Introduction
In Japan, cases of early gastric cancer associated
with a low possibility of lymph node metastasis have been
identified through pathological review of accumulated case reports
(1,2). In parallel, the development and spread
of endoscopic submucosal dissection (ESD) has enabled en
block resection with preserved gastric function (3–5). It
is, however, still difficult to accurately determine the invasion
depth of gastric cancer, with a number of cases requiring
additional surgical resection as a result of diagnostic ESD.
According to the Japanese Gastric Cancer Handling
Codes, gastric cancer with a vertical invasion depth of <500 μm
is defined as SM1 cancer, and differentiated SM1 gastric cancer
with a diameter of ≤3 cm is regarded as an extended indication
lesion (6). Therefore,
determination of invasion depth plays a very important role in
determining the treatment strategy for SM2 gastric cancer, for
which additional surgical resection is indicated.
The recent development of endoscopic devices has
enabled clearer visualization of fine mucosal and vascular
structures under magnified view, making magnifying endoscopy with
narrow-band imaging (NBI-ME) an innovative diagnostic tool. In
particular, a certain consensus has been reached regarding the
effectiveness of magnifying endoscopy for determining invasion
depth for intrapapillary capillary loop (IPCL) classification of
esophageal cancer (7) and
pit-pattern classification of colon cancer (8).
Although NBI-ME has also been shown to be useful for
qualitative diagnosis and for determining the range and tissue type
of gastric cancer, its usefulness for determining gastric cancer
invasion depth has not been established. The objective of this
study, therefore, was to evaluate the potential of NBI-ME for
determining invasion depth in differentiated early gastric
cancer.
Patients and methods
Of 42 patients who underwent ESD and were given a
final pathological diagnosis of SM cancer between April 2007 and
March 2011, 39 patients were evaluable by magnifying endoscopy. A
total of 15 patients with SM1 differentiated gastric cancer and 4
with undifferentiated mixed-type SM2 gastric cancer were excluded
from this study and the remaining 20 patients with SM2
differentiated gastric cancer were included.
In undifferentiated cancer, the surface of a tumor
frequently consists of non-cancerous epithelium. From the
histological features, the diagnosis of invasion depth for
undifferentiated cancer by NBI-ME that captures only microsurface
structure is limited. Therefore, we excluded undifferentiated mixed
type of SM2 gastric cancer.
There was no significant difference in patient
background factors between the groups (p>0.05). The maximum mean
tumor diameter tended to be larger in SM2 cancer than in SM1
cancer. Morphologically, there was a tendency of a higher
proportion of depressed-type cancer in both groups (Table I).
 | Table IPatient background. |
Table I
Patient background.
| SM1 | SM2 | P-value |
|---|
| No. | 15 | 20 | NS |
| Mean age (years) | 71.5 (50–90) | 77.5 (69–87)a | NS |
| Gender (M/F) | 14/1 | 16/4 | NS |
| Location (U/M/L) | 6/5/4 | 5/9/6 | NS |
| Maximum mean size
(mm) | 14.7 (3–45) | 26.9 (6–65)a | NS |
| Form
(protruding/depressed) | 4/11 | 4/16 | NS |
| Ul | 1 | 4 | NS |
| Pathology
(tub1/tub2/mixedb) | 8/1/6 | 8/1/11 | NS |
Endoscopic observation was performed using an
Olympus GIF-H260Z magnifying endoscope (Tokyo, Japan). The scope
was mounted with a tip hood (Elastic Touch M, Top Corp., Tokyo,
Japan) to maintain optimal focal distance. Lesions were imaged
under regular view, followed by regular magnified view and then by
NBI-ME (x60–80). Acetic acid (1.5% vinegar in tap water) spraying
was also used for lesions with obscure fine mucosal structure. A
total of 20 ml of the acetic acid solution was injected (about 10
ml at a time) through a forceps channel, with focus adjusted to the
region of interest under magnified view. ESD was performed in all
cases on the basis of preoperative diagnosis findings.
