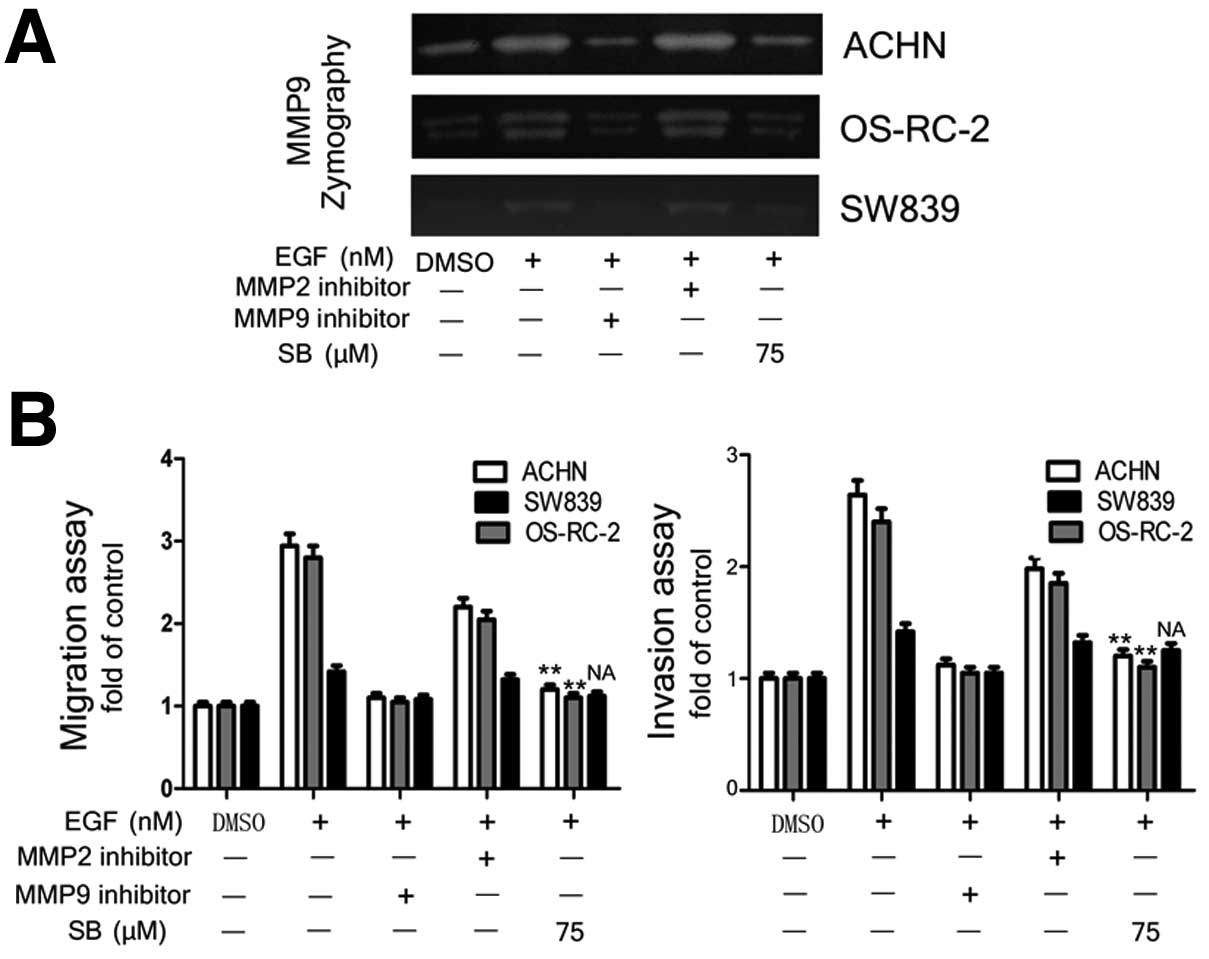
MMP-9 is the key molecule in exerting
silibinin effect on inhibiting EGFR signal-induced migration and
invasion of RCC cells
Since the EGFR-signal significantly increased MMP-9
mRNA expression and activity, not MMP-2, we further investigated
whether MMP-9 plays a role in RCC migration and invasion induced by
EGFR signaling. The ACHN, OS-RC-2 and SW839 cell were stimulated
with EGF, and then treated with inhibitors of MMP-2 and MMP-9, or
silibinin for 24 h, and the activities of MMP-2 and MMP-9 were
tested by zymography. Silibinin and MMP-9 inhibitor both showed
inhibition of EGFR signal-induced MMP-9 activity, cell migration
and invasion abilities in EGFR high expressing ACHN and OS-RC-2
cells (Fig. 6A), but the MMP-2
inhibitor did not. These results confirmed that MMP-9 is the key
molecule to mediate EGFR signal-induced RCC progression (Fig. 6B).
