Introduction
Colorectal cancer represents almost 10% of all
tumors and it is the third most common form of malignancy, behind
prostate and lung cancers worldwide (1,2). More
than 70% of colon cancers are related to diet and lifestyle and it
was suggested that changes in dietary and lifestyle patterns can
reduce colon cancer rates (3).
Persons with a diet high in vegetables, cereals, fruits and seeds
have a lower risk of colon cancer, and polyphenols in fruit led to
reduce colon cancer risk experimentally (4). Thus, the therapy of the human colon
cancer, induction of apoptosis is recognized as a very useful and
promising approach.
Apoptosis is a mode of programmed cell death that is
important for maintaining cell number, and deregulation of
apoptosis may contribute to development of neurodegenerative
disorders and cancer (5). There are
two protein families regulating apoptosis; one is the Bcl-2 family
which is involved in the initiation phase of apoptosis, and the
other is caspase family of proteases that are responsible for the
execution phase (6,7). It is well-known that cytochrome c
release from the mitochondrial inter-membrane space represents an
important checkpoint in apoptosis (8,9). Thus,
at this checkpoint, Bcl-2 family plays regulatory influence on this
process (10).
Diallyl trisulfide (DATS) is one of the main
activity compounds in garlic extract (11,12)
and has a broad-spectrum anti-neoplastic activity such as induction
of apoptosis in many human cancer cells (13–19).
DATS-induced apoptosis correlates with downregulation and
hyper-phosphorylation of Bcl-2 in human prostate cancer cells
(20). It was reported that
p38/MAPK and caspase-8 are involved in the process of DATS-induced
apoptosis in human CNE2 cells and interact with each other
(21). Recently, in our laboratory,
we have found that DATS inhibited migration and invasion of human
colon cancer colo 205 cells in vitro(22) and inhibited tumor growth in an
allograft animal model (23).
DATS-induced apoptosis has been shown in many human
cancer cells but the cytotoxic effects on human primary colorectal
cancer cells have not yet been defined. Therefore, the aim of this
study is to investigate the effect of DATS on the human primary
colorectal cancer cells and to elucidate its mechanism.
Materials and methods
Chemicals and reagents
Diallyl trisufile (DATS) (99% purity),
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
propidium iodide (PI) and 4′,6-diamidino-2-phenylindole (DAPI) were
purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). RPMI-1640
medium, fetal bovine serum (FBS), trypsin-EDTA, and
penicillin/streptomycin were purchased from Gibco-BRL/Invitrogen
Corp. (Grand Island, NY, USA). 2′,7′-Dichlorodihydrofluorescein
diacetate (DCFH-DA) and 3,3′-dihexyloxacarbocyanine iodide
(DiOC6) were purchased from Molecular Probes
(Invitrogen, Eugene, OR, USA). Antibodies to cytochrome c, Apaf-1,
caspase-9 and caspase-3, Bcl-2 and Bax were purchased from Cell
Signaling (USA). All other chemicals used were of analytical
grade.
Isolation of human primary colorectal
cancer cells
Three colorectal carcinoma specimens from three
patients were obtained from 2008 to 2009 from the Department of
Surgery, China Medical University Hospital, Taichung, Taiwan after
approval of the experiment by the hospital's Ethics Committee, and
with written, informed consent from patients (IRB NO:
DMR-96-IRB-72) (24). Each specimen
was dissected into 1-mm3 pieces, immersed in a 10-fold
volume of 0.25% trypsin solution (Sigma-Aldrich), maintained at 4°C
overnight and then incubated for 1 h at 37°C. Then the trypsin was
added to the cells (each well) followed by with FBS, the solution
containing released cells was collected by using centrifugation at
150 × g for 5 min. After centrifugation, cells in each tube were
re-suspended with RPMI-1640 supplemented with 10% FBS, and seeded
into a 10-cm culture dish. Undigested tissue from each patient was
immediately immersed in collagenase solution (500 U/ml in RPMI-1640
medium with 10% serum) (Sigma-Aldrich) in a plate and incubated at
37°C for 1 h. Released cells were collected, centrifuged,
re-suspended with RPMI-1640 medium supplemented with 10% FBS, and
seeded into a culture flask. When primary cultures became confluent
then cells were detached by trypsin
(0.25%)-ethylenediaminetetraacetic acid (EDTA) (0.02%) solution
(Sigma-Aldrich), examined and counted under phase-contrast
microscope, then were centrifuged and re-suspended with RPMI-1640
medium supplemented with 10% FBS and seeded into new culture flasks
(25–27).
Cell viability assay
Human primary colorectal cancer cells were seeded
onto 96-well plates at 1×104 cells/well 24 h before
treatment. The cultures were then rinsed in phenol-free RPMI-1640
medium and incubated with the DATS at the final concentrations 0,
10, 20 and 40 μM in RPMI-1640 culture medium for 24 h. At the end
of incubation, 20 μl of MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (5
mg/ml) was added to each well and incubated for 4 h at 37°C then
the MTT solution was removed and 200 μl dimethylsulfoxide (DMSO)
was added to dissolve the crystals. The absorbance of each well at
570 nm was measured by using a spectrophotometric plate reader
(Bio-Rad Laboratories, Tokyo, Japan) (24). All values were compared to the
corresponding controls. All assays were performed with 3
replicates.
DAPI staining for apoptosis
Human primary colorectal cancer cells were plated in
the 12-well plates at the density of 2×105 cells/well
for overnight then were treated with DATS (20 μM) for 24 h, and
cells in each well were then fixed in 4% paraformaldehyde for 30
min. Then cells from each treatment were added 0.1% Triton X-100
and maintained for 10 min and then incubated with 1 μg/ml of DAPI
staining solution for 30 min in the dark. Apoptotic cells from each
treatment and control were observed through fluorescence microscopy
(Zeiss, Oberkochen, Germany) as previously described (24,27).
