Introduction
Cancer cells increase their cellular metabolism rate
to produce a high level of energy and for biosynthesis of cellular
materials for continuous proliferation (1). The conversion of glucose metabolism
from oxidation to glycolysis is one of the representative
strategies for generation of ATP in cancer cells (2). However, in order to meet the high
metabolic demand, cancer cells must boost the processes for high
level of uptake of nutrition sources such as glucose and amino
acids. Indeed, it has been shown that the expression of GLUT1, a
transporter for glucose, is promoted in cancer cells (3,4).
Expression of glutamine transporters such as ASCT2 is also
facilitated (5,6), and this would support the cellular
abundance of glutamine, which is consumed as a source of nitrogen
and energy in tumor cells (7). The
coordination of expression of highly efficient transporters for
organic materials is requisite for powerful consumption of
resources to ensure sustained cancer cell proliferation (8), and disruption of this system would
lead to failure of tumor growth.
L-type amino acid transporter 1 (LAT1) is a member
of the solute carrier (SLC) family and incorporates neutral amino
acids into cells in a Na+-independent manner (9). Whereas the normal body displays low
levels and restricted expression of LAT1, high levels of LAT1
expression have been observed in a wide variety of cancer cells
(9,10). Therefore, it is speculated that LAT1
has a pivotal role in growth of tumor cells by promoting uptake of
essential amino acids. Indeed, the LAT1-specific inhibitor JPH203
(KYT0353) attenuated the incorporation of essential amino acids in
cancer cell lines and the growth of human tumor cells planted into
a nude mouse (11). For these
reasons, JPH203 has attracted attention as a potentially effective
drug for cancers with less toxicity, especially for therapy of
tumors with extremely poor prognosis such as pancreatic cancer and
gastric scirrhous cancer. However, the molecular mechanism
underlying the predominant enrichment of LAT1 in cancer cells has
remained unclear.
Here we defined c-Myc, a proto-oncogene that encodes
a transcription factor, as a critical regulator for LAT1 expression
in MIA Paca-2 human pancreatic cancer cells, which require LAT1 for
uptake of neutral amino acids. The LAT1 promoter has a canonical
c-Myc binding sequence, and knockdown of c-Myc mediated by siRNA
resulted in a reduction of LAT1, leading to disturbed neutral amino
acid incorporation. Moreover, overexpression of c-Myc increased
LAT1 promoter activity, whereas this effect was not observed with
mutation of c-Myc binding site. Taken together, these results
indicate that c-Myc plays a pivotal role for accelerated expression
of LAT1 in human pancreatic cancer cells.
Materials and methods
Cells
MIA Paca-2 cells were purchased from Japan Health
Sciences Foundation (Tokyo, Japan) and maintained in Dulbecco’s
modified Eagle’s medium supplemented with 10% fetal bovine serum, 2
mM L-glutamine, 0.1 mM non-essential amino acids, and
penicillin/streptomycin.
Antibodies
Anti-LAT1 antibody was described previously
(12). Anti-c-Myc antibody (9E11)
was purchased from Medical and Biological Laboratories (Nagoya,
Japan). Anti-GAPDH (rabbit polyclonal) was purchased from
Sigma-Aldrich (St.Louis, MO, USA).
siRNA transfection
Silencer select control siRNA (#1 and 2),
pre-designed c-Myc-specific siRNA (s9129 and s9130) and
LAT1-specific siRNA (s15653 and s15655) were purchased from Ambion
(Austin, TX, USA). siRNA was transfected into cells seeded in a
24-well plate with Lipofectamine RNAi MAX (Invitrogen, Carlsbad,
CA, USA). Cells were cultured for 3 days to knockdown endogenous
proteins and used for further experiments.
Western blot analysis
Cells were lysed in SDS sample buffer and boiled.
Cell lysate samples were resolved by SDS-polyacrylamide gel
electrophoresis and transferred to a PVDF membrane. Immunoblotting
was performed with a standard protocol.
Real-time (RT) PCR
Total RNA was extracted using an RNeasy mini kit
(Qiagen, Valencia, CA, USA). The cDNA was synthesized from total
RNA with a prime-script RT reagent kit (Takara Bio., Shiga, Japan).
RT-PCR was performed using SYBR Premix Ex Taq (Takara Bio.). The
primers for RT-PCR (HA097381-F and -R for LAT1, HA067812-F and -R
for GAPDH) were purchased from Takara Bio. The relative amount of
LAT1 mRNA was normalized to GAPDH.
