Introduction
Malignant pleural mesothelioma (MPM) is an extremely
invasive malignancy with complex mass geometry and asymmetric,
discontinuous growth. MPM easily transgresses anatomical
boundaries, including the pleura, pericardium and bone, typically
respected by tumors, indicating robust secretion of extracellular
proteases required for such aggressive local invasion. This pattern
of tumor growth poses challenges in mouse models, even when
measuring subcutaneous grafted tumors, since the invasive component
is often significantly underestimated by external caliper
measurement. Non-invasive caliper measurement is made impossible in
orthotopic MPM xenografts, implanted in the pleura and peritoneal
spaces. This is important since, in recent years, orthotopic models
of malignancy, that is tumors grown in the anatomically ‘natural’
site of growth, have been demonstrated to often yield more robust
growth. This phenomenon is likely due to locally available growth
stimuli, allowing for more ‘patient-like’ patterns of local and
metastatic invasion (1–6). For MPM, recent orthotopic pleural and
intraperitoneal models have been introduced (7) as they are both typical spontaneous
sites of human malignant mesothelioma (8) and do demonstrate robust tumor growth.
A recent study successfully addressed intraperitoneal and
intrapleural tumor mass visualization using red-shifted fluorescent
protein (RFP) transfected MSTO-211H tumor cells (9). However, further development of simple,
durable non-invasive methods for tracking of intraperitoneal MPM
cell mass is highly valuable for non-invasive in vivo
evaluation of tumor response in orthotopic models.
In this study we evaluate the feasibility of MPM
tumor imaging with ProSense 680, an optical imaging agent which
releases fluorescent signal when cleaved by extracellular protease
activity. The primary protease contributors to ProSense 680
signaling are members of the cathepsin family of cysteine proteases
(Fig. 3). Cathepsins are typically
expressed within the endo/lysosomal intracellular compartment
although are re-routed into the extracellular space in the context
of specific physiological and pathophysiological processes, such as
inflammation malignant local invasion and metastasis. The shunting
of the cysteine cathepsins from the endo/lysosomal to secretory
systems and release into the extracellular matrix is thought to
mediated by downregulation of the insulin-like growth factor
receptor-2. In the extracellular matrix, many of these proteases
are able to degrade the surrounding stroma, a necessary step for
malignant local and metastatic invasion. In particular cysteine
protease cathepsins B, L, S and aspartyl protease cathepsin D,
effect robust degradation of components of the extracellular matrix
(ECM), including collagen, fibronectin, proteoglycans, elastin and
basement membrane, including Type-IV-collagen and laminin (10). While their activity and regulation
is complex and not well understood, certain cathepsins have been
observed to be routinely highly expressed in malignancy including
cathepsins B, C, L, S and X/Z of which only cathepsin L is nearly
exclusively overexpressed in the setting of malignancy. The
overexpression of some of these cathepsins, such as cathepsins L
and B, are also associated with poor prognosis, increased tumor
invasiveness and elevated frequency of metastasis. As a result,
inhibitors of the cathepsin protease family are under development
as potential therapeutic agents.
We describe the first report of imaging human
malignant pleural mesothelioma with a physiologic, in vivo,
fluorescent optical imaging probe of extracellular cathepsin
protease activity. We also demonstrate feasibility of
intraperitoneal MPM cell mass tracking using a cell surface-labeled
fluorescent signal, approximating the signal in the extracellular
compartment.
Materials and methods
Cell lines and cell culture
The human CALU-6 cell line was utilized for in
vitro, and newly purchased from American Type Culture
Collection (ATCC, Manasas, VA). Cells were maintained in a
humidified sterile environment with 5% CO2, at 37°C.
RPMI-medium supplemented with 10% heat-inactivated fetal calf serum
with 1% penicillin and streptomycin (CM) was utilized to culture
cell lines. Passage of cell lines was performed at 1:5 dilution
after detachment using sterile 0.5% trypsin-EDTA solution.
Cell line transfection
The MSTO-211H cell line was transfected with the
firefly luciferase gene using a retroviral vector as described
previously (11).
Protocol approval was obtained from the IACUC at the
University of Pennsylvania for xenograft formation prior to the use
of animal models. Immunocompromised nu/nu 6-week-old female mice
were injected subcutaneously in each flank with 107
cells suspended in a 50% sterile, endotoxin-free Matrigel
solution/50% CellGro Ex Vivo Medium Solution or
intraperitoneally suspended in a 50% CellGro Ex Vivo/PBS
solution. During tumor induction, mice were maintained at the 5
Richards Animal Facility at the University of Pennsylvania. After
therapy/imaging completion, mice were euthanized with ketamine i.p.
followed by cervical dislocation.
Optical imaging
After obtaining a pre-injection optical imaging
acquisition in the target spectrum, optical imaging agent ProSense
680 (2 nmol/150 μl in 1X PBS), purchased from VisEn Medical, Inc.