Measurement of reactive oxygen species
(ROS) production
ROS production from DATS-treated and -untreated
cells was measured by flow cytometry following staining with 500 μl
of 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Molecular Probes,
Invitrogen). Human primary colorectal cancer cells were plated in
the 12-well plate at the density of 2×105 cells/well for
24 h then were treated with or without DATS (20 μM) for 0, 6 and 12
h. Then cells from each well were collected and were stained with
20 μM DCFH-DA for 30 min at 37°C and the fluorescence intensity in
cells was determined using the flow cytometer (24,27).
Mitochondrial membrane potential
assays
The levels of mitochondrial membrane potential
(ΔΨm) from DATS-treated and -untreated cells was
measured by flow cytometry following staining with 500 μl of
DiOC6 (1 μmol/l, Invitrogen) for ΔΨm. Human
primary colorectal cancer cells were plated in the 12-well plate at
the density of 2×105 cells/well for 24 h then were
treated with or without DATS (20 μM) for 0, 6 and 12 h. Then cells
from each well were collected and were stained with 500 μl of
DiOC6 (1 μmol/l, Invitrogen) for ΔΨm for 30
min at 37°C, and the fluorescence intensity in cells was determined
using the flow cytometer (Becton-Dickinson) (24,27).
Assays of caspase-9 and caspase-3
activity
Human primary colorectal cancer cells at the density
of 2×105 cells/well in 10-cm culture dish were treated
with 20 μM DATS and incubated for 0 and 24 h. The activities of
caspase-9 and caspase-3 were assessed according to the
manufacturer's instruction of caspase colorimetric kit (R&D
Systems Inc.). At the end of incubation, cells in each well were
harvested and lysed in 50 μl lysis buffer containing 2 mM DTT for
10 min then centrifuged, the supernatant containing 200 μg protein
were incubated with caspase-9 and caspase-3 substrate (Ac-DEVD-pNA
and Ac-IETD-pNA, respectively) in reaction buffer. Then all samples
from DATA-treated and -untreated cells were incubated in 96-well
flat bottom microplate at 37°C for 1 h. The level of released pNA
was measured with ELISA reader (Anthos Reader 2001, Anthos Labtec)
at 405 nm wavelength as previously described (28,29).
Western blot analysis
Human primary colorectal cancer cells at a density
of 1×107 cells in 75 T flasks were incubated with 20 μM
DATS for 0 and 24 h for examining the protein levels correlated
with apoptosis. At the end of incubation, cells from each treatment
were collected, and the total protein lysate was isolated, gel
electrophoresis and immunoblotting were conducted as previously
described (24,26,27).
The primary antibodies were anti-cytochrome c, anti-Apaf-1,
anti-caspase-9 and anti-caspase-3, anti-Bcl-2 and anti-Bax.
Immunoreactive proteins of all examined samples were visualized
with the ECL chemiluminescent detection system (Perkin-Elmer Life
Science, MA, USA) and BioMax Light Film (Eastman Kodak, New Heaven,
CT, USA) according to the manufacturer's instructions.
Statistical analyses
Data are presented as the mean ± SD for the
indicated number of separate experiment. Statistical analyses of
data were performed by Student's t-test, and P<0.05 was
considered significant.
Results
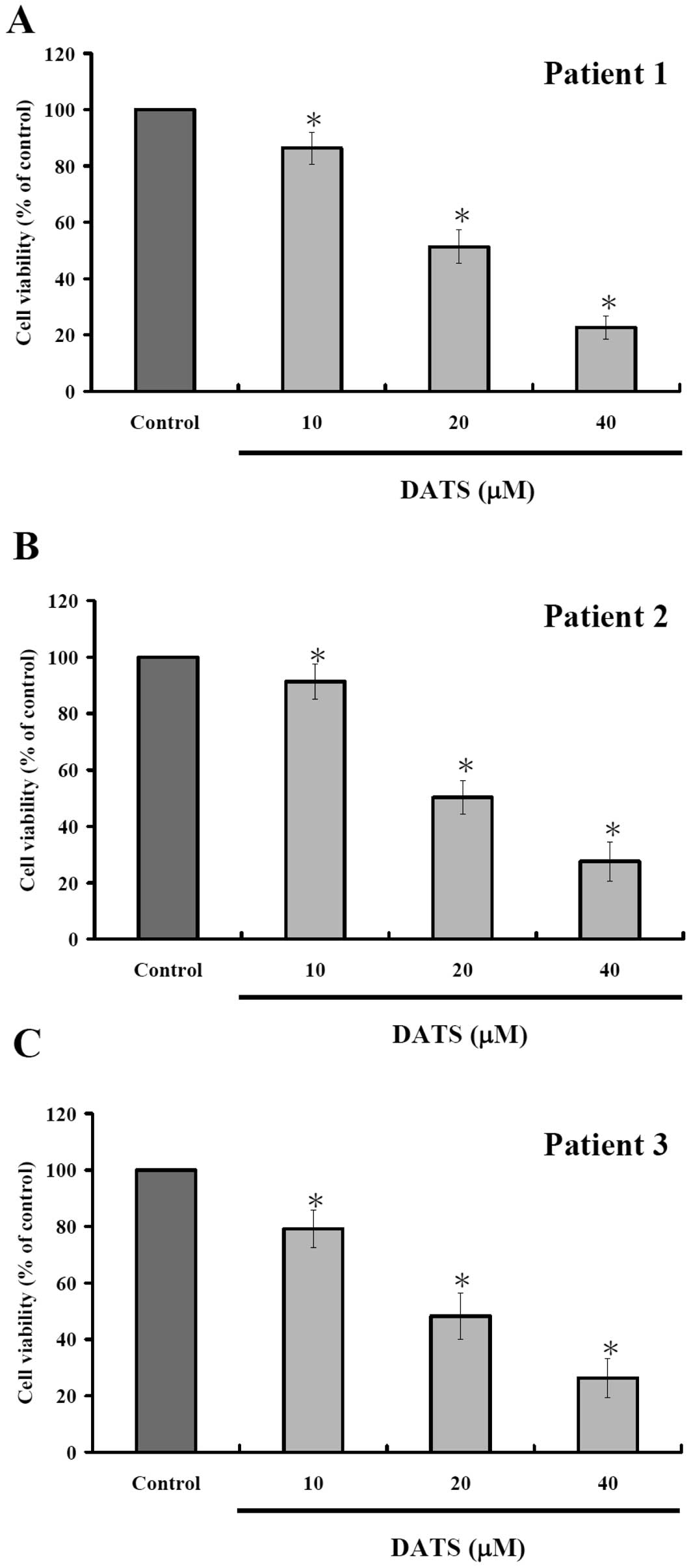
DATS decreases the percentage of
viability in human primary colorectal cancer cells
To measure DATS-mediated effects on human primary
colorectal cancer cells, the cells after incubated with 10, 20 and
40 μM DATS for 24 h were harvested and the percentage of viable
cells were measured by MTT assay. Results are shown in Fig. 1, indicating that increase of DATS
concentration led to decreased percentage of viable cells. As
expected, 24-h incubation showed apparent stronger dose-dependent
effects of DATS.