Histopathological diagnosis of resected specimens was performed by
a pathologist according to the Gastric Cancer Handling Codes.
NBI-ME images matching the invasion depth of
ESD-derived pathological specimens were retrospectively examined to
define the following three NBI-ME findings frequently observed in
differentiated SM2 cancer as its indicators (Table II).
 | Table IIDefinition of indicators for SM2
invasion. |
Table II
Definition of indicators for SM2
invasion.
| Definition |
|---|
| Indicator 1: loss of
fine mucosal structure; ‘non-structure’ | Areas in which no
glandular duct is observed, either under magnified view or after
acetic acid spraying, with a size corresponding to ≥2 residual
glandular ducts (Fig. 1). |
| Indicator 2:
scattered blood vessels; ‘scattery vessel’ | Presence of ≥2
scattered blood vessels in an area with obscure mucosal structure
(Fig. 2). |
| Indicator 3: severely
dilated and tortuous blood vessels; ‘multi-caliber vessel’ | Presence of tortuous
blood vessels with a diameter twice or more that of surrounding
tumor vessels (Fig. 3). |
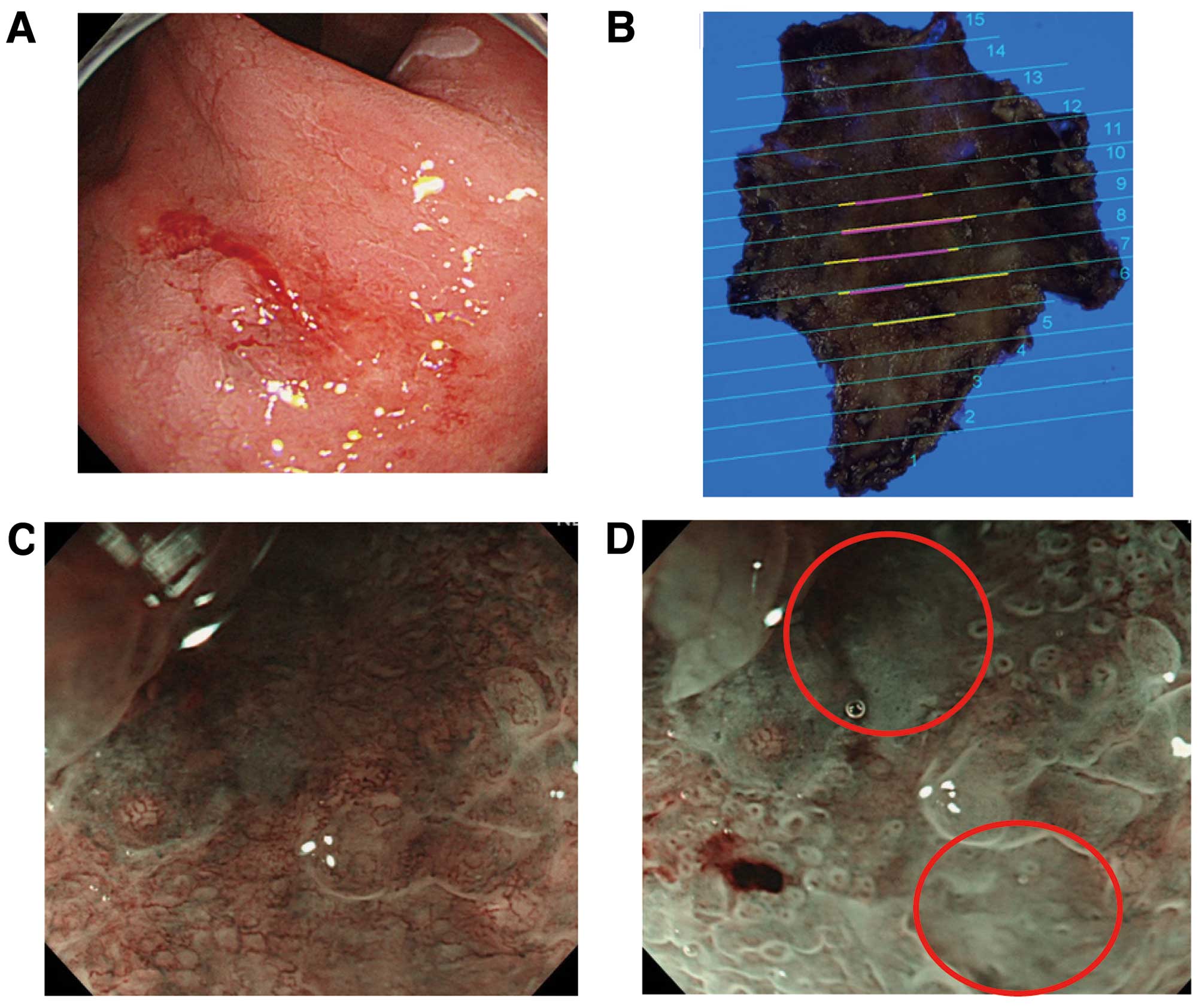
Typical findings of the indicator ‘non-structure’
are shown in Fig. 1. Conventional
endoscopic images showed a 10-mm red IIc lesion in the lesser
curvature of the gastric antrum (Fig.
1A). Mapping images of ESD-resected specimens revealed this
lesion to be a well-differentiated adenocarcinoma with mixed
invasion depth of M and SM2 (Fig.
1B). Preoperative NBI-ME (x80) of the SM2 invasion site showed
an obscure fine mucosal structure (Fig.
1C). Acetic acid spraying enabled clear visualization of
glandular ducts and revealed a ‘non-structure area’ (Fig. 1D).
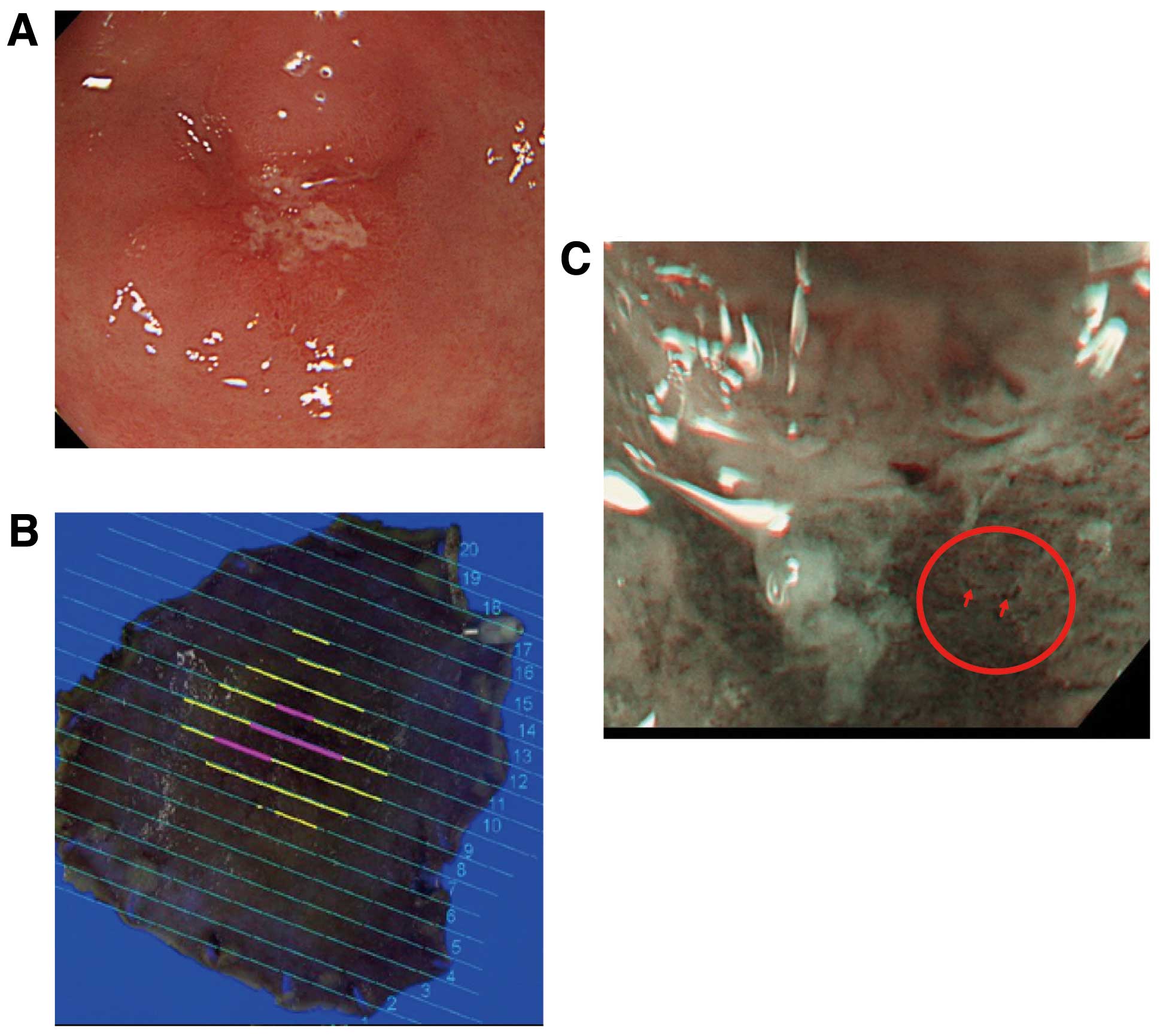
Typical findings of the indicator ‘scattery vessel’
are shown in Fig. 2. Conventional
endoscopic images showed a 15-mm erosive IIc lesion in the greater
curvature of the gastric antrum (Fig.