(Bedford, MA) was injected by tail vein and a time course of
imaging (20 min, 6 h, 24 h and 48 h) was acquired on the Maestro
(CRI, Woburn MA), with appropriate excitation and absorbance
filters. Mice were anesthetized with IP ketamine prior to each
imaging time point.
CellVue Maroon labeling of MSTO-211H per the
specifications of the manufacturers (Molecular Targeting
Technologies, Inc., West Chester, PA) prior to engraftment. Image
acquisition of the CellVue Maroon signal was acquired in the deep
red spectrum (excitation wavelength max=647 nm and emission
wavelength max=667 nm). Post-processing of optical images was
performed on the commercially available CRI Maestro software
(Woburn, MA). The acquired optical signal, obtained as an image
cube, was unmixed in order to separate dye signal from background
autofluorescence. After signal unmixing, tumor signal uptake was
quantitated with the CRI software.
RT-PCR
Total RNA was extracted from cultured MPM cell lines
using the RNeasy Mini Kit (Qiagen, Valencia, CA) following the
manufacturer's instructions. Reverse transcription was performed
with random hexamers and SuperScript II Reverse Transcriptase
(Invitrogen, Carlsbad, CA). Quantitative real-time polymerase chain
reaction (qPCR) analysis was performed in triplicate using an ABI
StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City,
CA) and SYBR Green PCR master mix (Applied Biosystems). Primers
used for amplification were obtained from published literature and
are as follows: cathepsin B, 5′-AAATCAGGCGTATACAA GCATGA-3′
(forward) and 5′-GCCCAGAATGCGGATGG-3′ (reverse); cathepsin L,
5′-CGGCATGAATGAAGA AGGAT-3′ (forward) and
5′-TGGGCTTACGGTTTTGAAAG-3′ (reverse); cathepsin D,
5′-ATCCCGCTGCACAAGTTCACGTCCATC CGC-3′ and
5′-CCACCAGCTTCTGCTGCATCAGGTTG TCGAAGACG-3′; cathepsin S,
5′-ATGAAACGGCTGGTTTG TGT-3′ and 5′-CTAGATTTCTGGGTAAGAGGGAAAG
CTAGC-3′; cathepsin K, 5′-CAGTGAAGAGGTGGTTC AGA-3′ (forward) and
5′-AGAGTCTTGGGGCTCTACCTT-3′ (reverse); cathepsin G,
5′-CTCAATATAATCAGCGGACC-3′ (forward) and 5′-CCAGCAGTTTGAAGCTTCTC-3′
(reverse); Klk5 (kallikrein-related peptidase 5), 5′-GCAAGACCCC
CCTGGATGTG-3′ (forward), 5′-TCCCAGAGGGCACG GTGTTA-3′ (reverse);
uPA, 5′-TTGCTCACCACAACGAC ATT-3′ (forward) and
5′-GGCAGGCAGATGGTCTGTAT-3′ (reverse); CD10 (membrane
metallo-endopeptidase), 5′-TGT GGCCAGATTGATTCGTC-3′ (forward) and
5′-TTGTAGGT TCGGCTGAGGCT-3′ (reverse); β-actin, 5′-AGAAAATCT
GGCACCACACC-3′ (forward) and 5′-GGGGTGTTGAAGG TCTCAAA-3′ (reverse);
TATA-binding protein, 5′-CAGGAGC CAAGAGTGAAGAAC-3′ (forward) and
5′-AGGAAATA ACTCTGGCTCATAACTACT-3′ (reverse); GP3DH, 5′-CAA
AGTTGTCATGGATGACC-3′ (forward) and 5′-CCATGG AGAAGGCTGGGG-3′
(reverse).
Statistical analysis RT-PCR data
The RT-PCR quantitative data were analyzed using an
Excel Spreadsheet (97–2003). The difference between the mean CT
values for the GADPDH controls and probe sets for each microarray
plate were performed and the log2 ratio calculated and termed the
‘actual value’. Actual values were then plotted on a bar graph for
interpretation.
Statistical analysis microarray data
RNA expression microarray data described previously
(12) and deposited into the public
database ArrayExpress (ID no. E-MTAB-47) was analyzed for
expression of cysteine proteases. Fresh frozen tissue tissue
samples were RNA extracted and run on the Affymetrix array HG-U133
Plus 2.0 microarray chip. A total of 12 human patient tissue
samples were chosen from this publically available dataset,
including 6 visceral pleura samples (A01.CEL, A04.CEL, A06.CEL,
A08.CEL, A10.CEL, A11.CEL), 5 malignant pleural mesothelioma
samples (A03.CEL, A12.CEL, A15.CEL, A16.CEL, A21.CEL) and 1 benign
pleural plaque (A07.CEL), and Affymetrix.cel (probe intensity)
files downloaded.
The patient samples were grouped into 3 categories,
malignant pleural mesothelioma (5),
normal pleura (6) and pleura plaque
(1). The .cel files were imported
into Partek Genomics Suite v6.5 (Partek Incorporated, St. Louis,
MO) and GCRMA applied to normalize and summarize the data to the
probeset level. We then applied a One-way ANOVA across the 3 groups
and additionally calculated pairwise contrasts between each pair of
groups. All calculated p-values were then corrected for False
Discovery Rate using the method of Benjamini-Hochberg (13) as implemented in Partek. Log2 ratio
and fold-change was then calculated.