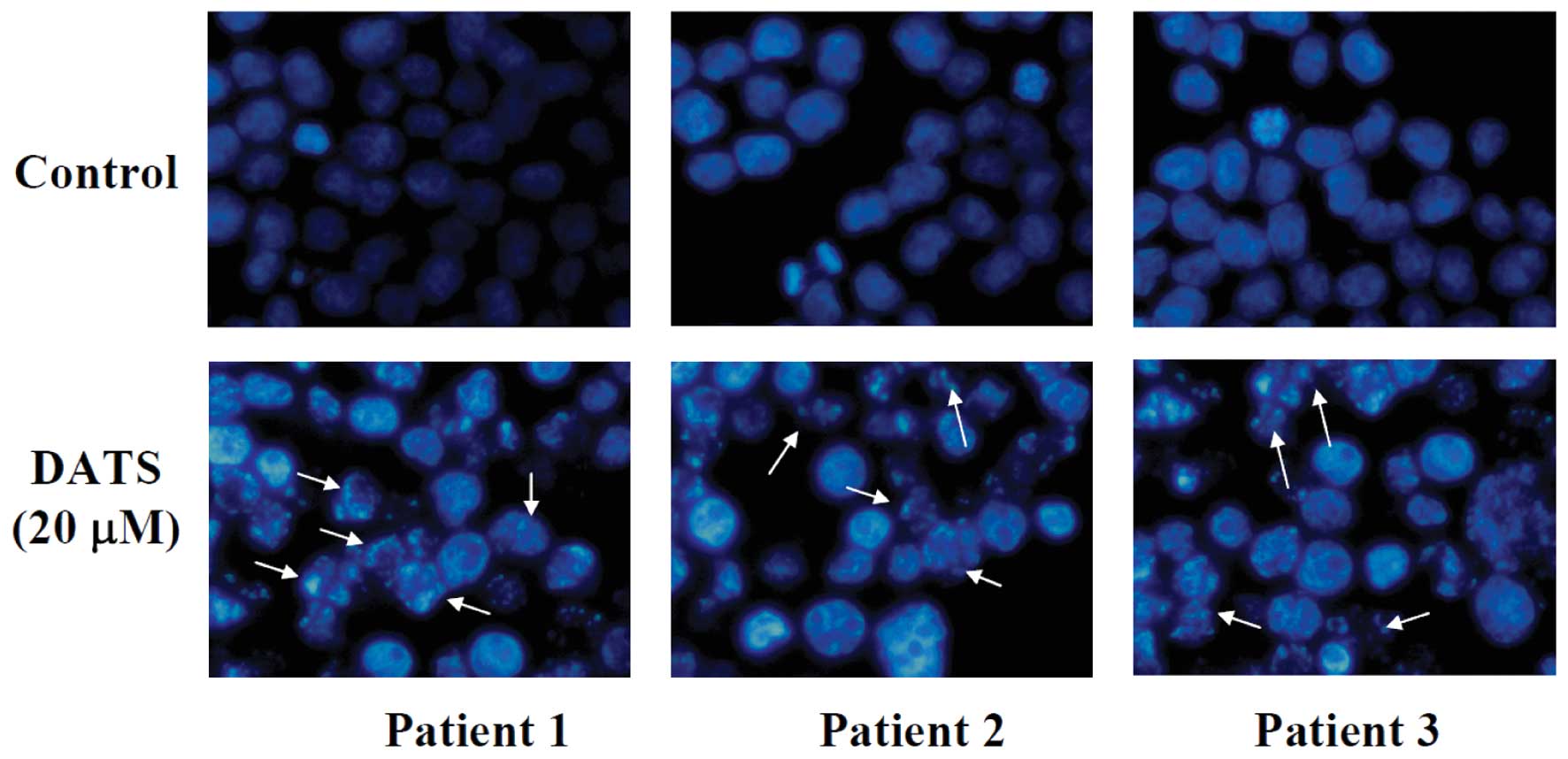
DATS induces apoptosis of human primary
colorectal cancer cells
To investigate the effect of DATS on nuclear
alterations, cells were stained with DAPI and results are shown in
Fig. 2, demonstrating that the
cells underwent remarkable nuclear changes upon treatment. In the
control (untreated) cells, the nuclei were intact, round, and
uniformly stained. However, after exposure to DATS, the cells
manifested nuclear shrinkage/condensation and nuclear
fragmentation. At 20 μM of DATS, a number of cells exhibited
nuclear shrinkage and chromatin condensation but these aberrant
nuclear alterations were not seen in the control cells. These
observations showed that DATS-induced apoptosis occurred in primary
colorectal cancer cells.
