Introduction
A number of epidemiologic studies have demonstrated
an association between non-Hodgkin’s lymphoma (NHL) and hepatitis C
virus (HCV) infection, suggesting that HCV plays a role in the
development of NHL (1–8). Low-grade marginal zone lymphoma has
been the lymphoma subtype most commonly associated with
HCV-infection, while limited data are available regarding
HCV-positive patients with diffuse large B-cell lymphoma (DLBCL)
(9). Clinicopathological
characteristics at presentation, tolerance to chemotherapy, natural
history and clinical outcome of patients with HCV-positive DLBCL
are still unclear. This is due to the heterogeneity in histology
and treatment strategies for DLBCL with HCV infection and a lack of
data based on large series of unselected patients (2). Previous studies involving lymphoma
patients with HCV infection have shown good tolerance to standard
chemotherapy (i.e., cyclophosphamide, doxorubicin, vincristine and
prednisone (CHOP) regimens) (10,11).
However, these studies were mainly conducted before the use of
rituximab in DLBCL patients. On the other hand, there are several
reports that DLBCL patients with HCV infection may exhibit a
characteristic clinical presentation, poor tolerance to intensive
chemotherapy and poorer survival (12,13).
Rituximab, a human/mouse chimeric monoclonal
antibody that reacts specifically with the CD20 antigen, was
approved for use in DLBCL patients in 2002 in Japan. Since its
introduction, rituximab has been widely used for the treatment of
DLBCL regardless of patient age (2,14). In
general, its associated toxicity is usually mild and limited to the
infusion period (14). In DLBCL
patients with HCV infection, rituximab has been reported to
increase alanine aminotransferase (ALT) levels and HCV viral load
(15). However, the prognostic
value of HCV infection in rituximab combination chemotherapy has
not been well established. To our knowledge there have been few
reports describing the clinical outcome in DLBCL patients with HCV
infection who received rituximab containing immunochemotherapy,
although hepatitis B virus (HBV) reactivation is a well-documented
complication that often develops after performing rituximab
containing immunochemotherapy in DLBCL patients, and has proved
fatal in some cases (9,16–19).
The aim of the present study was to compare clinical
outcome, treatment response and hepatotoxicity in patients with
DLBCL who received rituximab containing immunochemotherapy that had
HCV infection and those that did not have HCV infection.
Patients and methods
Patients
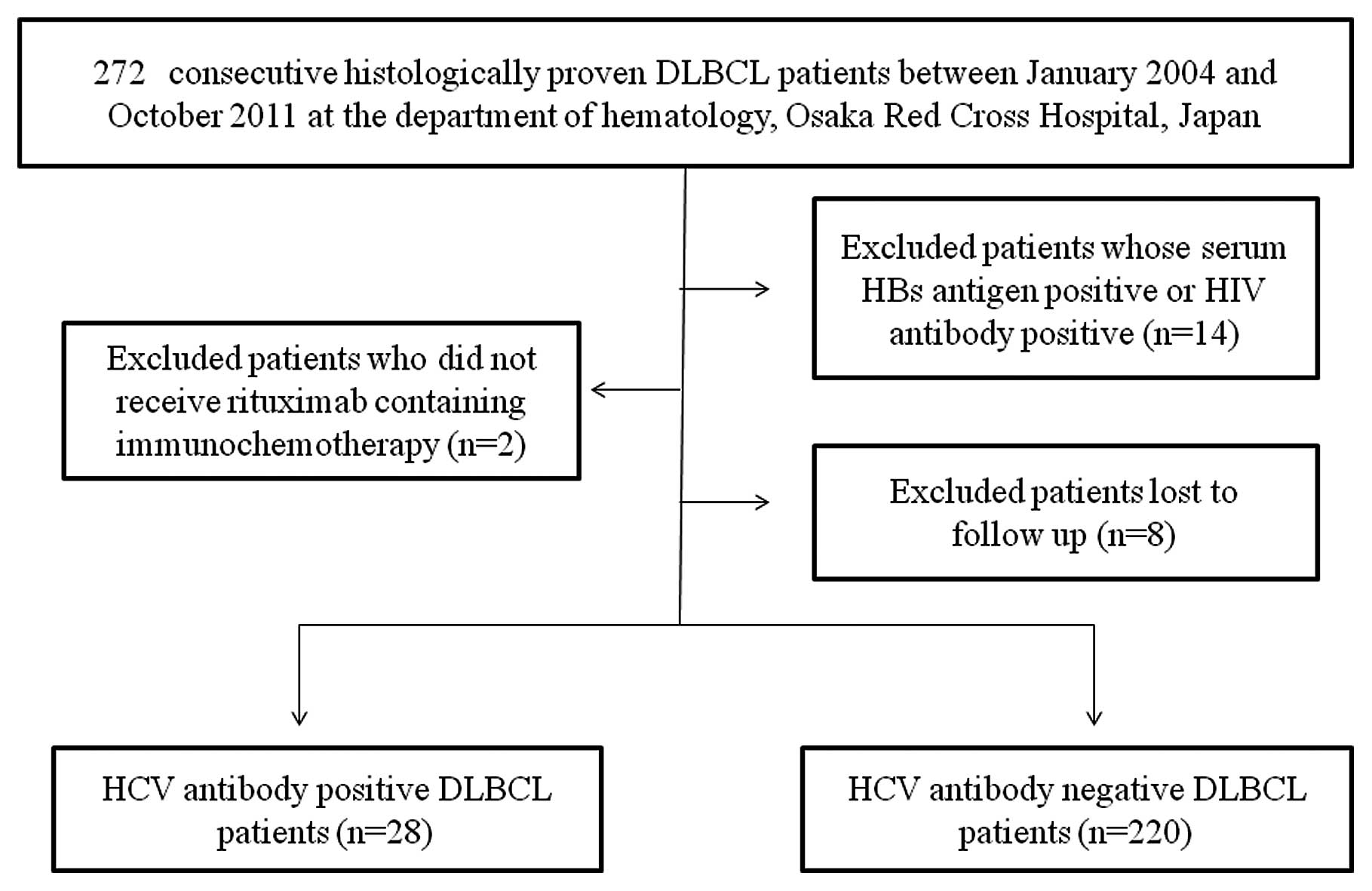
The subjects consisted of 272 consecutive
histopathologically proven DLBCL patients admitted to the
Department of Hematology, Osaka Red Cross Hospital, Japan between
January 2004 and October 2011. DLBCL was diagnosed by an expert
hematopathologist in our hospital, based on the World Health
Organization classification (20).