[14C]-L-leucine uptake
siRNA-transfected cells were cultured for 3 days.
The growth medium was removed and cells were washed 3 times with
Na+-free Hank’s balanced salt solution (HBSS) containing
125 mM choline-Cl, 25 mM HEPES, 4.8 mM KCl, 1.2 mM
MgSO4, 1.2 mM KH2PO4, 1.3 mM
CaCl2 and 5.6 mM glucose (pH 7.4) and further incubated
in the same solution at 37°C for 10 min. [14C]-L-leucine
uptake was initiated by incubating the cells in Na+-free
HBSS containing 1.0 μM [14C]-L-leucine (Moravek, Brea,
CA, USA) at 37°C for 1 min. Uptake was terminated by washing the
cells 3 times with ice-cold Na+-free HBSS. Cells were
lysed with 0.1 N NaOH and radioactivity was measured using an
LSC-5100 β-scintillation counter (Aloka, Tokyo, Japan). An aliquot
of cell lysate was used to determine protein concentration by the
BCA protein assay (Pierce Biotechnology, Rockford, IL, USA).
Reporter assay
Human LAT1 promoter fragment (−294/+1 relative to
the translation initiation site) was obtained by the PCR method
using human genome DNA as a template. The DNA fragment was placed
in the polylinker site of pGL3 basic vector (Promega, Madison, WI,
USA). A construct with mutation in the c-Myc consensus binding site
(located at −210 bp relative to translation initiation site) was
generated by replacing the sequence CACGTG with GGGTC by the PCR
method using pGL3 LAT1 (1-294) as a template. c-Myc expression
vector pCMV6 c-Myc was purchased from OriGene Technologies
(Rockville, MD, USA). For reporter assays, DNA constructs were
transfected into MIA PaCa-2 cells with FuGENE HD (Roche Applied
Science, Indianapolis, IN, USA) and cells were cultured for 20 h
and then harvested, and luciferase activity was assessed using an
Enhanced Luciferase Assay kit (Becton-Dickinson, San Diego, CA,
USA). The luciferase activity was normalized to the activity of