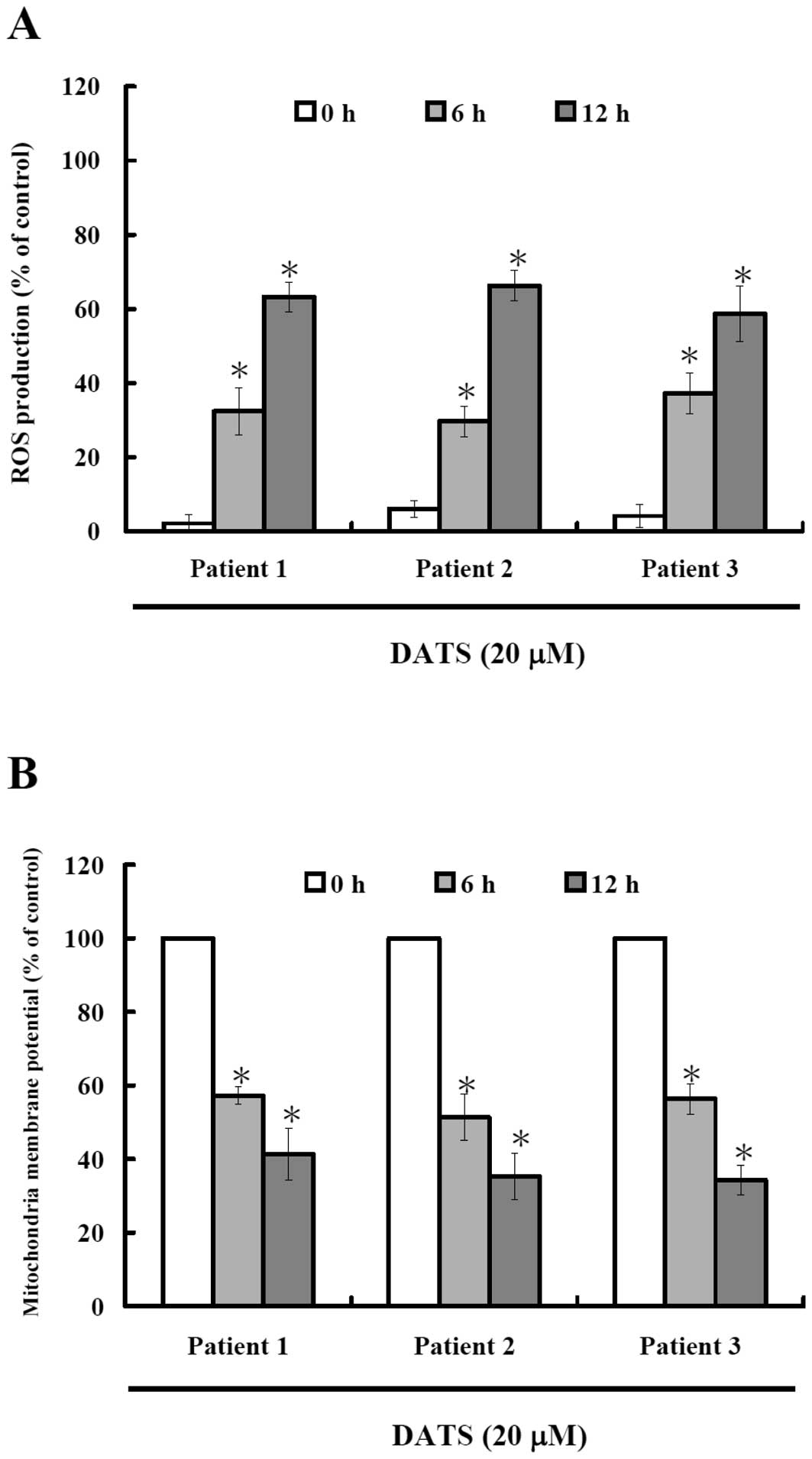
DATS induces reactive oxygen species
(ROS) production and decreases the level of mitochondrial membrane
potential (ΔΨm) in human primary colorectal cancer
cells
To investigate whether or not DATS-induced apoptosis
is via the production of ROS, we measured the intracellular
level of ROS during treatment with DATS by DCFH-DA and using a flow
cytometer and results are shown in Fig.
3A. Results indicate that DATS induced ROS production in a
time-dependent manner. The oxidation of DCF was dependent upon DATS
treatment time (Fig. 3A). To
investigate whether or not DATS-induced apoptosis is through
decreasing the levels of mitochondrial membrane potential, cells
were collected and stained with DiOC6 and results are
shown in Fig. 3B, indicating that
DATS decreased the levels of ΔΨm in human primary
colorectal cancer cells and these effects are time-dependent.