Of these 272 patients, 2 patients who did not undergo rituximab
containing immunochemotherapy, one patient whose serum human
immunodeficiency virus (HIV) antibody was positive, 13 patients
whose serum hepatitis B antigen was positive, and 8 patients who
had been lost to follow-up were excluded from the present study
(Fig. 1). A total of 248 DLBCL
patients were analyzed. All of them had undergone rituximab
containing immunochemotherapy and had no malignancies other than
DLBCL at the time of DLBCL diagnosis. There were 28 DLBCL patients
with HCV infection (the HCV group) and 220 DLBCL patients without
HCV infection (the control group) in the present study. HCV
infection was defined as the detection of anti-HCV antibodies with
commercially available second- or third-generation immunoassay kits
(Monolisa anti-HCV Plus, Sanofi Diagnostics Pasteur; and Axsym HCV
Version 3.0, Abbott Laboratories). Of the 28 DLBCL patients with
HCV infection, none received interferon (IFN) therapy for hepatitis
C during the follow-up period. Before performing rituximab
containing immunochemotherapy, written informed consent was
obtained from all patients. This retrospective study protocol
complied with all of the provisions of the Declaration of
Helsinki.
Virological study
All patients analyzed in the present study were
tested for the presence of serum antibodies against HCV, before the
initiation of immunochemotherapy for DLBCL. HCV genotype was
determined using the polymerase chain reaction (PCR) amplification
of the core region of the HCV genome by means of genotype-specific
PCR primers (21). Serum HCV-RNA
levels were quantified using the COBAS Amplicor HCV Monitor test,
version 2.0 (detection range 6–5000 KIU/ml: Roche Diagnostics,
Branchburg, NJ, USA). Serum HBV antigen, as well as HIV antibodies,
were also tested on all patients analyzed in the present study
using commercial enzyme immunoassays at the time of lymphoma
diagnosis.
Clinical staging
All records registered in our database were
retrospectively reviewed to verify clinical outcome, clinical
staging, presentation and treatment. Clinical staging evaluation
included routine laboratory tests, physical examination, bone
marrow aspirate and biopsy, chest radiographs and computed
tomography (CT) of the chest and whole abdomen. CT of the neck, an
abdominal ultrasonographic study, esophagogastroduodenoscopy,
colonoscopy, spleen biopsy and liver biopsy were performed when
clinically indicated. All patients were classified according to the
Ann Arbor staging system (22).
Treatment and response
In the present study, R-CHOP therapy (rituximab,
cyclophosphamide, doxorubicin, vincristine and prednisone) had been
performed in patients aged <65 years. R-THP-COP therapy
(rituximab, therarubicin, cyclophosphamide, vincristine and
prednisone) had been performed in patients aged ≥65 years. The
initial chemotherapy dose was determined mainly based on the
decisions of the attending physicians, considering factors such as
laboratory data, general condition and underlying diseases.
Sensitivity to chemotherapy was evaluated in each patient using CT
and positron emission tomography (PET)/CT with
18F-fluorodeoxyglucose imaging (23). Complete remission (CR) was defined
as the absence of disease for ≥1 month after the end of
immunochemotherapy. Partial response (PR) was defined as >50%
reduction in tumor area measurable in two dimensions. Progressive
disease (PD) was defined as enlargement of disease or the
development of disease in a previously involved site. Relapse was
defined as the occurrence of disease progression ≤1 month after CR
or PR.
Liver function tests and assessment of
liver toxicity
In all patients analyzed in the present study,
pretreatment levels of ALT and its highest levels during
immunochemotherapy were collected for analysis. Definition and
grading of hepatic toxicity relied on the standard National Cancer
Institute-World Health Organization (NCI-WHO) common toxicity
criteria grading scale. In patients with normal ALT at baseline,
significant liver toxicity was defined as a WHO toxicity of grade
≥2 (≥2.5xULN), and in patients with abnormal ALT values at
baseline, significant liver toxicity was defined as ≥3.5 times
elevation of ALT relative to the baseline value (24). Liver function tests were monitored
carefully before and during immunochemotherapy, as well as during
the follow-up period.
Follow-up
All patients were followed up every 3 months with
laboratory tests, CT scans and/or PET/CT after the completion of
immunochemotherapy. When lymphoma relapse was detected using
imaging modalities and/or laboratory tests, additional chemotherapy
was performed after histopathological examination.
Statistical analysis
The primary end-point was OS and the secondary
end-point was PFS. Differences between the two groups were analyzed
using the unpaired t-test for continuous variables, and the
categorical variables were analyzed using the χ2 test or
continuity correction method. The overall survival (OS) curves and
the progression-free survival (PFS) curves were generated using the
Kaplan-Meier method and compared using the log-rank test. OS was
calculated from the treatment initiation date until death from any
cause or the last follow-up. PFS was calculated from the treatment
initiation date to the date of documented disease progression,
relapse or the end date of the study. All statistical tests were
two-sided. All data were analyzed using SPSS software, version 9.0
(SPSS Inc., Chicago, IL, USA) for Microsoft Windows. Data are
expressed as means ± standard deviation. P<0.05 was considered
statistically significant.