Collectively, it can be concluded that silibinin
inhibited EGFR signal-induced invasion and migration of RCC cells
via blocking of EGFR/MMP-9 signaling and it suggests the
possibility of using silibinin as a new anti-EGFR drug to treat
metastatic RCC.
Discussion
In the present study, we first demonstrated the
effects of silibinin on progression of RCC cells induced by EGFR
signal in vitro. Employing three different human RCC cell
lines, ACHN, OS-RC-2 and SW839, with various levels of EGFR
expression, we demonstrated that, silibinin, a natural flavonoid
antioxidant isolated from milk thistle, could inhibit EGFR
signaling-induced migration and invasion of RCC cells via
suppressing EGFR/MMP-9 signaling.
The potential agents for treatment of advanced RCC
through targeting EGFR signaling have been studied (15,16).
The antibodies and small molecule inhibitors that targets EGFR
pathway have entered clinical trials of RCC patients (17,18).
Silibinin has been demonstrated to exert its
anticancer effects by targeting EGFR. It was reported that
silibinin inhibited ligand binding to EGFR and its internalization
into the cytoplasm, EGFR dimerization, ERK1/2 activation, as well
as cell proliferation in advanced human prostate carcinoma cells
(19). Moreover, silibinin was
reported to be effective in decreasing TGF-α and impaired
TGF-α-EGFR-ERK1/2 signaling in both androgen-dependent (LNCaP) and
-independent (DU145) advanced human prostate carcinoma cells
(20). Consistent with these
findings, we observed that silibinin inhibited EGFR and ERK1/2
phosphorylation, as well as cell proliferation of human RCC Caki1
cells.
Besides its antiproliferative effect, silibinin also
show anti-metastatic effects on a variety of malignant tumors
including prostate cancer, lung cancer, breast cancer, osteosarcoma
and oral cancer (11–13,21,22).
Still little is known about suppression effect of silibinin on EGFR
signal-induced RCC migration and invasion. Chang et
al(23) reported that silibinin
inhibited migration and invasion of RCC 786-O cells. However, RCC
786-O cell line was derived from a primary clear cell
adenocarcinoma, which represents a cell type with less invasive
potential. In this study, we employed three different human RCC
cell lines derived from both primary and metastatic tumor that
express various levels of EGFR. We demonstrated that, silibinin
could inhibit the migration and invasion of RCC cells induced by
EGFR signaling via blocking of EGFR/MMP-9 signaling. However, two
other EGFR signaling downstream proteins, STAT3 and AKT, were not
affected by silibinin, which is in agreement with previous results
that silibinin inhibited cell migration and invasion through ERK1/2
signal, not AKT and STAT3 phosphorylation (12,22,23).
Importantly, silibinin inhibited EGFR signal-induced
cell migration and invasion in a dose-dependent manner, however,
these effect, we believe, is EGFR expression-dependent based on our
observation that the inhibitory effect of silibinin was observed in
EGFR expressing cells (ACHN and OS0RC-2), but not in EGFR low
expressing cells (SW839), even at higher concentration of silibinin
(75 μM). Qi et al(24) also
observed that EGF conferred silibinin-induced cytotoxicity in
glioma cells that lack endogenous EGFR, which partly agree with our
finding, although the potential mechanism is not clear. It seems
that the migration and invasion of EGFR highly expressing cells
depend on EGFR signaling, but probably the EGFR low expressing
cells depend on other signal pathways to mediate their migration
and invasion. The more precise role of EGFR signal in RCC cell
survival and progression needs further study.