2A). Mapping images of ESD-resected specimens revealed this
lesion to be a well-differentiated adenocarcinoma consisting mainly
of M carcinoma with some SM2 invasion (Fig. 2B). Preoperative NBI-ME (x80) of the
SM2 invasion site showed two or more scattered blood vessels in an
area with obscure fine mucosal structure (Fig. 2C).
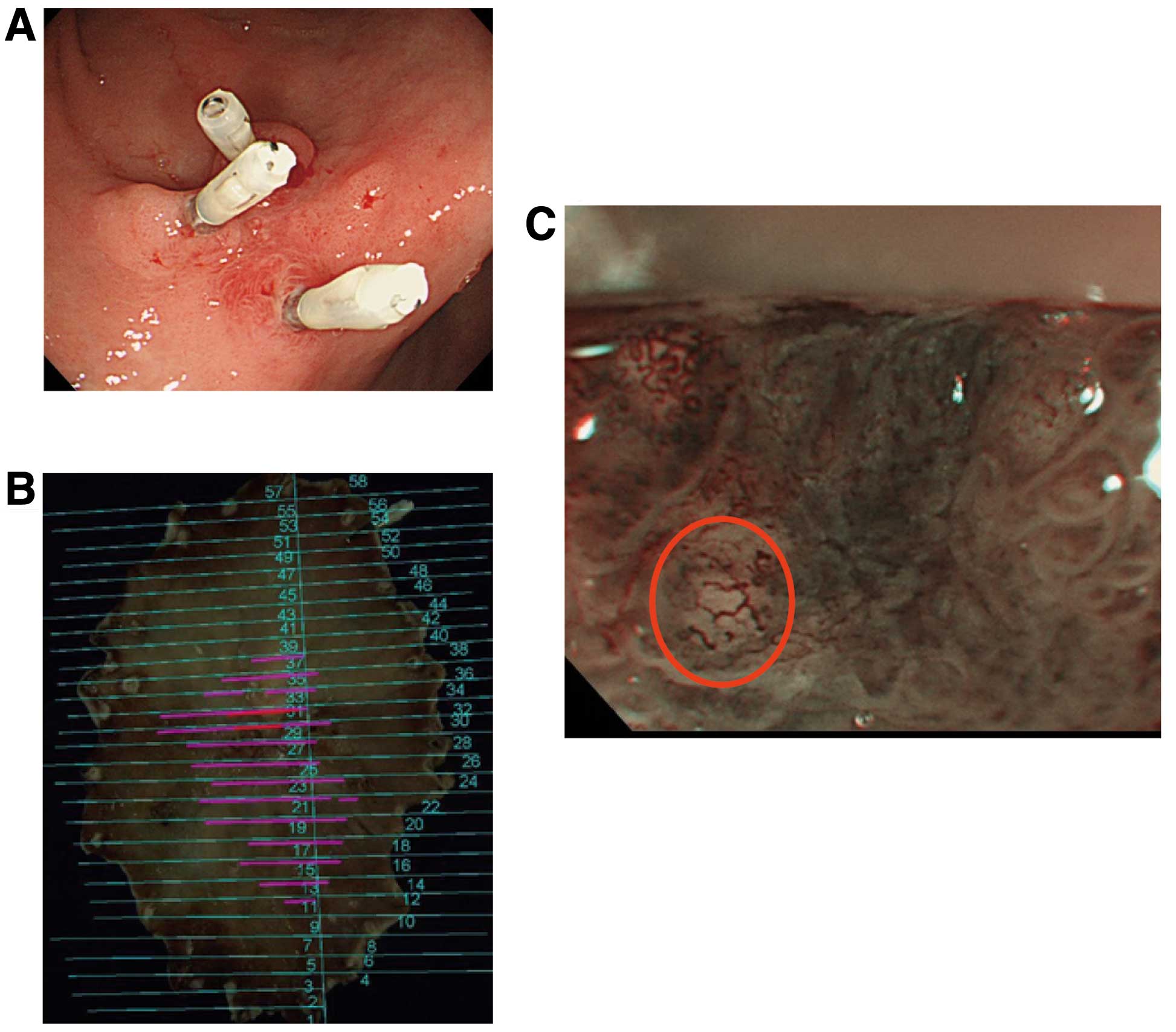
Typical findings of the indicator ‘multi-caliber
vessel’ are shown in Fig. 3.
Conventional endoscopic images showed a 25-mm red IIc lesion in the
anterior wall of the gastric angle in which clipping hemostasis had
been performed by a previous surgeon (Fig. 3A). Mapping images of ESD-resected
specimens revealed this lesion to be both a well- and
moderately-differentiated adenocarcinoma consisting mainly of M
carcinoma with some SM2 invasion (Fig.
3B). Preoperative NBI-ME (x80) of the SM2 invasion site showed
an abnormally dilated and tortuous blood vessel with a diameter
twice or more that of surrounding tumor vessels (Fig. 3C). We named this abnormal blood
vessel a ‘multi-caliber vessel’.
Evaluation
In D-GC, the histological architecture of gastric
microvilli become lower length in pattern. In contrast, in P-GC,
the histological architecture of gastric microvilli become enlonged
in pattern. From the histologic differences, we examined SM
differentiated gastric cancer according to morphology.
Evaluation: assessment I
The following items were evaluated separately in 27
patients with depressed-type gastric cancer (SM1/SM2: 11/16) and in
8 patients with protruding-type cancer (SM1/SM2: 4/4). I-1) The
relationship between the three indicators and invasion depth. I-2)
The relationship between scores for the three indicators and
invasion depth. An endoscopist blinded to regular endoscopic
findings and pathological diagnosis calculated scores for each
patient according to the presence (1 point) or absence (0 point) of
each indicator, with a maximum possible score of 3 points.