Results
Baseline characteristics
The baseline characteristics of the patients
enrolled in the present study are shown in Table I. There were significant differences
in age (P=0.030), platelet count (P=0.001) and white blood cell
count (P=0.017) between the HCV group and the control group. The
proportion of patients that had a high and high-intermediate
International prognostic index (IPI) (25) was almost the same between the two
groups (P=1.000). There were 157 out of the 248 (63.3%) DLBCL
patients with primary extranodal disease. The stomach was the most
frequently involved extranodal organ (44 out of 248, 17.7%),
followed by the small intestine (19 out of 248, 7.7%) and the
spleen (16 out of 248, 6.5%).
 | Table IBaseline characteristics between the
HCV group and the control group. |
Table I
Baseline characteristics between the
HCV group and the control group.
| HCV group (n=28) | Control group
(n=220) | P-value |
|---|
| Age (years) | 72.1±7.8 | 66.5±13.2 | 0.030a |
| Gender
(male/female) | 15/13 | 113 / 107 | 0.844b |
| DLBCL stage |
| I | 8 | 39 | 0.900b |
| II | 11 | 79 | |
| III | 5 | 42 | |
| IV | 6 | 60 | |
| IPI (H or HI/L or
LI) | 12/16 | 97 / 123 | 1.000a |
| White blood cells
(cells/mm3) | 5603.6±2297.9 | 6900.3±2732.5 | 0.017a |
| Hemoglobin
(g/dl) | 12.6±1.8 | 12.2±2.2 | 0.363a |
| Platelets
(x104/mm3) | 16.8±7.3 | 23.0±9.0 | 0.001a |
| AST (IU/l) | 46.3±32.9 | 45.1±179.6 | 0.972a |
| ALT (IU/l) | 35.6±33.1 | 33.4±152.2 | 0.938a |
| Total bilirubin
(mg/dl) | 0.82±0.39 | 0.75±0.63 | 0.574a |
| Albumin (g/dl) | 3.84±0.42 | 3.84±0.63 | 0.995a |
| Prothrombin time
(%) | 92.5±10.2 | 96.0±17.3 | 0.308a |
| LDH (IU/l) | 350.5±283.2 | 478.3±949.1 | 0.480a |