We found MMP-9 expression and activity, not MMP-2,
were activated by the phosphorylation of EGFR/ERK signal by EGF
stimulation in RCC cell lines. Silibinin, and an EGFR antagonist
significantly suppressed EGFR signal-induced MMP-9 mRNA expression
and activity, and subsequently decreased their invasion and
migration. Qiu et al(25)
showed EGF-stimulated MMP-9 expression and activity through PI3K
and MAPK signal pathway. Tian et al(26) also found the induction of MMP-9 via
EGF-induced pERK activation, and blocking of EGFR signal pathway
with shRNA (27) or specific
antagonist dramatically down-regulated MMP-9 expression (28). The evidence supports our findings
that EGFR signal regulates the expression and activity of
MMP-9.
Our study identified the vital role of EGFR/MMP-9
signal pathway in RCC progression, and provided first evidence that
silibinin inhibited EGFR signal-induced migration and invasion in
RCC cells via blocking of EGFR/MMP-9 signaling. The dramatic
anti-metastatic effect of silibinin on RCC, especially in EGFR
highly expressing cells, might suggest a potential effective
therapy to cure advanced RCC.
Acknowledgements
We thank Karen Wolf for assistance with manuscript
preparation (University of Rochester Medical Center, NY, USA); we
also thank Dr Jiangzhou Yu and Dr Soo O.K. Lee, for their helpful
review and discussion (University of Rochester Medical Center, NY,
USA). This sudy was supported by National Natural Science
Foundation of China (NSFC, no. 81101936).
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
MMP-9
|
matrix metallopeptidase 9
|
|
SB
|
silibinin
|
|
RCC
|
renal cell carcinoma
|
|
ERK
|
extracellular signal-regulated
kinase
|
References
|
1
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1999. CA Cancer J Clin. 49:8–31. 1999.
|
|
2
|
Maldazys JD and DeKernion JB: Prognostic
factors in metastatic renal carcinoma. J Urol. 136:376–379.
1986.
|
|
3
|
Li L, Gao Y, Zhang L, Zeng J, He D and Sun
Y: Silibinin inhibits cell growth and induces apoptosis by caspase
activation, down-regulating survivin and blocking EGFR-ERK
activation in renal cell carcinoma. Cancer Lett. 272:61–69.
2008.
|
|
4
|
Dancey JE: Epidermal growth factor
receptor and epidermal growth factor receptor therapies in renal
cell carcinoma: do we need a better mouse trap? J Clin Oncol.
22:2975–2977. 2004.
|
|
5
|
Ishikawa J, Maeda S, Umezu K, Sugiyama T
and Kamidono S: Amplification and overexpression of the epidermal
growth factor receptor gene in human renal-cell carcinoma. Int J
Cancer. 45:1018–1021. 1990.
|
|
6
|
Minner S, Rump D, Tennstedt P, et al:
Epidermal growth factor receptor protein expression and genomic
alterations in renal cell carcinoma. Cancer. 118:1268–1275.
2012.
|
|
7
|
Hutson TE: Targeted therapies. Oncologist.
16(Suppl 2): 14–22. 2011.
|
|
8
|
Itsumi M and Tatsugami K: Immunotherapy.
Clin Dev Immunol. 2010:2845812010.
|
|
9
|
Yang JC, Sherry RM, Steinberg SM, et al:
Low reponse rate randomized study of high-dose and low-dose
interleukin-2 in patients with metastatic renal cancer. J Clin
Oncol. 21:3127–3132. 2003.
|
|
10
|
Velmurugan B, Gangar SC, Kaur M, Tyagi A,
Deep G and Agarwal R: Silibinin exerts sustained growth suppressive
effect against human colon carcinoma SW480 xenograft by targeting
multiple signaling molecules. Pharm Res. 27:2085–2097. 2010.
|
|
11
|
Wu KJ, Zeng J, Zhu GD, et al: Silibinin
inhibits prostate cancer invasion, motility and migration by
suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin.
30:1162–1168. 2009.
|
|
12
|
Chen PN, Hsieh YS, Chiang CL, Chiou HL,
Yang SF and Chu SC: Silibinin inhibits invasion of oral cancer
cells by suppressing the MAPK pathway. J Dent Res. 85:220–225.