Results
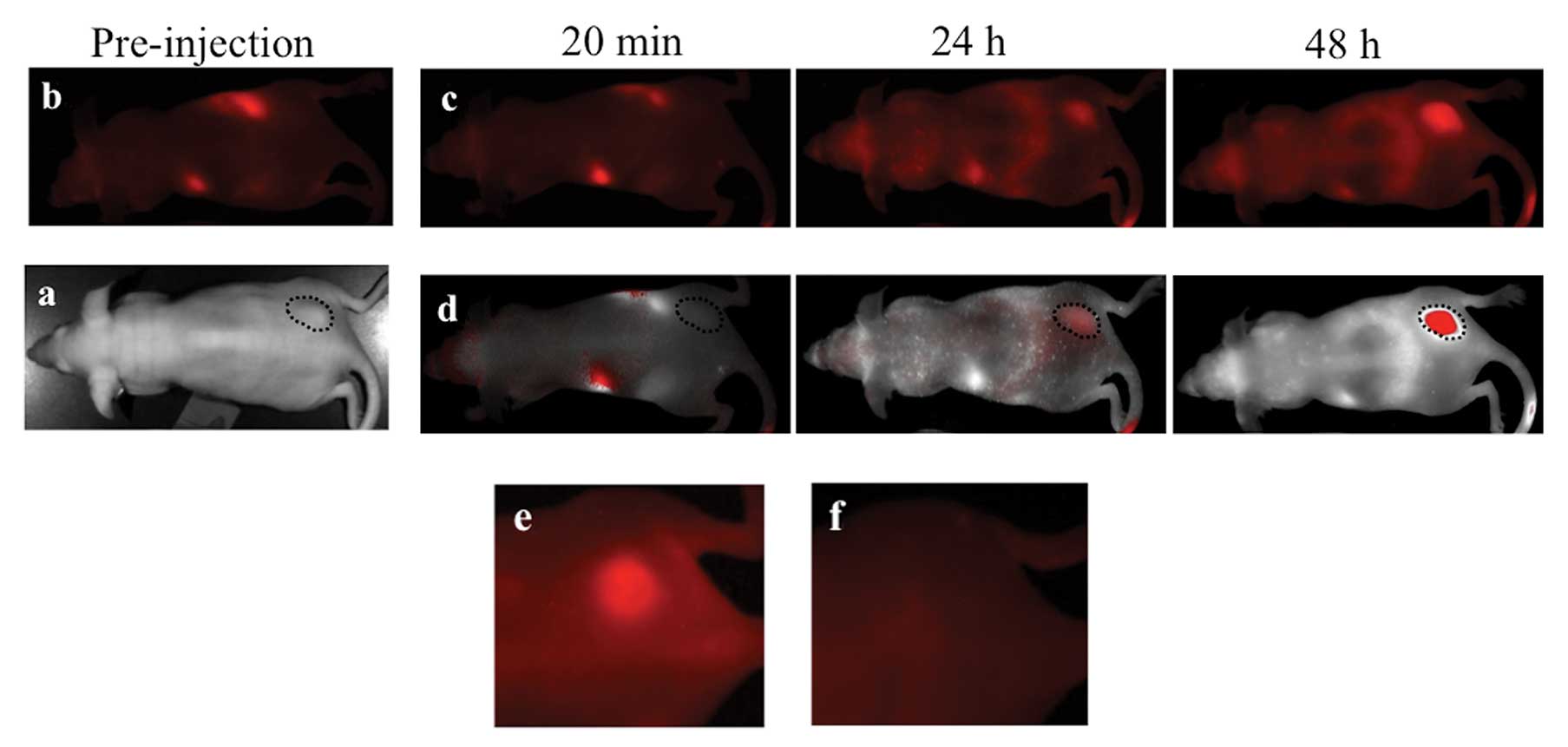
MPM xenografts were imaged with the ProSense 680
optical imaging agent which yielded robust, durable tumor signal
(Fig. 1a-d). This is a reflection
of the constitutively elevated levels of extracellular proteases in
malignant pleural mesothelioma. Once accumulated, ProSense 680
signal was still easily detectable within the xenografted tumors up
to 3 weeks after injection (Fig.
1e) however, this signal is not visible in the near-infrared
spectrum (Fig. 1f). This presents a
limitation in that a limited range of agents can be administered
coincidently or in tandem to allow the sufficient separation of the
spectral emission curves vital for specific individual probe signal
detection. The availability of a range of in vivo optical
imaging agents in the 750–800 nm emission wavelengths allows for
some flexibility in multispectral and longitudinal imaging with
other molecular imaging agents.