| sIL2R (U/ml) | 2653.1±3974.5 | 3389.0±5448.1 | 0.490a |
| Creatinine
(mg/dl) | 0.90±0.34 | 0.94±0.57 | 0.653a |
| Body mass index
(kg/m2) | 22.4±2.1 | 21.8±3.4 | 0.393a |
| Diabetes mellitus
(yes/no) | 11/17 | 56/164 | 0.173a |
HCV genotype and viral load
In 18 patients tested, HCV genotype was detected in
genotype 1 in 17 patients and genotype 2 in 1 patient,
respectively. In 18 patients who were tested for HCV viral load, 17
patients had a high viral load (≥100 kIU/ml) and one patient had a
low viral load (<100 kIU/ml), according to Japanese
criteria.
Overall survival
The median follow-up period was 3.4 years (0.3–7.7
years) in the HCV group and 2.6 years (0.2–7.9 years) in the
control group. Seven patients (25.0%) in the HCV group died during
the follow-up period. The causes of death in the HCV group were HCC
(4 patients), progression of malignant lymphoma (2 patients) and
miscellaneous (1 patient). Thirty-five patients (15.9%) in the
control group died during the follow-up period. The causes of death
in the control group were progression of malignant lymphoma (33
patients), pancreatic cancer (1 patient) and lung cancer (1
patient).
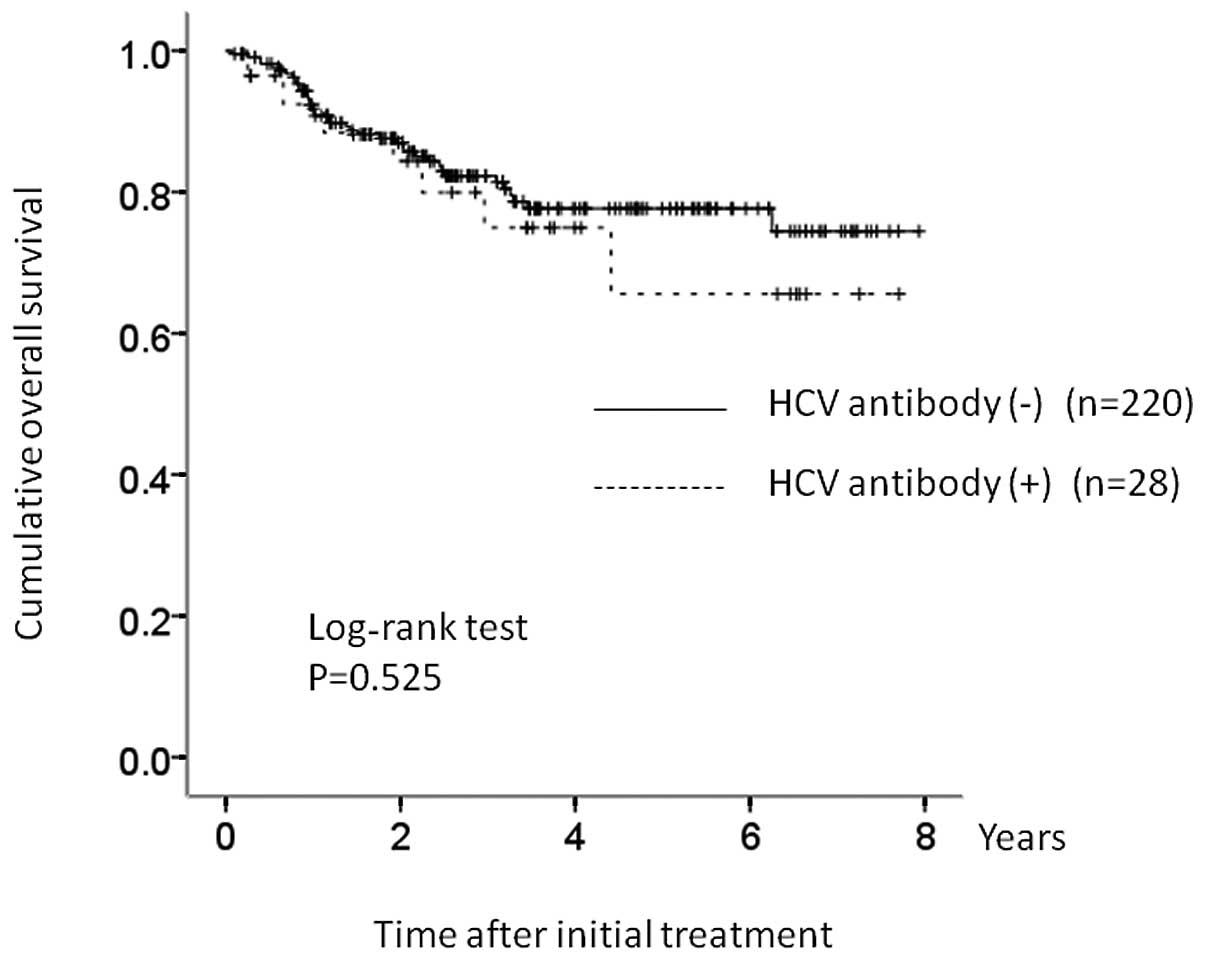
The 1-, 3- and 5-year OS rates were 90.2, 75.5 and
66.3%, respectively, in the HCV group and 90.3, 81.2 and 77.5%,
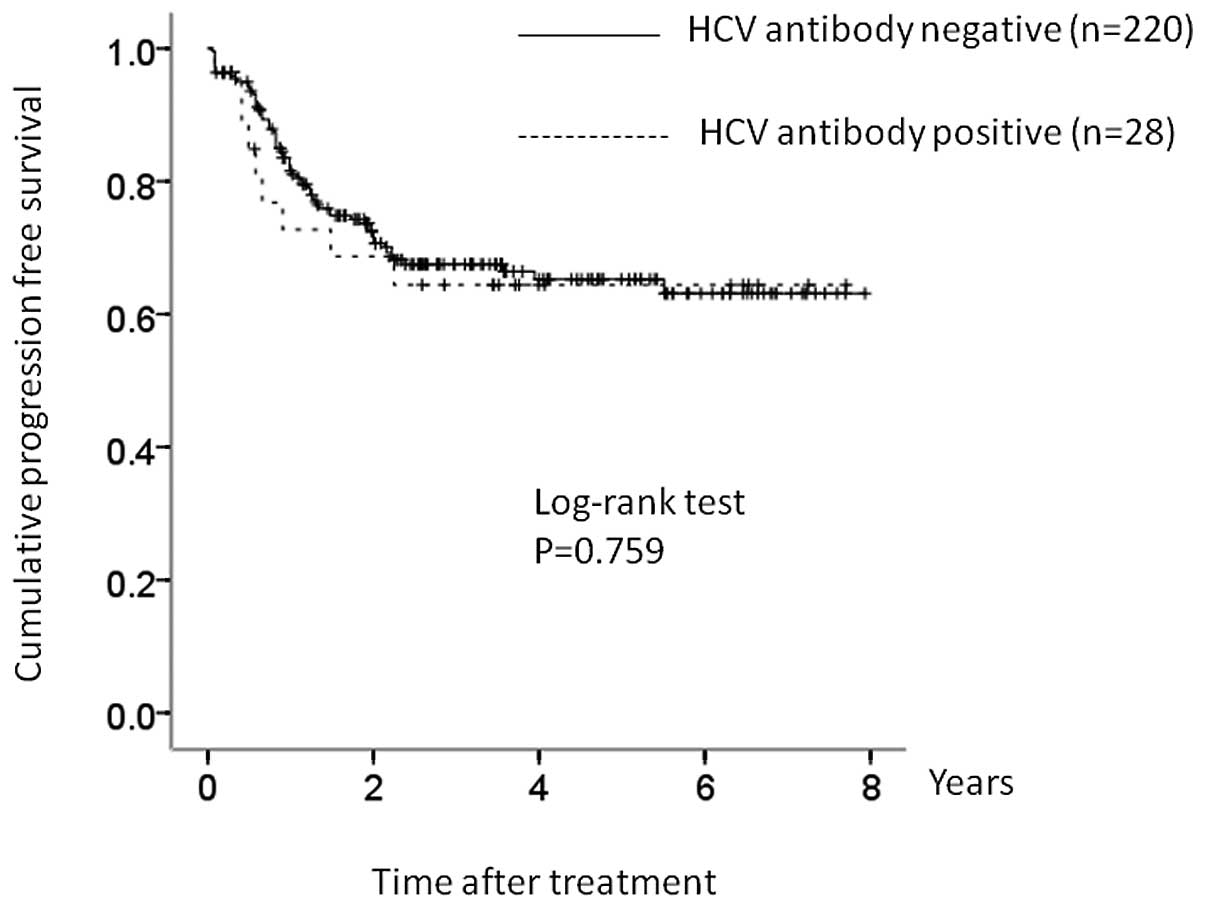
respectively, in the control group (Fig. 2). The corresponding PFS rates at 1,
3 and 5 years were 74.4, 63.8 and 63.8%, respectively, in the HCV
group and 80.7, 67.0 and 65.1%, respectively, in the control group
(Fig. 3). In terms of OS (P=0.525)
and PFS (P=0.759), there were no significant differences between
the two groups.