Evaluation: assessment II
The diagnostic accuracy of the determination of
invasion depth by regular endoscopy was compared with that by
magnifying endoscopy for the following items. II-1) After
randomizing SM1 and SM2 cases, four endoscopists blinded to
invasion depth were asked to determine this factor using regular
endoscopic images, after which diagnostic accuracy was determined
for each endoscopist. II-2) As in assessment I, four endoscopists
calculated scores according to the presence or absence of
indicators in each case. For each endoscopist, diagnostic accuracy
was determined by assuming that all patients with a score of ≥2
points (≥2 indicators) and showing significant differences, had SM2
cancer.
Statistical analysis
Intergroup comparison of patient background factors
was performed using the Student’s t-test or χ2 test. The
relationships between the three indicators and invasion depth and
between scores for the indicators and invasion depth were analyzed
using the χ2 test. The Student’s t-test was used to
compare diagnostic accuracy for invasion depth by regular endoscopy
and that by magnifying endoscopy. A p-value of <0.05 was
considered significant.
Results
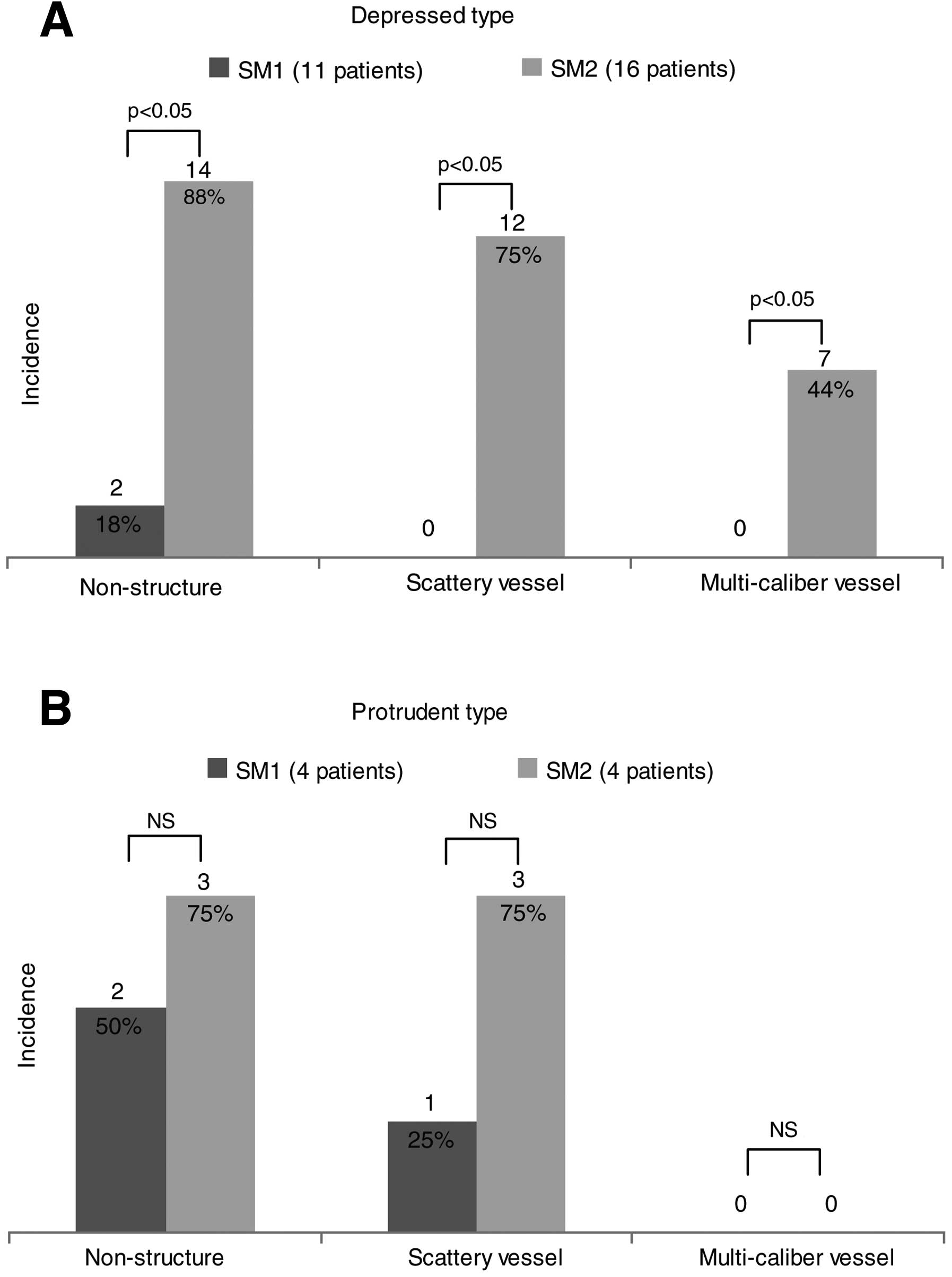