ProSense 680 functions by integrating a quenched
fluorophore-containing protease sensor in the cell surface lipid
bilayer. Upon encountering an extracellular cathepsin protease,
proteolysis ensues at multiple sites along its PEGylated polylysine
backbone releasing fluorescence signal. While ProSense 680
activation can be induced by a range of proteases, including
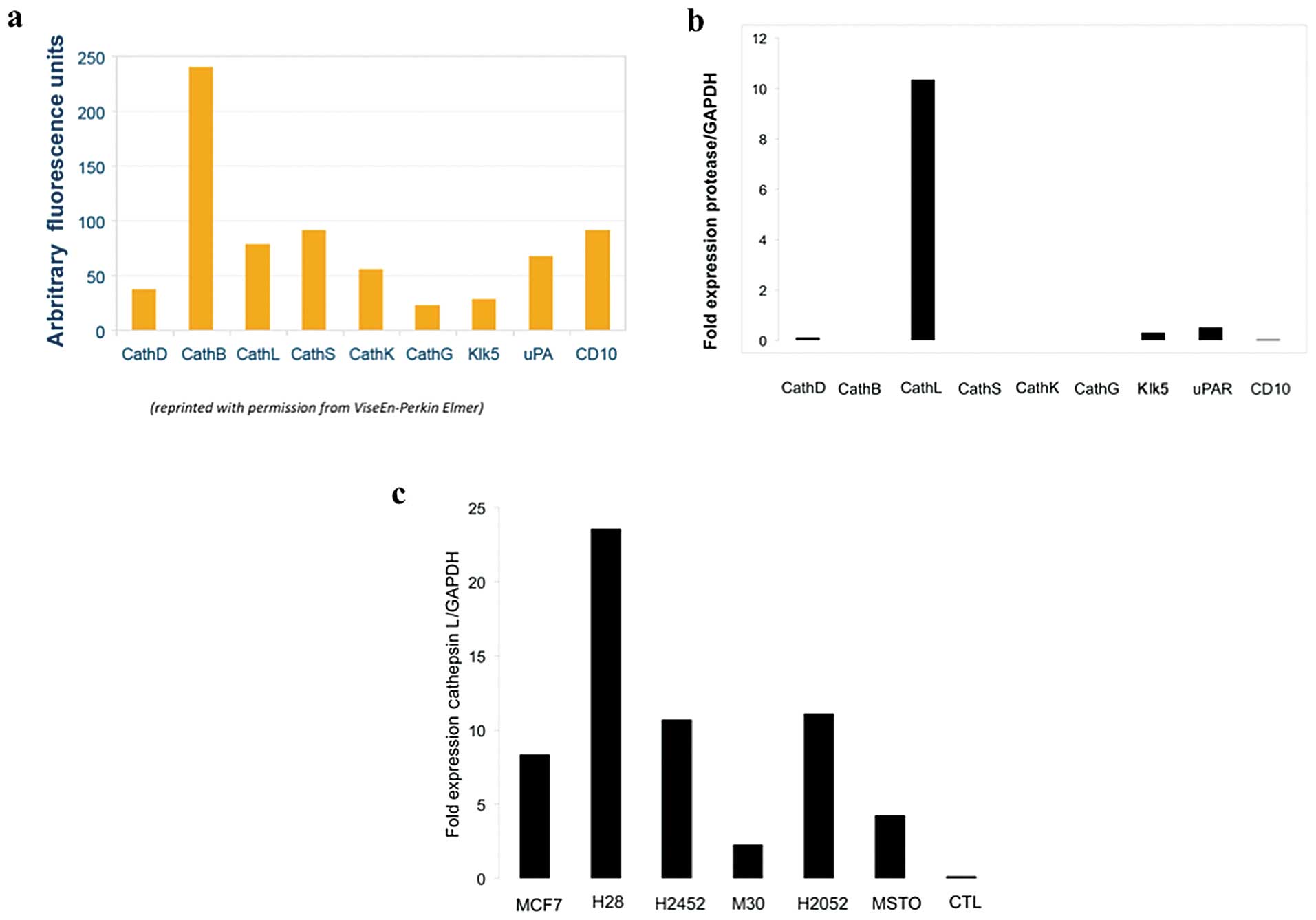
cathepsins B, D, L, S, K, G and Klk5 and uPA, the greatest activity
of this agent is derived from interaction with cathepsins B and L
and to a lesser extent cathepsin S (Fig. 2a). RNA expression analysis of the
MPM cell lines used for xenograft formation was performed in order
to determine the relative contributions of candidate extracellular
proteases to ProSense signal in our xenograft mouse model of human
MPM.