cotransfected CMV-β-galactosidase (Invitrogen).
Statistical analysis
All statistical significance was tested with
Student’s t-test. In siRNA transfection experiments, samples
treated with siRNA for gene knockdown were compared to both
control-1 and control-2 siRNA-treated samples.
Results
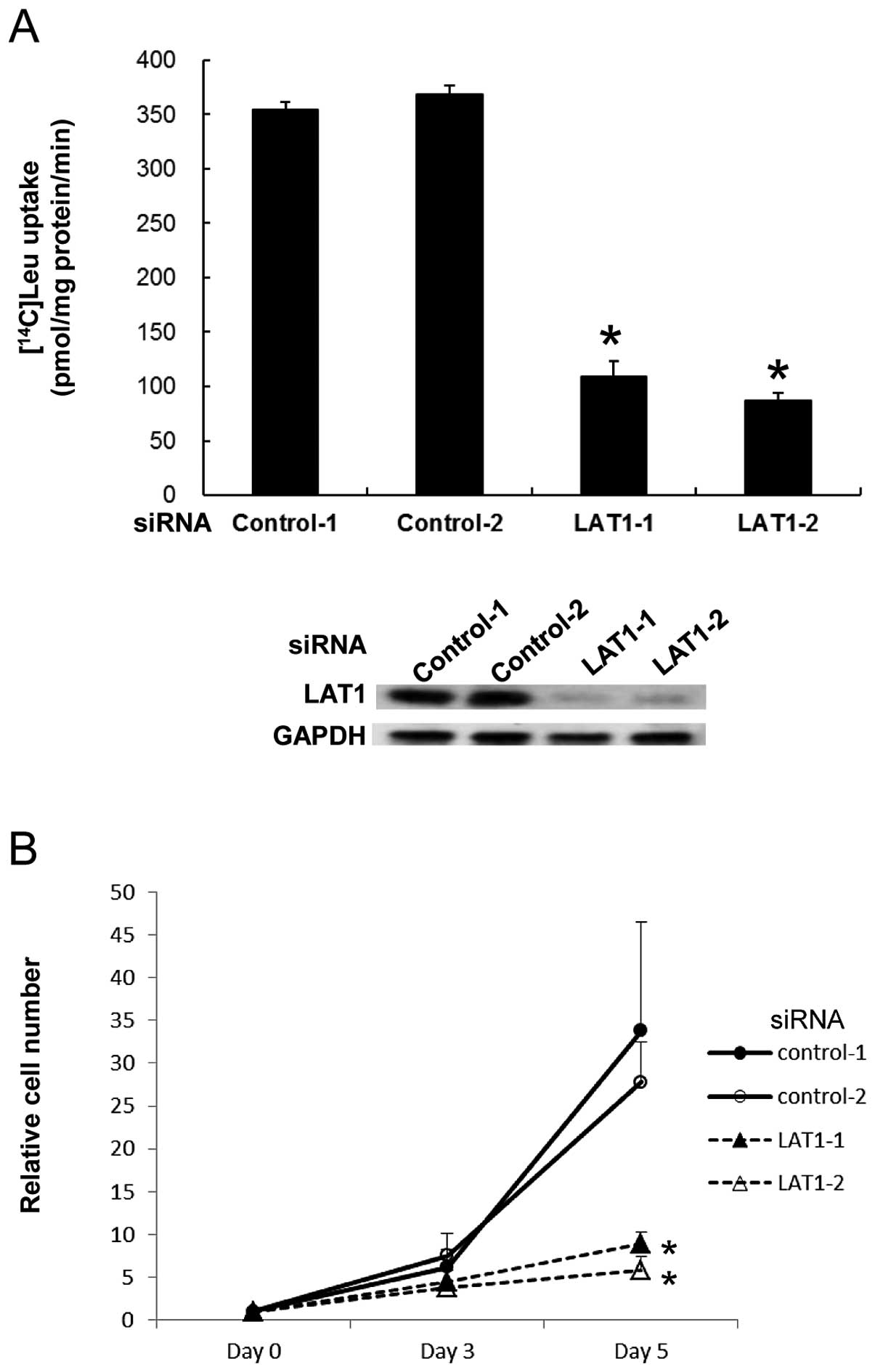
LAT1 is indispensable for incorporation
of neutral amino acid in MIA Paca-2 cells
MIA Paca-2 cells are human pancreatic cancer cells
that express a high level of LAT1. To address the functional
significance of LAT1 in these cells, we initially evaluated the
effect of LAT1 reduction by using siRNA on the uptake of L-leucine,
a representative neutral amino acid. We transfected siRNA specific
for LAT1 mRNA with two different sequences to exclude targeting any
genes other than LAT1 and analyzed [14C]-labeled
L-leucine uptake. The incorporation of [14C]-L-leucine
was severely impaired by knockdown of LAT1 (Fig. 1A). This result indicates that LAT1
is absolutely required for neutral amino acid uptake in MIA Paca-2
cells.
Since cancer cells are continuously proliferating,
insufficiency of amino acids resulting from disturbed expression of
their transporters would limit the growth of cells. Given that MIA
Paca-2 cells rely on LAT1 for uptake of neutral amino acids, we
investigated whether disturbed LAT1 expression results in
inhibition of cell proliferation. MIA Paca-2 cells were transfected
with LAT1 siRNA and the number of cells was counted. Cell
proliferation was markedly impaired by LAT1 siRNA with both
sequences (Fig. 1B), indicating
that deprivation of neutral amino acids by decreased LAT1
expression leads to inhibition of tumor growth.
Taken together, these results suggest that LAT1 is
an essential transporter to provide sufficient neutral amino acids
that are crucial for continuous proliferation of human pancreatic
cancer cells.