Assessment I-1: relationship between
indicators and invasion depth
In patients with depressed-type gastric cancer,
indicator ‘non-structure’ was observed significantly more
frequently in SM2 cases (14/16, 88%) than in SM1 cases (2/11, 18%)
(p=0.002). Indicator ‘scattery vessel’ was also significantly more
frequently observed in SM2 cases (12/16, 75%) than in SM1 cases
(0/11, 0%) (p<0.0001). Similarly, indicator ‘multi-caliber
vessel’ was significantly more frequently observed in SM2 cases
(7/16, 44%) than in SM1 cases (0/11, 0%) (p=0.01). Thus, in
patients with depressed-type gastric cancer, all indicators were
significantly more frequently observed in SM2 than in SM1 cases
(Fig. 4A).
On the other hand, in patients with protruding-type
gastric cancer, indicator ‘non-structure’ was observed in 2 of 4
(50%) SM1 cases and in 3 of 4 (75%) SM2 cases, with no significant
difference in frequency (p=0.465). Indicator ‘scattery vessel’ was
observed in 1 of 4 (25%) SM1 cases and in 3 of 4 (75%) SM2 cases,
again with no significant difference in frequency (p=0.157).
Indicator ‘multi-caliber vessel’ was not observed in any of the
patients in either group. Thus, in patients with protruding-type