RT-PCR of RNA extracted from MPM cell lines, H28,
H2052, M30, MSTO-211H and H2452 was performed using GAPDH as a
control and revealed elevated levels of cathepsin L relative to
other tested extracellular proteases (Fig. 2b). Very low levels of uPAR
(urokinase plasminogen activator surface receptor), Klk5, CD10,
cathepsin D, but no cathepsin B or S expression, was detected in
the 5 malignant pleural mesothelioma cell lines. When cathepsin L
expression was compared between the tested human MPM cell lines,
the highest levels of expression was found in H28 and lowest in M30
(Fig. 2c). RT-PCR analysis of
cathepsin family expression of the 5 human MPM cell lines used in
this study demonstrated that the extracellular ProSense 680 signal
observed within our in vivo MPM xenografted tumors is likely
primarily a result of cathepsin L activity. Post-processing of
publically available RNA expression microarray data on normal
pleura, pleural plaque and malignant pleural mesothelioma was
evaluated using a 2-fold change cut-off for
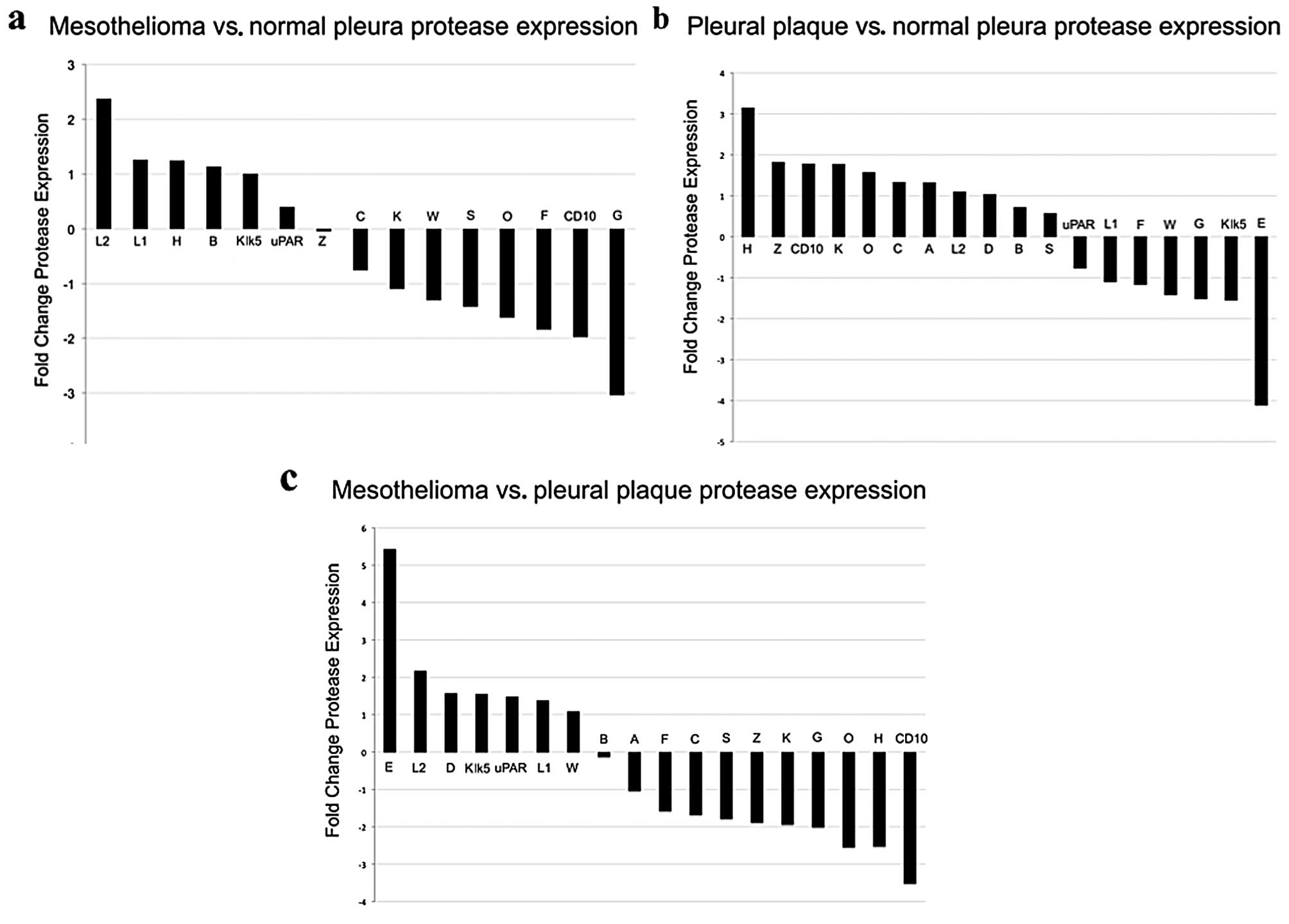
overexpression/underexpression. Data analysis demonstrated elevated
levels of expression of cathepsin L, composed of relatively more L2
transcript than L1 transcript, in MPM relative to normal pleura
(Fig. 3a) and relative to pleural
plaque (Fig. 3b).
Discussion
Cathepsin L, is expressed preferentially in the
setting of malignancy and inflammation. Overexpression of cathepsin
L is seen almost exclusively in malignant cells, and is correlated
with tumor aggressiveness manifested by local invasion and
metastasis (14) thought to be a
result of cathepsin L-mediated degradation of the extracellular
matrix along with similar proteins such as other members of the
cathepsin family and metalloproteinases. Interestingly, IL-6, a
cytokine found in abundance in MPM pleural effusions (15), has been shown to increase the
expression and secretion of cathepsin L (16,17).