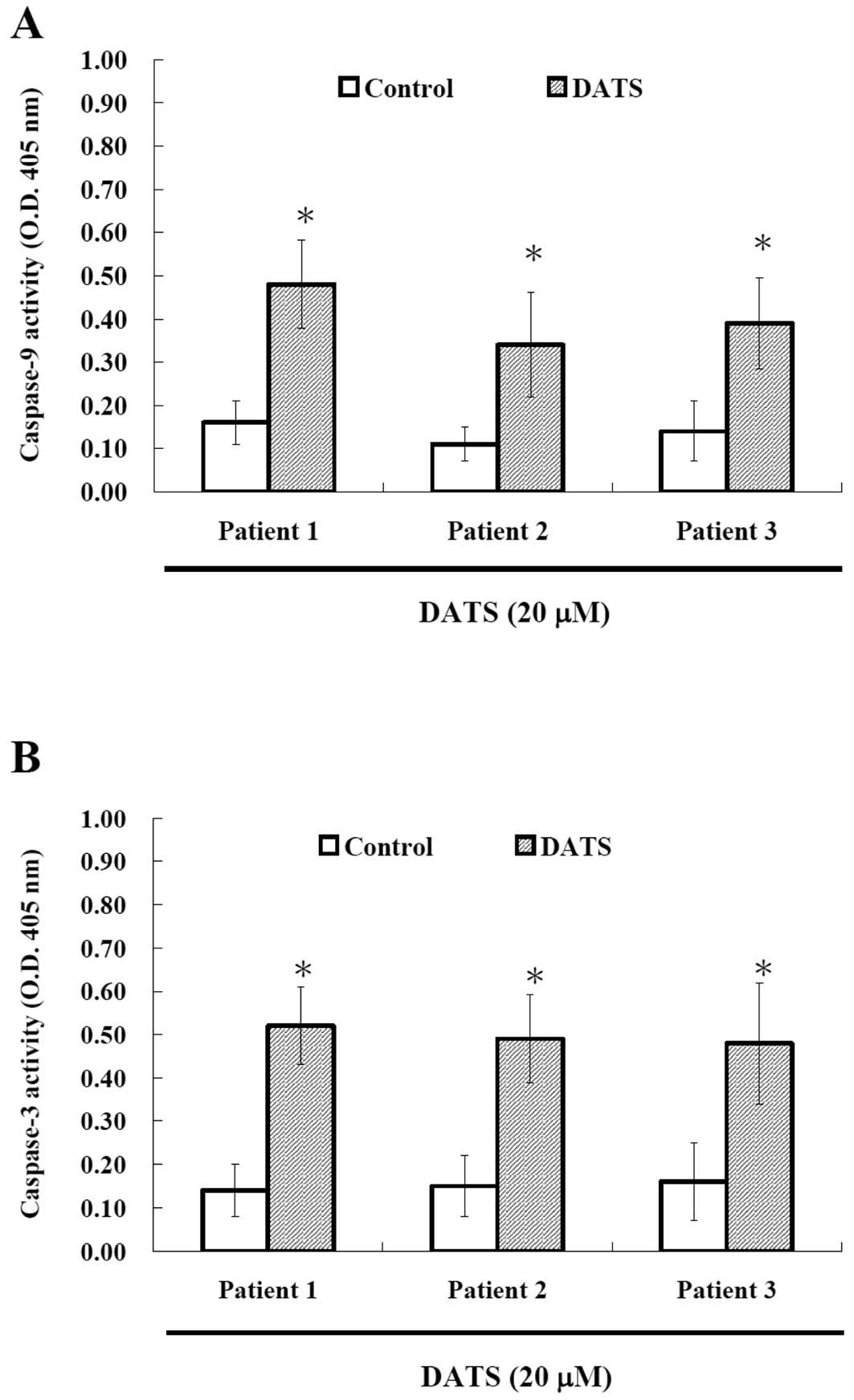
DATS affects caspase-9 and -3 activities
in human primary colorectal cancer cells
To investigate whether or not DATS-induced apoptosis
is via the activation of caspases, the cells after treatment
with DATS were harvested and caspase-9 and -3 activities were
measured and results are shown in Fig.
4. Results indicated that DATS promoted the activation of
caspase-9 and caspase-3 in the examined cancer cells. Based on this
observation, DATS induced apoptosis was via activation of caspase-9
and caspase-3.
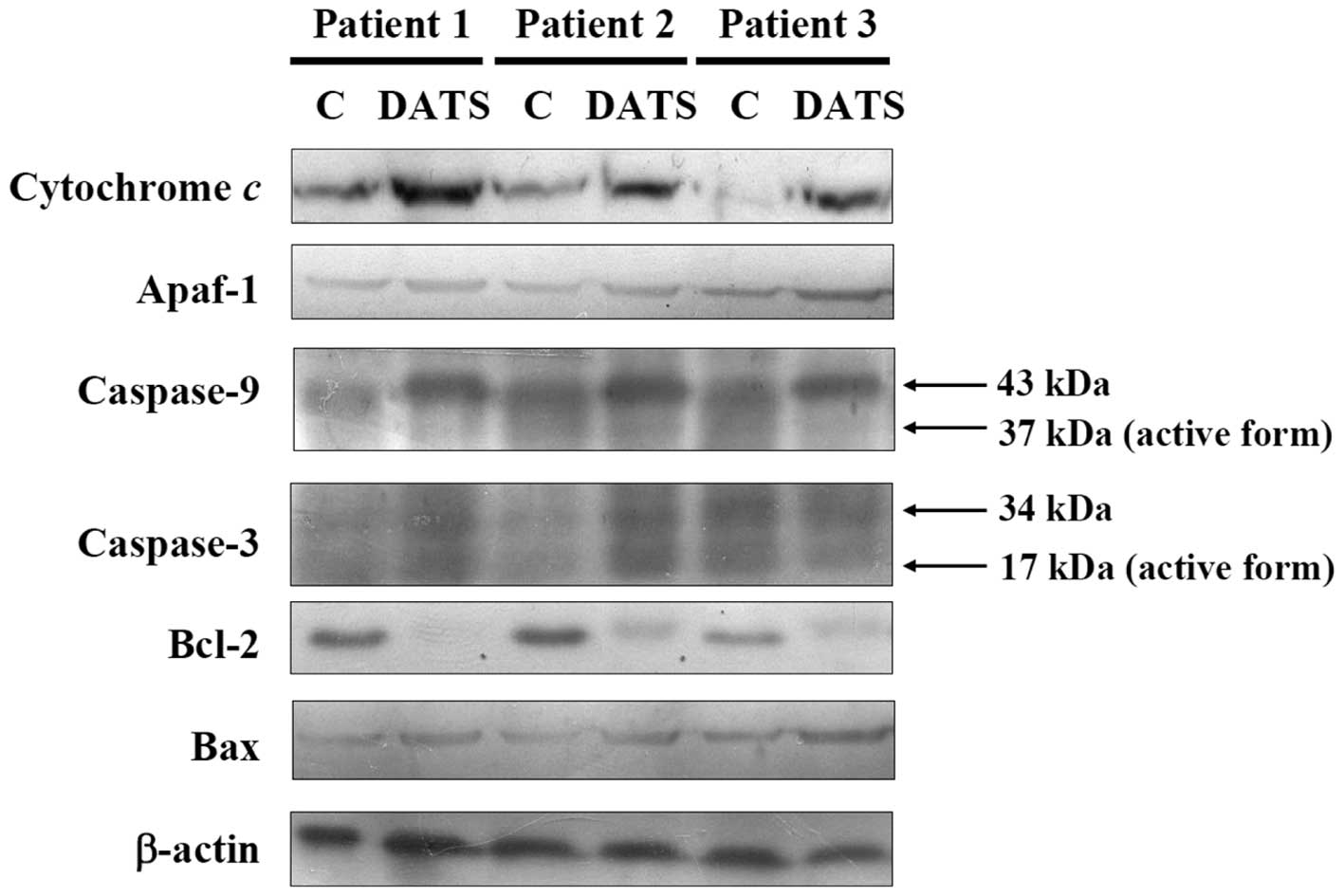
DATS affects apoptosis-associated
proteins in human primary colorectal cancer cells
For further examining the effects of DATS-induced
apoptosis in the human primary colorectal cancer cells through
apoptosis-associated protein levels, the cells after exposure to 20
μM DATS for 24 h were harvested for western blotting and result are
shown in Fig. 5. DATS increased the
protein levels of cytochrome c, caspase-9 and caspase-3, showing
that DATS promoted the release of cytochrome c from mitochondria,
and then led to the activation of caspase-9 and caspase-3.
Furthermore, DATS promoted the pro-apoptotic protein Bax and
inhibited the anti-apoptotic protein, leading to apoptosis in the
three examined human colorectal cancer cell types.
Discussion
Numerous studies have shown that DATS induces
cytotoxic effects in many human cancer cells through cell cycle
arrest and induction of apoptosis. However, there is no information
to show DATS induced apoptosis in human primary colorectal cancer
cells. Thus, the major objective of the present study was to test
anticancer responses to DATS on human primary colorectal cancer
cells. DATS is a well documented highly promising
cancer-chemopreventive constituent of processed garlic. Recently it
was reported that DATS treatment suppresses STAT3 phosphorylation
in prostate cancer cells in culture and in vivo, but
activation of this oncogenic transcription factor is largely
dispensable for cellular responses to DATS (30).
The present study revealed that DATS treatment
decreased the percentage of viable cells (Fig. 1), induced apoptosis based on DAPI