c-Myc is crucial for expression of
LAT1
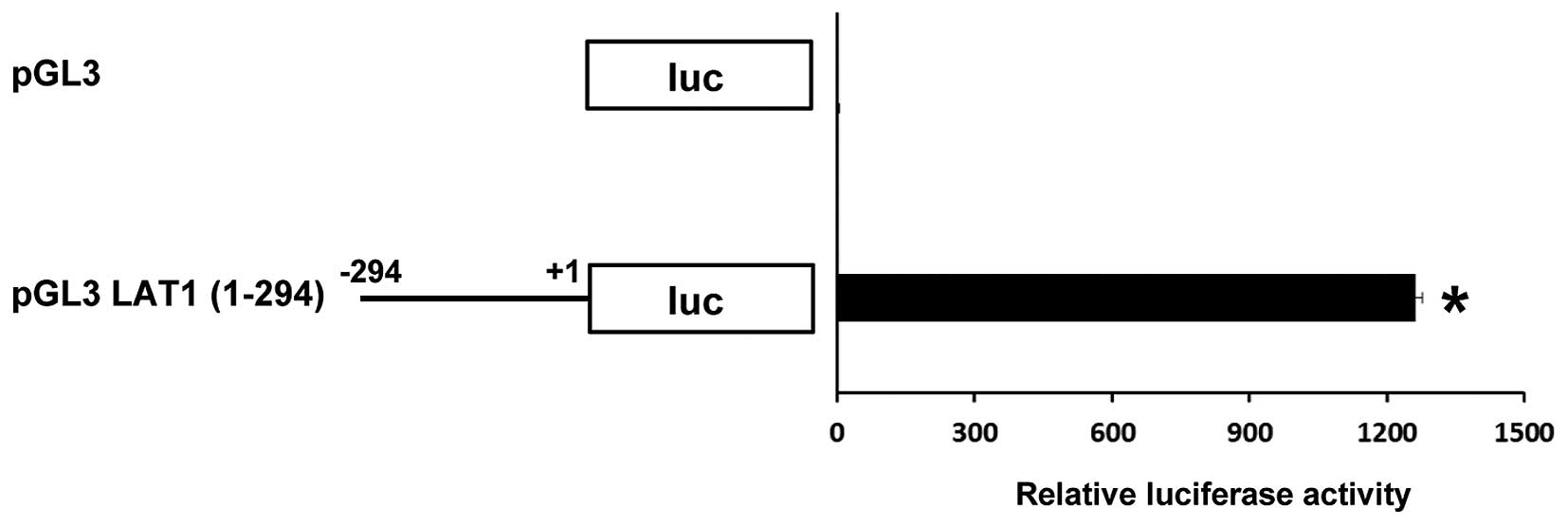
In many cases, the promoter region for gene
expression is observed adjacent to the transcriptional starting
point. In line with this notion, it has been shown that the LAT1
gene promoter is located at a region of ~300 bp upstream of the
LAT1 translational site (13).
Indeed, this region has strong ability as a promoter to drive the
expression of posterior cDNA coding luciferase in MIA Paca-2 cancer
cells (Fig. 2).
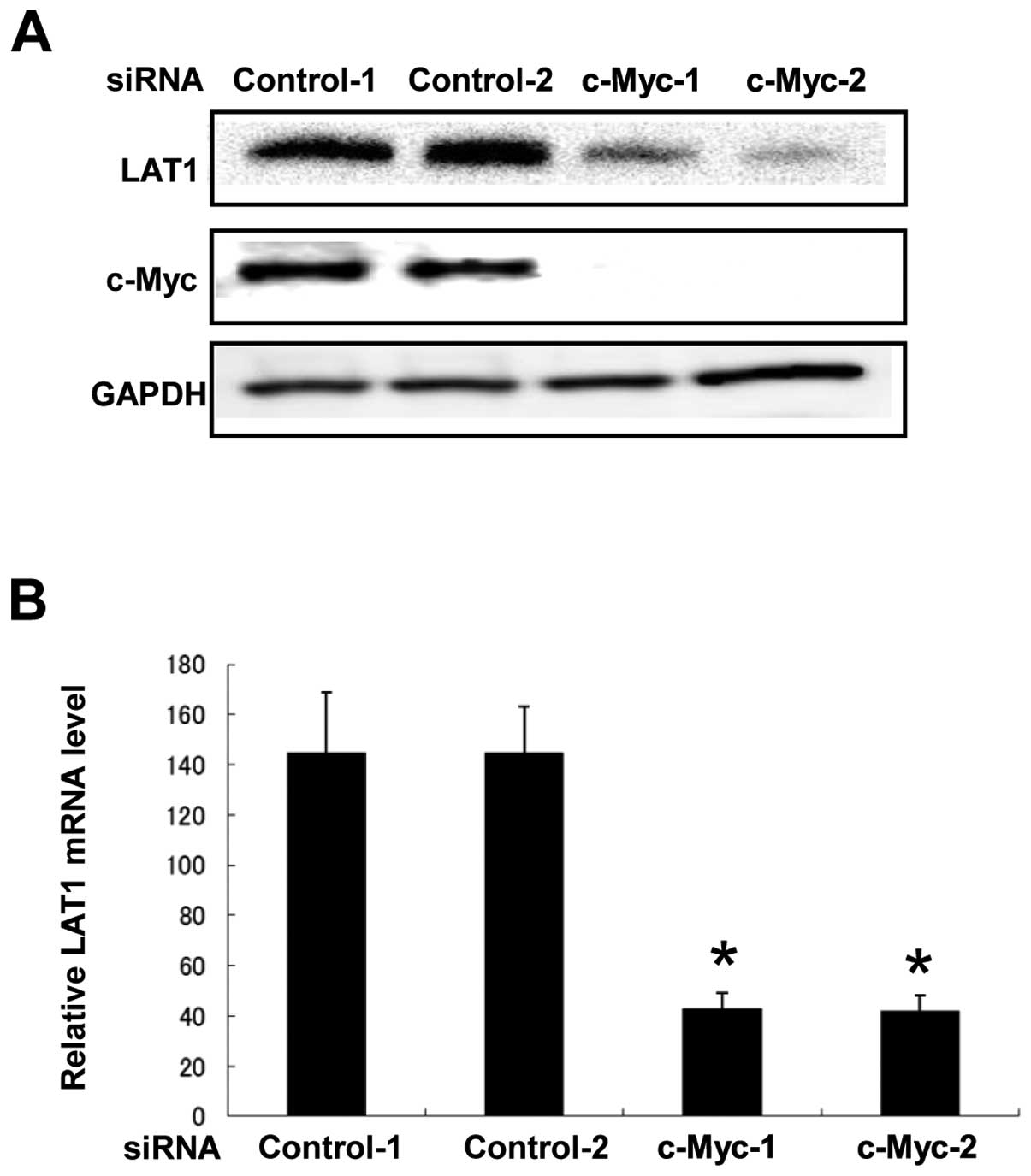
To identify the factors that regulate transcription
of the LAT1 gene, we analyzed the sequence of the LAT1 promoter and
found that there is a consensus c-Myc binding sequence (CACGTG)
(14,15) located 210 bp upstream of the LAT1
translation site. This finding prompted us to examine whether c-Myc
participates in the regulation of LAT1 expression in cancer cells,
since c-Myc is a proto-oncogene that has strong ability to enforce
malignant alteration. To address this, we transfected siRNA for
knockdown of c-Myc expression into MIA Paca-2 cells and analyzed
LAT1 protein level. As shown in Fig.
3A, suppression of c-Myc expression by siRNA with two different
sequences dramatically decreased the expression of LAT1 protein.