2006.
|
|
13
|
Singh RP, Raina K, Sharma G and Agarwal R:
Silibinin inhibits established prostate tumor growth, progression,
invasion, and metastasis and suppresses tumor angiogenesis and
epithelial-mesenchymal transition in transgenic adenocarcinoma of
the mouse prostate model mice. Clin Cancer Res. 14:7773–7780.
2008.
|
|
14
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000.
|
|
15
|
Asakuma J, Sumitomo M, Asano T, Asano T
and Hayakawa M: Modulation of tumor growth and tumor induced
angiogenesis after epidermal growth factor receptor inhibition by
ZD1839 in renal cell carcinoma. J Urol. 171:897–902. 2004.
|
|
16
|
Prewett M, Rothman M, Waksal H, Feldman M,
Bander NH and Hicklin DJ: Mouse-human chimeric anti-epidermal
growth factor receptor antibody C225 inhibits the growth of human
renal cell carcinoma xenografts in nude mice. Clin Cancer Res.
4:2957–2966. 1998.
|
|
17
|
Rowinsky EK, Schwartz GH, Gollob JA, et
al: Safety, pharmacokinetics, and activity of ABX-EGF, a fully
human anti-epidermal growth factor receptor monoclonal antibody in
patients with metastatic renal cell cancer. J Clin Oncol.
22:3003–3015. 2004.
|
|
18
|
Motzer RJ, Amato R, Todd M, et al: Phase
II trial of antiepidermal growth factor receptor antibody C225 in
patients with advanced renal cell carcinoma. Invest New Drugs.
21:99–101. 2003.
|
|
19
|
Singh RP and Agarwal R: A cancer
chemopreventive agent silibinin, targets mitogenic and survival
signaling in prostate cancer. Mutat Res. 555:21–32. 2004.
|
|
20
|
Tyagi A, Sharma Y, Agarwal C and Agarwal
R: Silibinin impairs constitutively active TGFalpha-EGFR autocrine
loop in advanced human prostate carcinoma cells. Pharm Res.
25:2143–2150. 2008.
|
|
21
|
Singh RP, Gu M and Agarwal R: Silibinin
inhibits colorectal cancer growth by inhibiting tumor cell
proliferation and angiogenesis. Cancer Res. 68:2043–2050. 2008.
|
|
22
|
Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC
and Lu KH: Silibinin suppresses human osteosarcoma MG-63 cell
invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of
MMP-2. Carcinogenesis. 28:977–987. 2007.
|
|
23
|
Chang HR, Chen PN, Yang SF, et al:
Silibinin inhibits the invasion and migration of renal carcinoma
786-O cells in vitro, inhibits the growth of xenografts in vivo and
enhances chemosensitivity to 5-fluorouracil and paclitaxel. Mol
Carcinog. 50:811–823. 2011.
|
|
24
|
Qi L, Singh RP, Lu Y, et al: Epidermal
growth factor receptor mediates silibinin-induced cytotoxicity in a
rat glioma cell line. Cancer Biol Ther. 2:526–531. 2003.
|
|
25
|
Qiu Q, Yang M, Tsang BK and Gruslin A:
EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves
activation of both PI3K and MAPK signalling pathways. Reproduction.
128:355–363. 2004.
|
|
26
|
Tian YC, Chen YC, Chang CT, et al:
Epidermal growth factor and transforming growth factor-beta1
enhance HK-2 cell migration through a synergistic increase of
matrix metalloproteinase and sustained activation of ERK signaling
pathway. Exp Cell Res. 313:2367–2377. 2007.
|
|
27
|
Kang CS, Pu PY, Li YH, et al: An in vitro
study on the suppressive effect of glioma cell growth induced by
plasmid-based small interference RNA (siRNA) targeting human
epidermal growth factor receptor. J Neurooncol. 74:267–273.
2005.
|
|
28
|
Sumitomo M, Asano T, Asakuma J, Asano T,
Horiguchi A and Hayakawa M: ZD1839 modulates paclitaxel response in
renal cancer by blocking paclitaxel-induced activation of the
epidermal growth factor receptor-extracellular signal-regulated
kinase pathway. Clin Cancer Res. 10:794–801. 2004.
|