staining (Fig. 2), promoted the ROS
generation and decrease the levels of ΔΨm (Fig. 3) and promoted the activities of
caspase-9 and -3 (Fig. 4) that were
analyzed by flow cytometric assay. To clarify the underlying
mechanism for DATS-induced apoptosis, we used western blotting to
confirm that DATS promoted the release of cytochrome c from
mitochondria and promoted the activation of caspase-9 and -3,
further, DATS increased the pro-apoptotic protein Bax and inhibited
the anti-apoptotic protein Bcl-2 leading to apoptosis (Fig. 5).
Fig. 3 indicates
that DATS induced ROS generation in the 3 isolated cancer cell
types and these effects are time-dependent. This is in agreement
with other recent reports indicating that DATS can be reduced in
cancer cells to hydroperthiol that leads to
H2O2 generation, thereby influencing
transmission of signals regulating cell proliferation and apoptosis
(31). Other reports also show that
the cytotoxicity caused by DATS is mediated by the generation of
ROS and subsequent activation of the ASK1-JNK-Bim signal
transduction pathway in human breast carcinoma MDA-MB-231 cells
(32). Reports also exist showing
that in vivo healthy mice injected intraperitoneally with
allyl sulfides for ten days. The experiment revealed that DATS as
well as DADS diminished lipid peroxidation and ROS level in normal
mouse liver (31). Thus, it is
possible that DATS-induced elevation of the intracellular level of
ROS is due to disruption of mitochondrial electron transport chain
activity, as Fig. 3 clearly
demonstrates that DATS decreased the levels of ΔΨm. This
needs to be determined in future studies. It is well known that the
ratio of Bax/Bcl-2 is involved at the level of ΔΨm in
mitochondria (10). Our results
from western blotting clearly showed that DATS increased the
pro-apoptotic protein Bax and decreased anti-apoptotic protein
Bcl-2 (Fig. 5). Thus, DATS affects
the levels of ΔΨm in mitochondria via the changes in the
ratio of Bax/Bcl-2. DATS has also clearly been demonstrated to
promote caspase-3 activation (31).
Our data showed that DATS promoted the activities of caspase-9 and
caspase-3 (Fig. 4), and also
confirmed by western blotting (Fig.