In our analysis of human RNA microarray expression data available
on surgically obtained human tissues cathepsin L was elevated
(greater than 2-fold increase) in MPM but not normal pleura or
pleural plaque.
Other proteases that were potential ProSense 680
signal contributors did not reach 2-fold change including
cathepsins D, B, S, uPAR, Klk5 and CD10. Cathepsin D, a lysosomal
aspartic protease and potential contributor to ProSense signal, was
slightly elevated in expression in MPM tissue samples (less than
2-fold) relative to normal pleura and pleural plaque. However,
while cathepsin D can contribute to ProSense 680 signal, it is
proportionally a much weaker contributor than for cathepsin L
(Fig. 2a). Other potential
contributors to ProSense signal were either unchanged or
underexpressed in human surgical tissues of MPM relative to pleural
plaque or normal pleura. Cathepsin H and CD10, were underexpressed
in MPM relative to pleural plaque (Fig.
3b) and cathepsin H was overexpressed in pleural plaque
(Fig. 3c) relative to normal
pleura. Cathepsin H is a serine protease responsible for overall
degradation of lysosomal proteins and implicated in some
malignancies.
Other cathepsins typically elevated in expression in
the setting of inflammation, cathepsins H (18), C (18), K (19), B (20,21)
and S (21), were also either
decreased or little changed in expression in MPM tumor tissues
relative to normal pleura and pleural plaque. Cathepsin G,
frequently expressed highly in inflammation, was decreased in both
MPM (Fig. 3a) and pleural plaque
(Fig. 3c) relative to normal
pleura. The robust MPM tumor avidity for ProSense, and lack of
expression of inflammatory cathepsins able to significantly
contribute to ProSense signal, suggests extracellular protease
imaging as a useful means of MPM imaging.
In order to determine the feasibility of cell mass
tracking using optical imaging agents in the intraperitoneal space,
MSTO-211H human MPM tumor cells were coated with the CellVue
Maroon, a non-specific cell surface labeling fluorophore that
inserts non-specifically in the cell membrane prior to tumor
xenografting. CellVue Maroon labeled MSTO-211H cells were then
xenografted into nu/nu mice subcutaneously in the flank (Fig. 4a) and intraperitoneal (Fig. 4b) locations and tracked with serial
optical image acquisition. Qualitatively, signal from
MSTO-211H-labeled fluorescent probe CellVue Maroon was easily
tracked for 3 weeks after grafting, although signal was not as
robust as that generated from bioluminescence imaging. The dilution
of CellVue Maroon signal, a result of cellular division and
increasing cell mass, did not appear to impair qualitative tumor
visualization. Following sacrifice, necropsy evaluation
demonstrated correlation of tumor CellVue signal and cell mass
location both in the subcutaneous and intraperitoneal locations
after 3 weeks of growth. For comparison, imaging with
bioluminescence signal (Fig. 4c) in
the subcutaneous and intraperitoneal locations was performed using
MSTO-211H transfected with the bioluminescent fluorophore,
luciferase. Luciferase-transfected MSTO-211H demonstrated robust,
tumor-specific signal that was easily tracked and appeared
undiluted throughout the duration of the experiment.
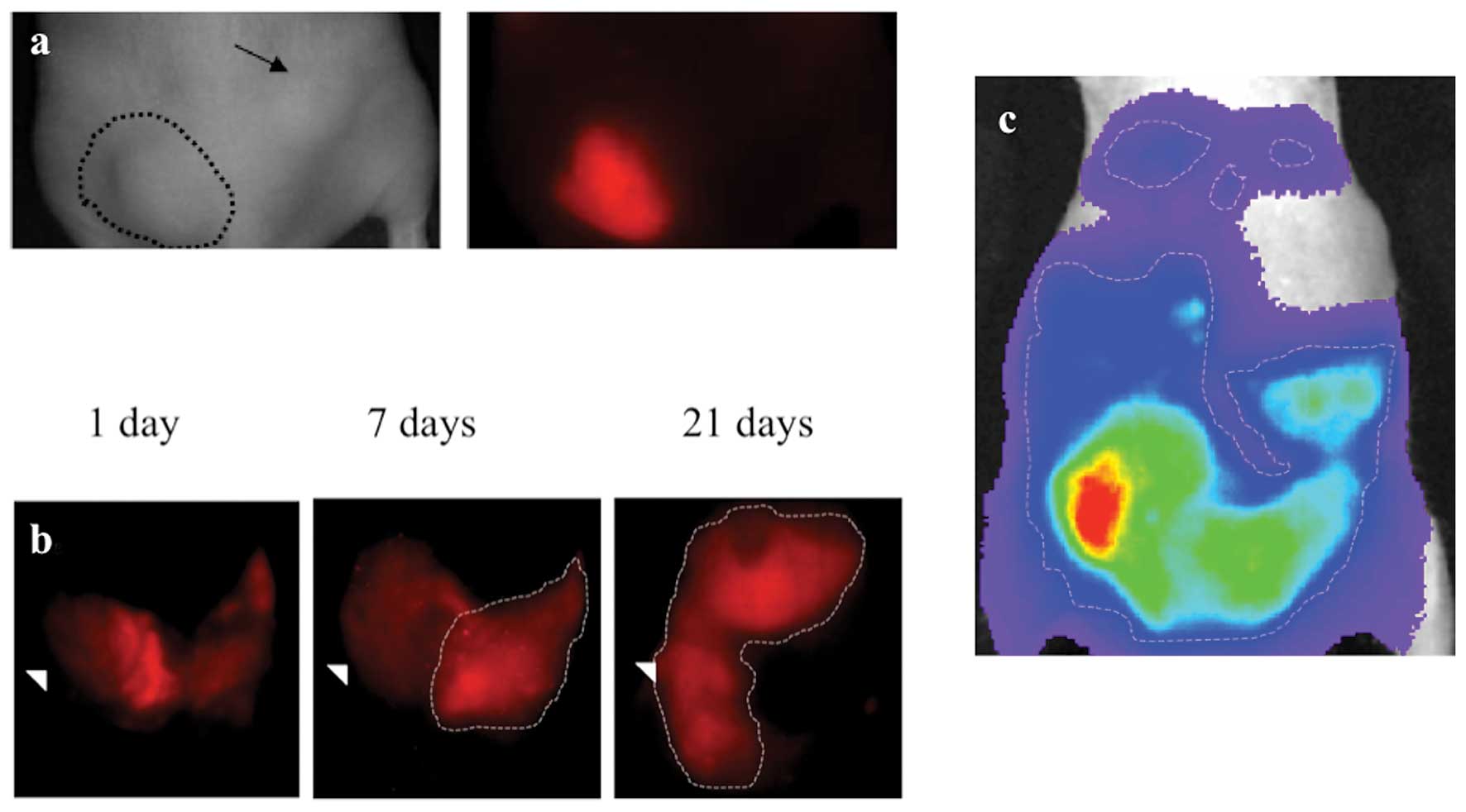 | Figure 4Subcutanous and orthotopic engraftment
of MSTO-211H labelled with CellVue cell surface labeling agent.