Treatment response
In the HCV group, a CR was obtained in 24 patients
(85.7%) and a PR was obtained in 2 patients (7.1%) after the
front-line therapy. The objective response rate (ORR) in the HCV
group was 92.9% (26/28). In the control group, a CR was obtained in
204 patients (92.7%) and a PR was obtained in 7 patients (3.2%)
after the front-line therapy. The ORR in the control group was
95.9% (211/220). In terms of ORR, there was no significant
difference between the two groups (P=0.619).
Liver dysfunction
As shown in Table I,
the pretreatment transaminase levels were not significantly
different between the two groups. In the HCV group, 7 patients
(25.0%) developed hepatotoxicity during immunochemotherapy. In the
HCV group, immunochemotherapy was not discontinued in any of the
patients owing to hepatotoxicity. In the control group, 35 patients
(15.9%) developed hepatotoxicity during chemotherapy, and none of
the patients had immunochemotherapy discontinued owing to
hepatotoxicity. In terms of hepatotoxicity, there was no
significant difference between the two groups (P=0.281).
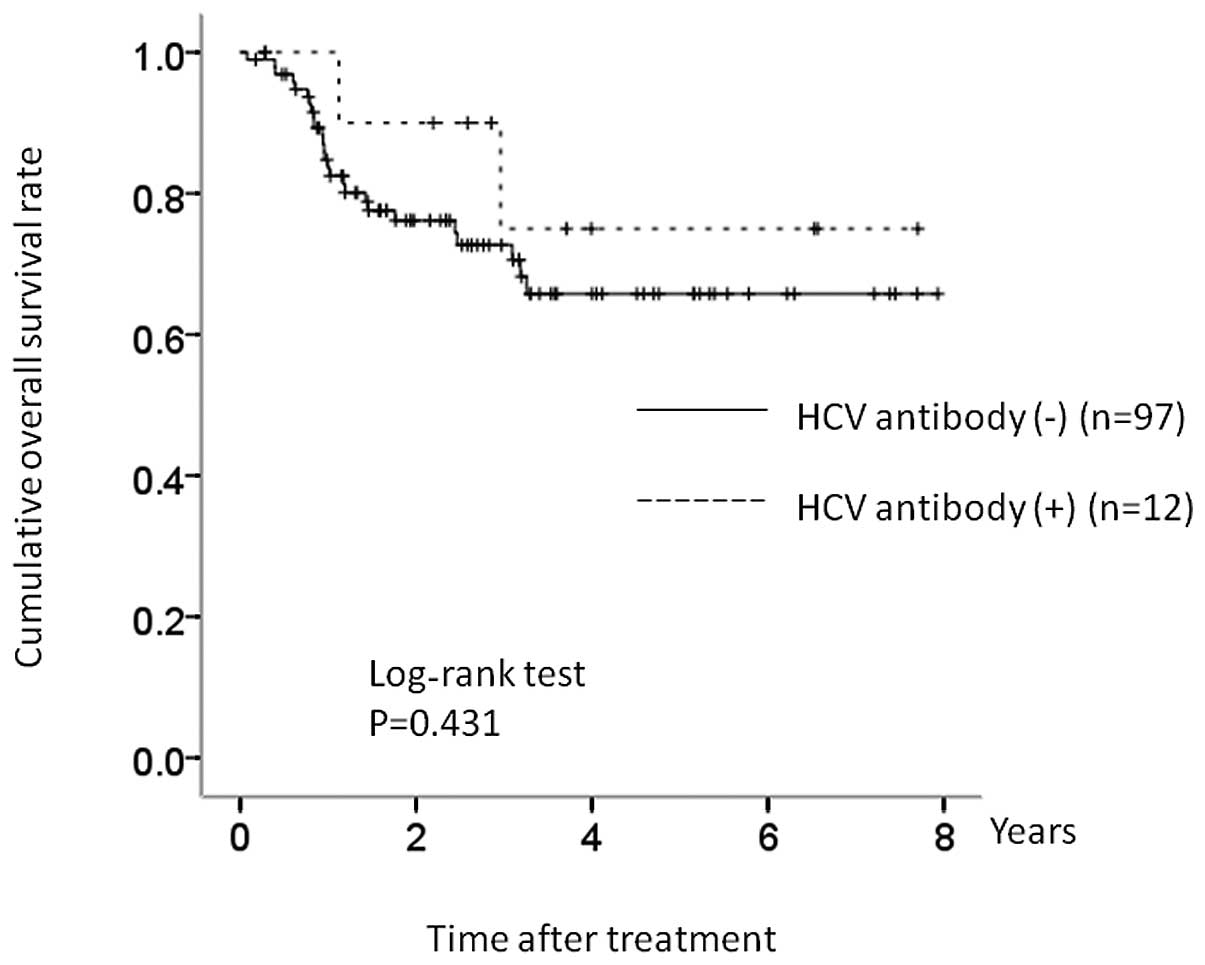
Subgroup analysis according to IPI: high
or high/intermediate IPI group
There were 12 patients (42.9%) with high or
high/intermediate IPI values in the HCV group, and 97 patients
(44.1%) with high or high/intermediate IPI values in the control
group. In terms of OS, there was no significant difference between
the two groups (P=0.431) (Fig.
4).