This result indicates that c-Myc is critical for up-regulation of
LAT1 expression in MIA Paca-2 cancer cells.
To determine whether reduction of LAT1 protein
expression induced by c-Myc siRNA is derived from a reduced amount
of LAT1 mRNA, we performed RT-PCR using RNA from MIA Paca-2 cells
transfected with c-Myc siRNA. We found that transfection of c-Myc
siRNA resulted in reduction of LAT1 mRNA expression (Fig. 3B). This result suggests that c-Myc
regulates LAT1 expression at the mRNA level.
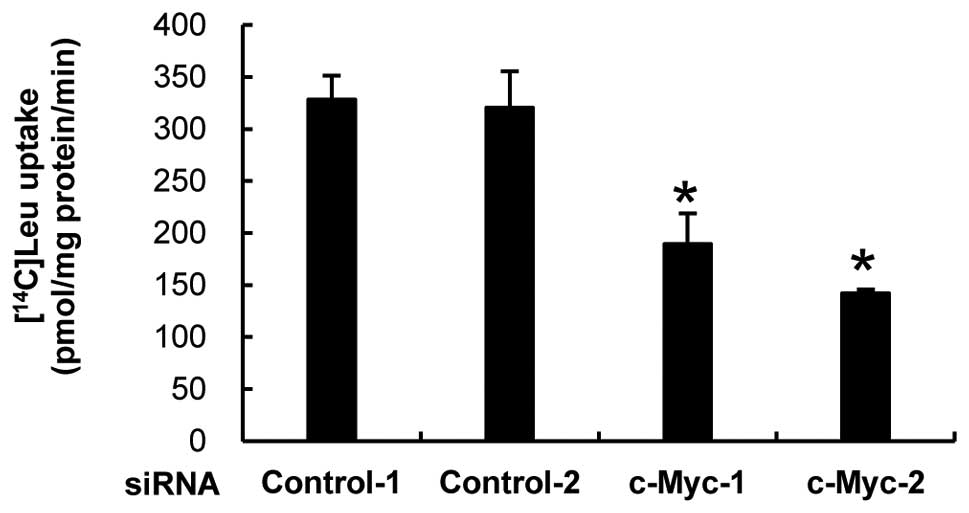
Down-regulation of LAT1 by c-Myc
knockdown impairs the uptake of neutral amino acid
Based on our finding that LAT1 is essential for
incorporation of neutral amino acids in MIA Paca-2 cells, we
postulated that reduction of LAT1 expression caused by knockdown of
c-Myc protein could affect the efficiency of neutral amino acid
incorporation. To test this hypothesis, we transfected c-Myc siRNA
into MIA Paca-2 cells and measured [14C]-labeled leucine
uptake. c-Myc siRNA-transfected cells had less ability than that of
control siRNA-transfetcted cells for uptake of leucine (Fig. 4). This result indicates that
c-Myc-mediated up-regulation of LAT1 expression is strictly
required to maintain the maximum level of amino acid incorporation
in MIA Paca-2 cancer cells.