Human pleural mesothelioma cell line, MSTO-211H labelled with
CellVue Maroon cell surface-labeled tumor implanted (a)
subcutaneously in the left flank is faintly visible as a raised
mass on the white light image (encircled with dashed line), on the
left image, and deep red emission spectrum, on the right image,
whereas the right flank unlabeled tumor (arrow) is only seen in the
white light image since it contains no deep red fluorophore.
MSTO-211H labelled with CellVue Maroon cell surface-labeled tumor
implanted in the (b) intraperitoneal space and viewed in supine
positioning 1 d, 7 d and 21 d following engraftment demonstrates
the growth of tumor as initially layering fluorophore-labelled cell
along the peritoneal covered bowel surfaces migrates and evolves
into diffuse solid masses (encircled with dashed line). The
arrowhead serves as a fixed point of reference, located at the
lateral boundary of the right lower quadrant, to allow comparison
between time points. For comparison, in a different mouse, (c) a
heat map of luciferase signal emitted from luciferase expressing
MSTO-211H cells imaged in supine positioning following
intraperitoneal engraftment. The areas of highest signal (lighter
colors) corresponds to area of greater tumor bulk whereas blues and
purples colors depict areas of lower signal and therefore less
tumor bulk. |
CellVue, which coats the cell surface and thus
approximates the extracellular space, demonstrated robust tumor
cell mass fluorescent signal. The dilution of CellVue Maroon
signal, a result of cellular division and increasing cell mass, did
not appear to impair tumor visualization. While bioluminescent
transfection mediated cellular imaging is generally known to have
significant advantages over fluorescence, including robust signal,
extremely low background and lack of signal dilution over time,
fluorescent cell surface-labeling of cells has the advantage of
ease of use while maintaining low background and durable signal.
Caveats exist for both methods. For luciferase imaging, these
caveats include the stability of cellular luciferase-transfection,
imaging dependence on intravenous substrate injection and reports
of potential biological impacts of luciferase upon tumor growth
(22). For fluorescent-label cell
surface coating, caveats include potential loss of label with
concurrent IP delivery of drugs and interference with
co-administered IV fluorescent physiological tracers. Nevertheless,
cell surface coating of tumor cell approximates signal from the
extracellular space.
In this study we demonstrated a novel method of
imaging human MPM xenografts using an in vivo fluorescence
imaging agent of extracellular protease activity, ProSense 680. We
also demonstrated feasibility of cell mass tracking of MPM in the
orthotopic intraperitoneal location using a fluorescent signal
which address a significant caveat of intraperitoneal tumor
engraftment since these tumors cannot be directly measured.
Orthotopic modeling of human disease is often favored due to more
physiologic, ‘patient-like’ disease patterning and often, as in the
case of MPM, more robust tumor growth likely due to locally
available tumor growth signals. Future directions include a
quantitative study of fluorescently-labeled intraperitoneal cell
mass, cell mass depth and measured signal intensity in order to
define a non-invasive, method of quantitative intraperitoneal tumor
mass measurement.