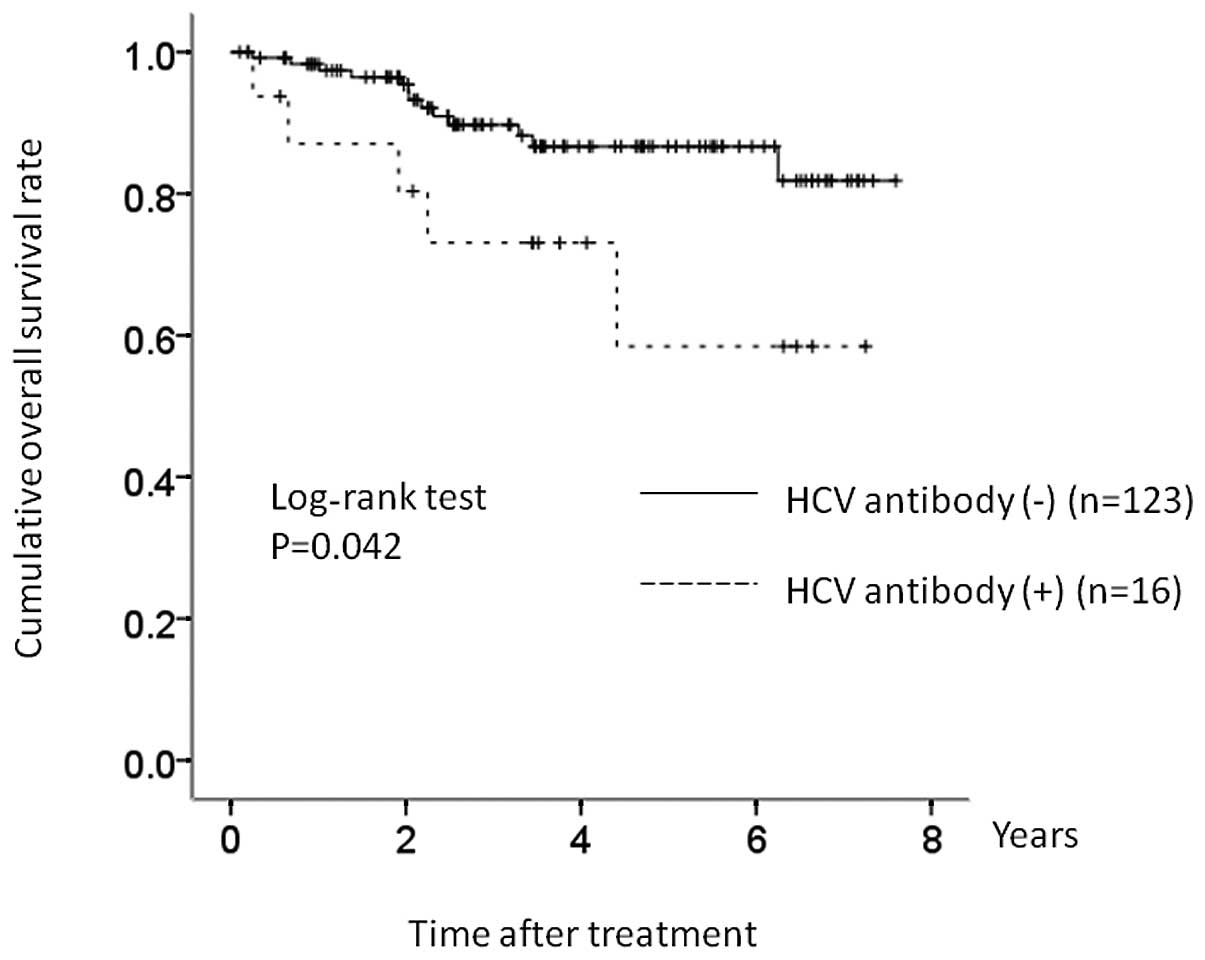
Subgroup analysis according to IPI: low
or low/intermediate IPI group
There were 16 patients (57.1%) with low or
low/intermediate IPI values in the HCV group, and 123 patients
(55.9%) with low or low/intermediate IPI values in the control
group. In terms of OS, there was a significant difference between
these two groups (P=0.042) (Fig.
5).
Discussion
Over the past two decades, considerable evidence has
accumulated with regard to the association between HCV and several
hematologic malignancies, most notably B-cell NHL (9). However, there have been few reports
regarding the clinical outcome of DLBCL in patients with HCV
infection who have undergone rituximab containing
immunochemotherapy (2,12).
In the present study, 28 patients (10.3%) out of the
272 DLBCL patients were anti-HCV-positive. The prevalence of HCV is
reported to be higher in patients with B-NHL (~15%) than in the
general population (1~2%), particularly in geographical areas with
a high incidence of HCV infection (8–10). Our
results were similar to those reported in previous studies.
In terms of OS and PFS, there were no significant
differences between the HCV group and the control group in the
present study. Our results suggested that in DLBCL patients, HCV
infection was not a significant risk factor for prognosis in
rituximab containing immunochemotherapy.
In the present study, favorable objective response
to immunochemotherapy was obtained in the HCV group as compared
with previous reports (2,12,26),
and there was no significant difference between the HCV group and
the control group in terms of ORR. Besson et al reported
that the CR rate of DLBCL patients with HCV infection did not
differ from control patients (12);
our results were similar and suggested that the addition of
rituximab did not seem to affect the treatment response of DLBCL
patients with HCV infection.
Although the addition of rituximab to the
chemotherapy regime for ML patients heralded a new treatment era,
hepatotoxicity due to immunochemotherapy is an important
consideration. Besson et al (12) reported that the hepatotoxicity of
HCV-positive ML patients undergoing chemotherapy could not be
attributed to pretreatment liver abnormalities or to a specific
drug, whereas Ennishi et al (2) reported that hepatotoxicity in
HCV-positive ML patients undergoing chemotherapy was more likely to
occur if pretreatment transaminase levels were high. In the present
study, in the HCV group, three patients out of five (60.0%) with
high pretreatment transaminase levels developed hepatotoxicity
during immunochemotherapy, whereas in the control group only one
patient out of twenty (5.0%) with high pretreatment transaminase
levels developed hepatotoxicity during immunochemotherapy. Our
results are similar to those reported by Ennishi et al
(2) and indicate that careful
monitoring of liver function during immunochemotherapy will be
required, especially in HCV-positive DLBCL patients with high
pretreatment transaminase levels.