5). It is well documented that apoptosis can be divided into
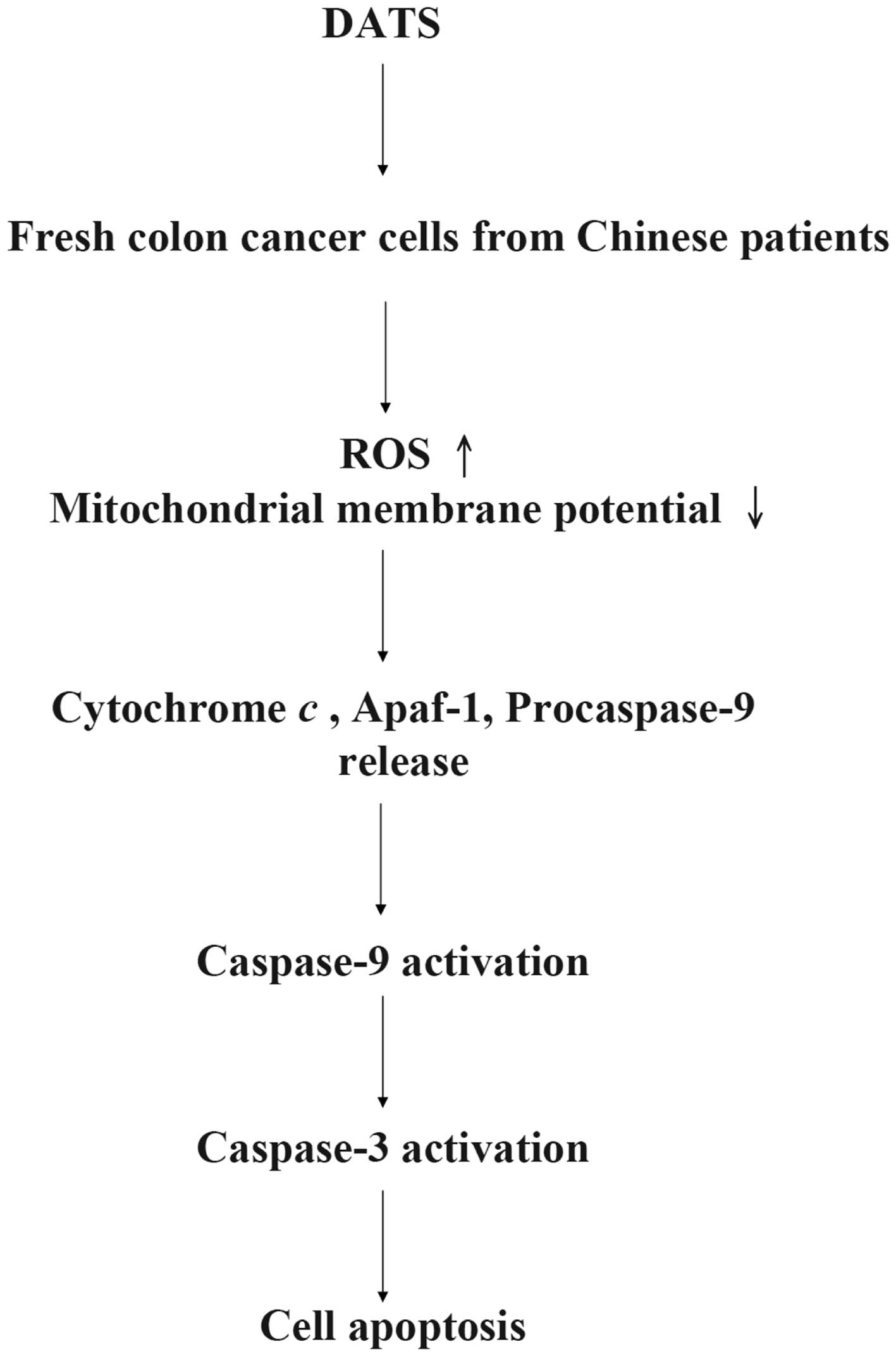
caspase-dependent and caspase-independent pathways (33), based on our results we suggest that
DATS induces apoptosis through the caspase-dependent pathway
(Fig. 6).
In summary, the present study shows the cytotoxic
effects of DATS via ROS generation, dysfunction of mitochondria
(decreased the levels of ΔΨm in mitochondria) due to the
increase in the ratio of Bax/Bcl-2, promoting the activation of
caspase-9 and -3 leading to apoptosis in human primary colorectal
cancer cells.
Acknowledgements
This work was supported by the grant NSC
95-2320-B-039-030-MY2 from National Science Council, Republic of
China (Taiwan) and by the grant CMU97-127 from China Medical
University, Taichung, Taiwan, R.O.C.
References
|
1
|
Jaramillo S, Lopez S, Varela LM, et al:
The flavonol isorhamnetin exhibits cytotoxic effects on human colon
cancer cells. J Agric Food Chem. Oct 5–2010.(Epub ahead of
print).
|
|
2
|
Nautiyal J, Banerjee S, Kanwar SS, et al:
Curcumin enhances dasatinib-induced inhibition of growth and
transformation of colon cancer cells. Int J Cancer. 128:951–961.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Duijnhoven FJ, Bueno-De-Mesquita HB,
Ferrari P, et al: Fruit, vegetables, and colorectal cancer risk:
the European Prospective Investigation into Cancer and Nutrition.