Consensus c-Myc binding sequence is
required for maximum activity of LAT1 promoter
As described above, a consensus c-Myc binding
sequence is present in the LAT1 promoter. In addition, our studies
demonstrated that reduction of c-Myc results in declined LAT1
expression at the mRNA level. These observations suggest that c-Myc
can drive transcription of the LAT1 gene by enhancing the LAT1
promoter activity. To examine this, we performed a reporter assay
driven by the LAT1 promoter. The construct for reporter assay used
in Fig. 2 and a vector for
overexpression of c-Myc were co-transfected into MIA Paca-2 cells
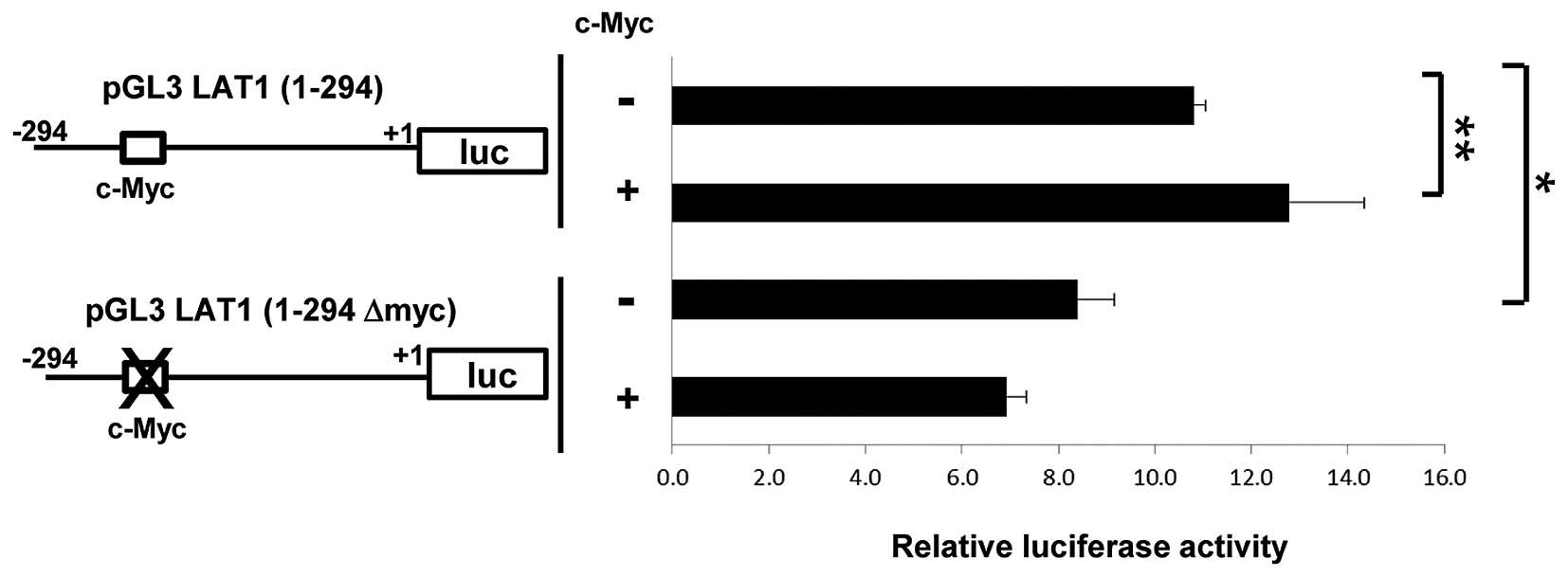
and luciferase activity was analyzed. The overexpression of c-Myc
increased LAT1 promoter activity (Fig.
5). On the other hand, mutation in the c-Myc binding sequence
in LAT1 promoter resulted in significant reduction of promoter
activity. In addition, overexpression of c-Myc could not promote
activity of LAT1 promoter with mutation in c-Myc binding site.
These results indicate that c-Myc enhances LAT1 promoter activity
and a consensus binding sequence of c-Myc is crucial for maximum
activity of LAT1 promoter.