In our study, there were no patients in the HCV
group who required discontinuation of immunochemotherapy owing to
hepatotoxicity or other causes. These results suggest that
rituximab containing immunochemotherapy is safe in DLBCL patients
with HCV infection, although Arcaini et al reported that a
significant proportion of patients with HCV-positive NHL developed
liver toxicity (13). This often
led to interruption or discontinuation of treatment, and was a
limiting factor in the application of immunochemotherapy programs
(13).
In the present study, in terms of OS, there was a
significant difference in the low/low intermediate IPI group,
although there was no significant difference in the high/high
intermediate IPI group. Our results suggested that the survival of
DLBCL patients with favorable prognostic value may be more affected
by HCV infection than those with poor prognostic value.
In the present study, four patients in the HCV group
died of HCC, and all of them achieved a CR after the front-line
therapy. Significant immunosuppression may change the tempo of HCV
natural history and accelerate complications such as liver
cirrhosis and HCC. In these patients, IFN therapy may be required
with the objective of HCV eradication and suppression of HCC
occurrence (27,28). La Mura et al reported that
after complete response to chemotherapy, antiviral treatment in
HCV-positive NHL might be an important strategy (29). Collaboration between hematologists
and hepatologists is essential to optimize outcome.
Interestingly, out of 18 patients tested for HCV
genotype in the present study, 17 (94.4%) had HCV genotype 1.
Pellicelli et al reported that DLBCL patients with HCV
infection had a higher prevalence of HCV genotype 1 as compared
with patients with indolent B-NHL, in which HCV genotype 2 was the
more frequent genotype (30); our
results were similar to their report, although the reasons for this
are unclear.
The present study had several limitations. First, it
was a retrospective study. Second, the sample sizes between the HCV
group and the control group were not balanced. Third, the cause of
hepatotoxicity during immunochemotherapy in the HCV group was
unclear, because drug induced liver injury or HCV reactivation
since HCV-RNA was not tested during chemotherapy in many patients
in the HCV group. Therefore, to clarify these issues, larger
prospective studies will be needed in the future. However, it was
confirmed in the present study that in both the HCV and control
groups patients could achieve favorable clinical outcome, and in
the HCV group they had good tolerance to immunochemotherapy. In
conclusion, HCV infection may not influence the clinical course in
DLBCL patients who received rituximab containing
immunochemotherapy.
Acknowledgements
The authors would like to thank Hitomi Kaneko for
data collection.
References
|
1
|
Mele A, Pulsoni A, Bianco E, Musto P,
Szklo A, Sanpaolo MG, Iannitto E, De Renzo A, Martino B, Liso V, et
al: Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian
multicenter case-control study. Blood. 102:996–999. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ennishi D, Maeda Y, Niitsu N, Kojima M,
Izutsu K, Takizawa J, Kusumoto S, Okamoto M, Yokoyama M, Takamatsu
Y, et al: Hepatic toxicity and prognosis in hepatitis C
virus-infected patients with diffuse large B-cell lymphoma treated
with rituximab-containing chemotherapy regimens: a Japanese
multicenter analysis. Blood. 116:5119–5125. 2010. View Article : Google Scholar
|
|
3
|
De Vita S, Sacco C, Sansonno D, Gloghini
A, Dammacco F, Crovatto M, Santini G, Dolcetti R, Boiocchi M,
Carbone A and Zagonel V: Characterization of overt B-cell lymphomas
in patients with hepatitis C virus infection. Blood. 90:776–782.
1997.PubMed/NCBI
|
|
4
|
Pioltelli P, Zehender G, Monti G,
Monteverde A and Galli M: HCV and non-Hodgkin lymphoma. Lancet.
347:624–625. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuo K, Kusano A, Sugumar A, Nakamura S,
Tajima K and Mueller NE: Effect of hepatitis C virus infection on
the risk of non-Hodgkin’s lymphoma: a meta-analysis of
epidemiological studies. Cancer Sci. 95:745–752. 2004.
|
|
6
|
Gisbert JP, Garcia-Buey L, Pajares JM and
Moreno-Otero R: Prevalence of hepatitis C virus infection in B-cell
non-Hodgkin’s lymphoma: systematic review and meta-analysis.
Gastroenterology. 125:1723–1732. 2003.
|
|
7
|
Izumi T, Sasaki R, Tsunoda S, Akutsu M,
Okamoto H and Miura Y: B cell malignancy and hepatitis C virus
infection. Leukemia. 11:516–518. 1997.PubMed/NCBI
|
|
8
|
Galli M, Pioltelli P, Zehender G, Monti G
and Monteverde A: HCV and lymphomagenesis. Lancet. 348:2751996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartridge-Lambert SK, Stein EM, Markowitz
AJ and Portlock CS: Hepatitis C and non-Hodgkin lymphoma: the
clinical perspective. Hepatology. 55:634–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawatani T, Suou T, Tajima F, Ishiga K,
Omura H, Endo A, Ohmura H, Ikuta Y, Idobe Y and Kawasaki H:
Incidence of hepatitis virus infection and severe liver dysfunction
in patients receiving chemotherapy for hematologic malignancies.