Am J Clin Nutr. 89:1441–1452. 2009.
|
|
4
|
Davis CD and Milner JA: Gastrointestinal
microflora, food components and colon cancer prevention. J Nutr
Biochem. 20:743–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fadeel B and Orrenius S: Apoptosis: a
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huttemann M, Pecina P, Rainbolt M, et al:
The multiple functions of cytochrome c and their regulation in life
and death decisions of the mammalian cell: from respiration to
apoptosis. Mitochondrion. 11:369–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kao ST, Yeh CC, Hsieh CC, et al: The
Chinese medicine Bu-Zhong-Yi-Qi-Tang inhibited proliferation of
hepatoma cell lines by inducing apoptosis via G0/G1 arrest. Life
Sci. 69:1485–1496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antony ML and Singh SV: Molecular
mechanisms and targets of cancer chemoprevention by garlic-derived
bioactive compound diallyl trisulfide. Indian J Exp Biol.
49:805–816. 2011.PubMed/NCBI
|
|
12
|
Seki T, Hosono T, Hosono-Fukao T, et al:
Anticancer effects of diallyl trisulfide derived from garlic. Asia
Pac J Clin Nutr. 17(Suppl 1): 249–252. 2008.PubMed/NCBI
|
|
13
|
Li N, Guo R, Li W, et al: A proteomic
investigation into a human gastric cancer cell line BGC823 treated
with diallyl trisulfide. Carcinogenesis. 27:1222–1231. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakamoto K, Lawson LD and Milner JA: Allyl
sulfides from garlic suppress the in vitro proliferation of human
A549 lung tumor cells. Nutr Cancer. 29:152–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hosono T, Fukao T, Ogihara J, et al:
Diallyl trisulfide suppresses the proliferation and induces
apoptosis of human colon cancer cells through oxidative
modification of beta-tubulin. J Biol Chem. 280:41487–41493. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pinto JT and Rivlin RS: Antiproliferative
effects of allium derivatives from garlic. J Nutr. 131:S1058–S1060.
2001.PubMed/NCBI
|
|
17
|
Chun HS, Kim HJ and Choi EH: Modulation of
cytochrome P4501-mediated bioactivation of benzo[a]pyrene by
volatile allyl sulfides in human hepatoma cells. Biosci Biotechnol
Biochem. 65:2205–2212. 2001.PubMed/NCBI
|
|
18
|
Antosiewicz J, Herman-Antosiewicz A,
Marynowski SW and Singh SV: c-Jun NH(2)-terminal kinase signaling
axis regulates diallyl trisulfide-induced generation of reactive
oxygen species and cell cycle arrest in human prostate cancer
cells. Cancer Res. 66:5379–5386. 2006. View Article : Google Scholar
|
|
19
|
Xiao D, Zeng Y, Hahm ER, Kim YA,
Ramalingam S and Singh SV: Diallyl trisulfide selectively causes
Bax- and Bak-mediated apoptosis in human lung cancer cells. Environ
Mol Mutagen. 50:201–212. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao D, Choi S, Johnson DE, et al: Diallyl
trisulfide-induced apoptosis in human prostate cancer cells
involves c-Jun N-terminal kinase and extracellular-signal regulated
kinase-mediated phosphorylation of Bcl-2. Oncogene. 23:5594–5606.
2004. View Article : Google Scholar
|
|
21
|
Ji C, Ren F and Xu M: Caspase-8 and
p38MAPK in DATS-induced apoptosis of human CNE2 cells. Braz J Med
Biol Res. 43:821–827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai KC, Hsu SC, Kuo CL, et al: Diallyl
sulfide, diallyl disulfide, and diallyl trisulfide inhibit
migration and invasion in human colon cancer colo 205 cells through
the inhibition of matrix metalloproteinase-2, -7, and -9
expressions. Environ Toxicol. Jun 21–2011.(Epub ahead of
print).
|
|
23
|
Wu PP, Liu KC, Huang WW, et al: Diallyl
trisulfide (DATS) inhibits mouse colon tumor in mouse CT-26 cells
allograft model in vivo. Phytomedicine. 18:672–676. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai KC, Chiu YJ, Tang YJ, et al:
Houttuynia cordata Thunb extract inhibits cell growth and induces
apoptosis in human primary colorectal cancer cells. Anticancer Res.
30:3549–3556. 2010.PubMed/NCBI
|
|
25
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
27
|
Ho YT, Lu CC, Yang JS, et al: Berberine
induced apoptosis via promoting the expression of caspase-8, -9 and
-3, apoptosis-inducing factor and endonuclease G in SCC-4 human
tongue squamous carcinoma cancer cells. Anticancer Res.
29:4063–4070. 2009.
|
|
28
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar
|
|
29
|
Ying WZ and Sanders PW: Cytochrome c
mediates apoptosis in hypertensive nephrosclerosis in Dahl/Rapp
rats. Kidney Int. 59:662–672. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chandra-Kuntal K and Singh SV: Diallyl
trisulfide inhibits activation of signal transducer and activator
of transcription 3 in prostate cancer cells in culture and in vivo.
Cancer Prev Res (Phila). 3:1473–1483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iciek M, Kwiecien I, Chwatko G,
Sokolowska-Jezewicz M, Kowalczyk-Pachel D and Rokita H: The effects
of garlic-derived sulfur compounds on cell proliferation, caspase 3
activity, thiol levels and anaerobic sulfur metabolism in human
hepatoblastoma HepG2 cells. Cell Biochem Funct. 30:198–204. 2011.
View Article : Google Scholar
|
|
32
|
Lee BC, Park BH, Kim SY and Lee YJ: Role
of Bim in diallyl trisulfide-induced cytotoxicity in human cancer
cells. J Cell Biochem. 112:118–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao D and Singh SV: Diallyl trisulfide, a
constituent of processed garlic, inactivates Akt to trigger
mitochondrial translocation of BAD and caspase-mediated apoptosis
in human prostate cancer cells. Carcinogenesis. 27:533–540. 2006.
View Article : Google Scholar
|