Discussion
We have demonstrated the physiological importance of
LAT1 as an essential factor to transport neutral amino acids into
MIA Paca-2 human pancreatic cancer cells. Inhibition of LAT1
expression by siRNA severely decreased neutral amino acid
incorporation, leading to suppression of cell growth. We also
demonstrated that knockdown of c-Myc, a proto-oncogene, resulted in
a reduction of LAT1 protein as well as mRNA, which led to impaired
neutral amino acid incorporation. Moreover, mutation of the c-Myc
binding site in the LAT1 promoter region suppressed maximum
promoter activity and diminished the effect of c-Myc
overexpression. These results indicate that c-Myc is a crucial
transcription factor for up-regulation of LAT1 in human pancreatic
cancer cells.
In order to satisfy rapid cellular metabolism,
cancer cells must take up as much nutrients as possible. Therefore,
it is a huge advantage to make utmost use of special molecular
devices such as highly effective transporters to maximize the
efficiency of nutrition incorporation. Insufficient nutrition
resulting from inability to use facilitative transporters would
limit the proliferation of cancer cells. Consistent with this
notion, we revealed that siRNA-mediated LAT1 reduction remarkably
impaired uptake of neutral amino acids, which subsequently induced
arrest of the growth of pancreatic cancer cells. Although previous
studies using a potential LAT1-specific inhibitor have shown the
ability of this drug to inhibit tumor growth (11). Evidence that excludes any effect of
this drug on molecules other than LAT1 has not been obtained. Our
results obtained by using LAT1 siRNA are important in that they
strongly support the drug model of LAT1 being essential for
delivery of neutral amino acids into cancer cells.
Results of many studies have shown that LAT1
exhibits preferred expression in cancer cells (10,12,16–18).
However, the molecular machinery regulating LAT1 expression in
cancer cells has remained a long-standing puzzle. In this study, we
showed that c-Myc is a critical factor for promotion of LAT1
expression in human pancreatic cancer cells. Our observation about
regulation of LAT1 by virtue of a proto-oncogene would provide a
clue that accounts for the high expression level of LAT1 in cancer
cells. Many genes associated with the cell cycle such as cyclins
are representative targets of c-Myc (19–21).
However, c-Myc also enhances biosynthesis as well as energy
generation. For example, expression of genes required for glucose
transport and the glycolytic pathway is up-regulated by c-Myc
(22,23). Therefore, c-Myc conducts
tumorigenesis by controlling the expression of genes involved in
the cell cycle in concert with metabolic genes, and such ability to
concurrently regulate a set of genes all required for cell
proliferation is an enormous contribution to stable growth of
cancer cells. Our results showed that LAT1 is also a c-Myc target
as a central transporter of essential neutral amino acids expressed
in human pancreatic cancer cells.
Since overexpression of c-Myc enhanced the LAT1
promoter activity and mutation of c-Myc binding site diminished
this effect, one possible explanation for up-regulation of LAT1 by
c-Myc is its binding to consensus sequence in LAT1 promoter and
driving the transcription of LAT1 mRNA. However, although mutation
of the c-Myc binding sequence significantly reduced LAT1 promoter
activity, the effect was slight. Therefore, we cannot exclude the
possibility that c-Myc regulates the mRNA level of LAT1 by some
other mechanisms in addition to direct binding to the promoter
region. For example, since one of the targets regulated by c-Myc is
microRNA (24), c-Myc might
increase LAT1 expression by down-regulating microRNA, which
interacts with and diminishes LAT1 mRNA. Alternatively, because
c-Myc controls some of target gene expression epigenetically
(25,26), c-Myc might regulate LAT1 gene
expression by modulating chromatin conformations of LAT1 promoter.
Further study is required to decide more details of the mechanism
for regulation of LAT1 by c-Myc.
In conclusion, we identified c-Myc as a crucial
regulator for expression of LAT1. Our results will be helpful in
investigation and further development of drugs that target LAT1
more effectively in cancer therapy.
Acknowledgements
This work was supported in part by grants of TR
Project from the New Energy and Industrial Technology Development
Organization (NEDO), Japan.
References
|
1
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Airley RE and Mobasheri A: Hypoxic
regulation of glucose transport, anaerobic metabolism and
angiogenesis in cancer: novel pathways and targets for anticancer
therapeutics. Chemotherapy. 53:233–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Physiol. 202:654–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Witte D, Ali N, Carlson N and Younes M:
Overexpression of the neutral amino acid transporter ASCT2 in human
colorectal adenocarcinoma. Anticancer Res. 22:2555–2557.