Acknowledgements
We would like to acknowledge Dr John Tobias, Interim
Director, University of Pennsylvania Bioinformatics Core for his
assistance in analysis of the microarray data.
References
|
1
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grisanzio C, Seeley A, Chang M, et al:
Orthotopic xenografts of RCC retain histological, immunophenotypic
and genetic features of tumours in patients. J Pathol. 225:212–221.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan E, Patel A, Heston W and Larchian W:
Mouse orthotopic models for bladder cancer research. BJU Int.
104:1286–1291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoffman RM: Orthotopic metastatic mouse
models for anticancer drug discovery and evaluation: a bridge to
the clinic. Invest New Drugs. 17:343–359. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang M, Jiang P, Sun FX, et al: A
fluorescent orthotopic bone metastasis model of human prostate
cancer. Cancer Res. 59:781–786. 1999.PubMed/NCBI
|
|
6
|
Gros SJ, Dohrmann T, Rawnaq T, et al:
Orthotopic fluorescent peritoneal carcinomatosis model of
esophageal cancer. Anticancer Res. 30:3933–3938. 2010.PubMed/NCBI
|
|
7
|
Nakataki E, Yano S, Matsumori Y, et al:
Novel orthotopic implantation model of human malignant pleural
mesothelioma (EHMES-10 cells) highly expressing vascular
endothelial growth factor and its receptor. Cancer Sci. 97:183–191.
2006. View Article : Google Scholar
|
|
8
|
Moolgavkar SH, Meza R and Turim J: Pleural
and peritoneal mesotheliomas in SEER: age effects and temporal
trends, 1973–2005. Cancer Causes Control. 20:935–944.
2009.PubMed/NCBI
|
|
9
|
Yamaoka N, Kawasaki Y, Xu Y, et al:
Establishment of in vivo fluorescence imaging in mouse
models of malignant mesothelioma. Int J Oncol. 37:273–279.
2010.
|
|
10
|
Sloane BF: Cathepsin B and cystatins:
evidence for a role in cancer progression. Semin Cancer Biol.
1:137–152. 1990.PubMed/NCBI
|
|
11
|
Wang W and El-Deiry WS: Bioluminescent
molecular imaging of endogenous and exogenous p53-mediated
transcription in vitro and in vivo using an HCT116 human colon
carcinoma xenograft model. Cancer Biol Ther. 2:196–202. 2003.
View Article : Google Scholar
|
|
12
|
Roe OD, Anderssen E, Helge E, et al:
Genome-wide profile of pleural mesothelioma versus parietal and
visceral pleura: the emerging gene portrait of the mesothelioma
phenotype. PLoS One. 4:e65542004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klipper-Aurbach Y, Wasserman M,
Braunspiegel-Weintrob N, et al: Mathematical formulae for the
prediction of the residual beta cell function during the first two
years of disease in children and adolescents with insulin-dependent
diabetes mellitus. Med Hypotheses. 45:486–490. 1995.
|
|
14
|
Lankelma JM, Voorend DM, Barwari T, et al:
Cathepsin L, target in cancer treatment? Life Sci. 86:225–233.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DeLong P, Carroll RG, Henry AC, et al:
Regulatory T cells and cytokines in malignant pleural effusions
secondary to mesothelioma and carcinoma. Cancer Biol Ther.
4:342–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi T, Naruishi K, Arai H, Nishimura
F and Takashiba S: IL-6/sIL-6R enhances cathepsin B and L
production via caveolin-1-mediated JNK-AP-1 pathway in human
gingival fibroblasts. J Cell Physiol. 217:423–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerber A, Wille A, Welte T, Ansorge S and
Buhling F: Interleukin-6 and transforming growth factor-β1 control
expression of cathepsins B and L in human lung epithelial cells. J
Interferon Cytokine Res. 21:11–19. 2001.
|
|
18
|
D'Angelo ME, Bird PI, Peters C, Reinheckel
T, Trapani JA and Sutton VR: Cathepsin H is an additional
convertase of pro-granzyme B. J Biol Chem. 285:20514–20519. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asagiri M, Hirai T, Kunigami T, et al:
Cathepsin K-dependent toll-like receptor 9 signaling revealed in
experimental arthritis. Science. 319:624–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagai A, Murakawa Y, Terashima M, et al:
Cystatin C and cathepsin B in CSF from patients with inflammatory
neurologic diseases. Neurology. 55:1828–1832. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin SL, Moffitt KL, McDowell A, et al:
Association of airway cathepsin B and S with inflammation in cystic
fibrosis. Pediatr Pulmonol. 45:860–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brutkiewicz S, Mendonca M, Stantz K, et
al: The expression level of luciferase within tumour cells can
alter tumour growth upon in vivo bioluminescence imaging.
Luminescence. 22:221–228. 2007. View
Article : Google Scholar : PubMed/NCBI
|