Eur J Haematol. 67:45–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuckerman E, Zuckerman T, Douer D, Qian D
and Levine AM: Liver dysfunction in patients infected with
hepatitis C virus undergoing chemotherapy for hematologic
malignancies. Cancer. 83:1224–1230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Besson C, Canioni D, Lepage E, Pol S,
Morel P, Lederlin P, van Hoof A, Tilly H, Gaulard P, Coiffier B, et
al: Groupe d’Etude des Lymphomes de l’Adulte Programs:
Characteristics and outcome of diffuse large B-cell lymphoma in
hepatitis C virus-positive patients in LNH 93 and LNH 98 Groupe
d’Etude des Lymphomes de l’Adulte programs. J Clin Oncol.
24:953–960. 2006.
|
|
13
|
Arcaini L, Merli M, Passamonti F, Bruno R,
Brusamolino E, Sacchi P, Rattotti S, Orlandi E, Rumi E, et al:
Impact of treatment-related liver toxicity on the outcome of
HCV-positive non-Hodgkin’s lymphomas. Am J Hematol. 85:46–50.
2009.PubMed/NCBI
|
|
14
|
Tobinai K and Hotta T: Clinical trials for
malignant lymphoma in Japan. Jpn J Clin Oncol. 34:369–378. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lake-Bakaar G, Dustin L, McKeating J,
Newton K, Freeman V and Frost SD: Hepatitis C virus and alanine
aminotransferase kinetics following B-lymphocyte depletion with
rituximab: evidence for a significant role of humoral immunity in
the control of viremia in chronic HCV liver disease. Blood.
109:845–846. 2007. View Article : Google Scholar
|
|
16
|
Tsutsumi Y, Kanamori H, Mori A, Tanaka J,
Asaka M, Imamura M and Masauzi N: Reactivation of hepatitis B virus
with rituximab. Expert Opin Drug Saf. 4:599–608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai MS, Chao TY, Kao WY, Shyu RY and Liu
TM: Delayed hepatitis B virus reactivation after cessation of
preemptive lamivudine in lymphoma patients treated with rituximab
plus CHOP. Ann Hematol. 83:769–774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niitsu N, Hagiwara Y, Tanae K, Kohri M and
Takahashi N: Prospective analysis of hepatitis B virus reactivation
in patients with diffuse large B-cell lymphoma after rituximab
combination chemotherapy. J Clin Oncol. 28:5097–5100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Renzo A, Perna F, Persico M, Notaro R,
Mainolfi C, de Sio I, Ciancia G, Picardi M, Del Vecchio L, Pane F
and Rotoli B: Excellent prognosis and prevalence of HCV infection
of primary hepatic and splenic non-Hodgkin’s lymphoma. Eur J
Haematol. 81:51–57. 2008.PubMed/NCBI
|
|
20
|
Harris NL, Jaffe ES, Diebold J, Flandrin
G, Muller-Hermelink HK, Vardiman J, Lister TA and Bloomfield CD:
World Health Organization classification of neoplastic diseases of
the hematopoietic and lymphoid tissues: report of the Clinical
Advisory Committee meeting-Airlie House, Virginia, November 1997. J
Clin Oncol. 17:3835–3849. 1999.
|
|
21
|
Ohno O, Mizokami M, Wu RR, Saleh MG, Ohba
K, Orito E, Mukaide M, Williams R and Lau JY: New hepatitis C virus
(HCV) genotyping system that allows for identification of HCV
genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol.
35:201–207. 1997.PubMed/NCBI
|
|
22
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the Committee on Hodgkin’s
Disease Staging Classification. Cancer Res. 31:1860–1861. 1971.
|
|
23
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai
K, Vose JM, Connors JM, Federico M and Diehl V: International
Harmonization Project on Lymphoma: revised response criteria for
malignant lymphoma. J Clin Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Labarga P, Soriano V, Vispo ME, Pinilla J,
Martin-Carbonero L, Castellares C, Casado R, Maida I, Garcia-Gasco
P and Barreiro P: Hepatotoxicity of antiretroviral drugs is reduced
after successful treatment of chronic hepatitis C in HIV-infected
patients. J Infect Dis. 196:670–676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
[No authors listed]. A predictive model
for aggressive non-Hodgkin’s lymphoma. The International
Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med.
329:987–994. 1993.
|
|
26
|
Visco C, Arcaini L, Brusamolino E,
Burcheri S, Ambrosetti A, Merli M, Bonoldi E, Chilosi M, Viglio A,
Lazzarino M, et al: Distinctive natural history in hepatitis C
virus positive diffuse large B-cell lymphoma: analysis of 156
patients from northern Italy. Ann Oncol. 17:1434–1440. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshida H, Shiratori Y, Moriyama M,
Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, et
al: Interferon therapy reduces the risk for hepatocellular
carcinoma: national surveillance program of cirrhotic and
noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study
Group. Ann Intern Med. 131:174–181. 1999. View Article : Google Scholar
|
|
28
|
Nishiguchi S, Shiomi S, Nakatani S, Takeda
T, Fukuda K, Tamori A, Habu D and Tanaka T: Prevention of
hepatocellular carcinoma in patients with chronic active hepatitis
C and cirrhosis. Lancet. 357:196–197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
La Mura V, De Renzo A, Perna F, D’Agostino
D, Masarone M, Romano M, Bruno S, Torella R and Persico M:
Antiviral therapy after complete response to chemotherapy could be
efficacious in HCV-positive non-Hodgkin’s lymphoma. J Hepatol.
49:557–563. 2008.PubMed/NCBI
|
|
30
|
Pellicelli AM, Marignani M, Zoli V, Romano
M, Morrone A, Nosotti L, Barbaro G, Picardi A, Gentilucci UV,
Remotti D, et al: Hepatitis C virus-related B cell subtypes in non
Hodgkin’s lymphoma. World J Hepatol. 3:278–284. 2011.PubMed/NCBI
|