2002.PubMed/NCBI
|
|
6
|
Li R, Younes M, Frolov A, Wheeler TM,
Scardino P, Ohori M and Ayala G: Expression of neutral amino acid
transporter ASCT2 in human prostate. Anticancer Res. 23:3413–3418.
2003.PubMed/NCBI
|
|
7
|
Levine AJ and Puzio-Kuter AM: The control
of the metabolic switch in cancers by oncogenes and tumor
suppressor genes. Science. 330:1340–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ganapathy V, Thangaraju M and Prasad PD:
Nutrient transporters in cancer: relevance to Warburg hypothesis
and beyond. Pharmacol Ther. 121:29–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanai Y, Segawa H, Miyamoto K, Uchino H,
Takeda E and Endou H: Expression cloning and characterization of a
transporter for large neutral amino acids activated by the heavy
chain of 4F2 antigen (CD98). J Biol Chem. 273:23629–23632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yanagida O, Kanai Y, Chairoungdua A, et
al: Human L-type amino acid transporter 1 (LAT1): characterization
of function and expression in tumor cell lines. Biochim Biophys
Acta. 1514:291–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oda K, Hosoda N, Endo H, et al: L-type
amino acid transporter 1 inhibitors inhibit tumor cell growth.
Cancer Sci. 101:173–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ichinoe M, Mikami T, Yoshida T, et al:
High expression of L-type amino-acid transporter 1 (LAT1) in
gastric carcinomas: comparison with non-cancerous lesions. Pathol
Int. 61:281–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nii T, Segawa H, Taketani Y, et al:
Molecular events involved in up-regulating human
Na+-independent neutral amino acid transporter LAT1
during T-cell activation. Biochem J. 358:693–604. 2001.PubMed/NCBI
|
|
14
|
Blackwell TK, Kretzner L, Blackwood EM,
Eisenman RN and Weintraub H: Sequence-specific DNA binding by the
c-Myc protein. Science. 250:1149–1151. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alex R, Sözeri O, Meyer S and Dildrop R:
Determination of the DNA sequence recognized by the bHLH-zip domain
of the N-Myc protein. Nucleic Acids Res. 20:2257–2263. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakata T, Ferdous G, Tsuruta T, et al:
L-type amino-acid transporter 1 as a novel biomarker for high-grade
malignancy in prostate cancer. Pathol Int. 59:7–18. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaira K, Oriuchi N, Imai H, et al:
Prognostic significance of L-type amino acid transporter 1 (LAT1)
and 4F2 heavy chain (CD98) expression in early stage squamous cell
carcinoma of the lung. Cancer Sci. 100:248–254. 2009. View Article : Google Scholar
|
|
18
|
Kaji M, Kabir-Salmani M, Anzai N, et al:
Properties of L-type amino acid transporter 1 in epidermal ovarian
cancer. Int J Gynecol Cancer. 20:329–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouchard C, Thieke K, Maier A, et al:
Direct induction of cyclin D2 by Myc contributes to cell cycle
progression and sequestration of p27. EMBO J. 18:5321–5333. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bouchard C, Dittrich O, Kiermaier A,
Dohmann K, Menkel A, Eilers M and Lüscher B: Regulation of cyclin
D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP
recruitment and histone acetylation at the cyclin D2 promoter.
Genes Dev. 15:2042–2047. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Menssen A and Hermeking H:
Characterization of the c-Myc-regulated transcriptome by SAGE:
identification and analysis of c-Myc target genes. Proc Natl Acad
Sci USA. 99:6274–6279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JW, Zeller KI, Wang Y, Jegga AG,
Aronow BJ, O’Donnell KA and Dang CV: Evaluation of myc E-box
phylogenetic footprints in glycolytic genes by chromatin
immunoprecipitation assays. Mol Cell Biol. 24:5923–5936. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Osthus RC, Shim H, Kim S, et al:
Deregulation of glucose transporter 1 and glycolytic gene
expression by c-Myc. J Biol Chem. 275:21797–21800. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O’Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005.PubMed/NCBI
|
|
25
|
Frank SR, Schroeder M, Fernandez P,
Taubert S and Amati B: Binding of c-Myc to chromatin mediates
mitogen-induced acetylation of histone H4 and gene activation.
Genes Dev. 15:2069–2082. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frank SR, Parisi T, Taubert S, et al: Myc
recruits the TIP60 histone acetyltransferase complex to chromatin.
EMBO Rep. 4:575–580. 2003. View Article : Google Scholar : PubMed/NCBI
|