gastric cancer, no significant difference was observed in the
frequency of each indicator between groups (Fig. 4B).
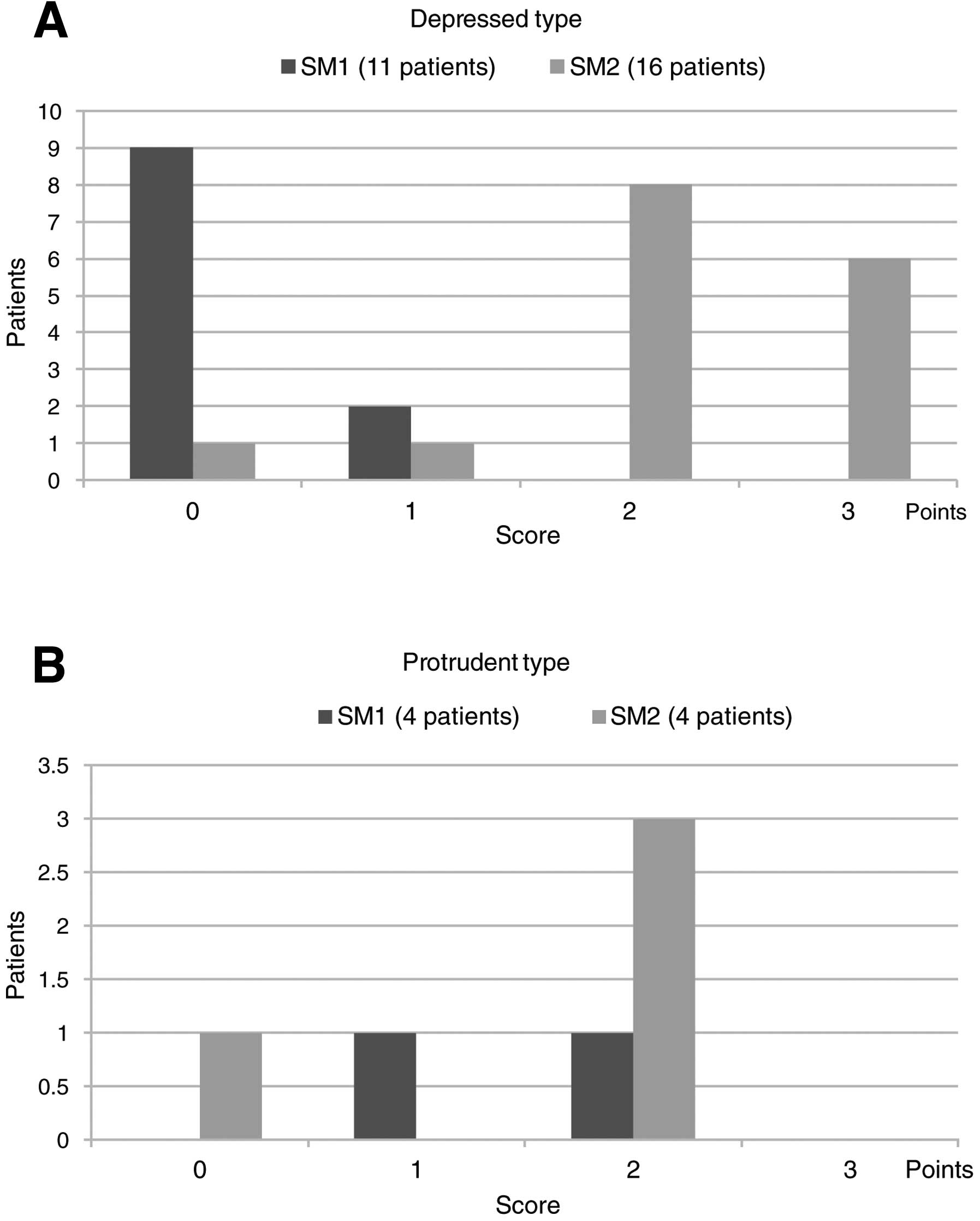
Assessment I-2: relationship between
indicator score and invasion depth
Of the 11 patients with SM1 depressed-type gastric
cancer, 9 and 2 patients had a score of 0 and 1 point,
respectively, with no patients having a score of ≥2 points. Of the
16 patients with SM2 depressed-type cancer, 1, 1, 8 and 6 patients
had 0, 1, 2 and 3 points, respectively. Of the 8 patients with 2
points, 6 were positive for indicators ‘non-structure’ and
‘scattery vessel’ and 2 patients were positive for ‘non-structure’
and ‘multi-caliber vessel’. Thus, all patients with a score of ≥2
points had SM2 cancer, indicating a significant difference in score
distribution between SM1 and SM2 cases (p<0.0001) (Fig. 5A).
Of patients with protruding-type gastric cancer, 2,
1 and 1 of 4 patients with SM1 cancer had 0, 1 and 2 points,
respectively, and 1, 0, 3 and 0 of 4 patients with SM2 cancer had
0, 1, 2 and 3 points, respectively. Thus, among patients with
protruding-type gastric cancer, no significant difference was
observed in score distribution between SM1 and SM2 cases
(P>0.05) (Fig. 5B).
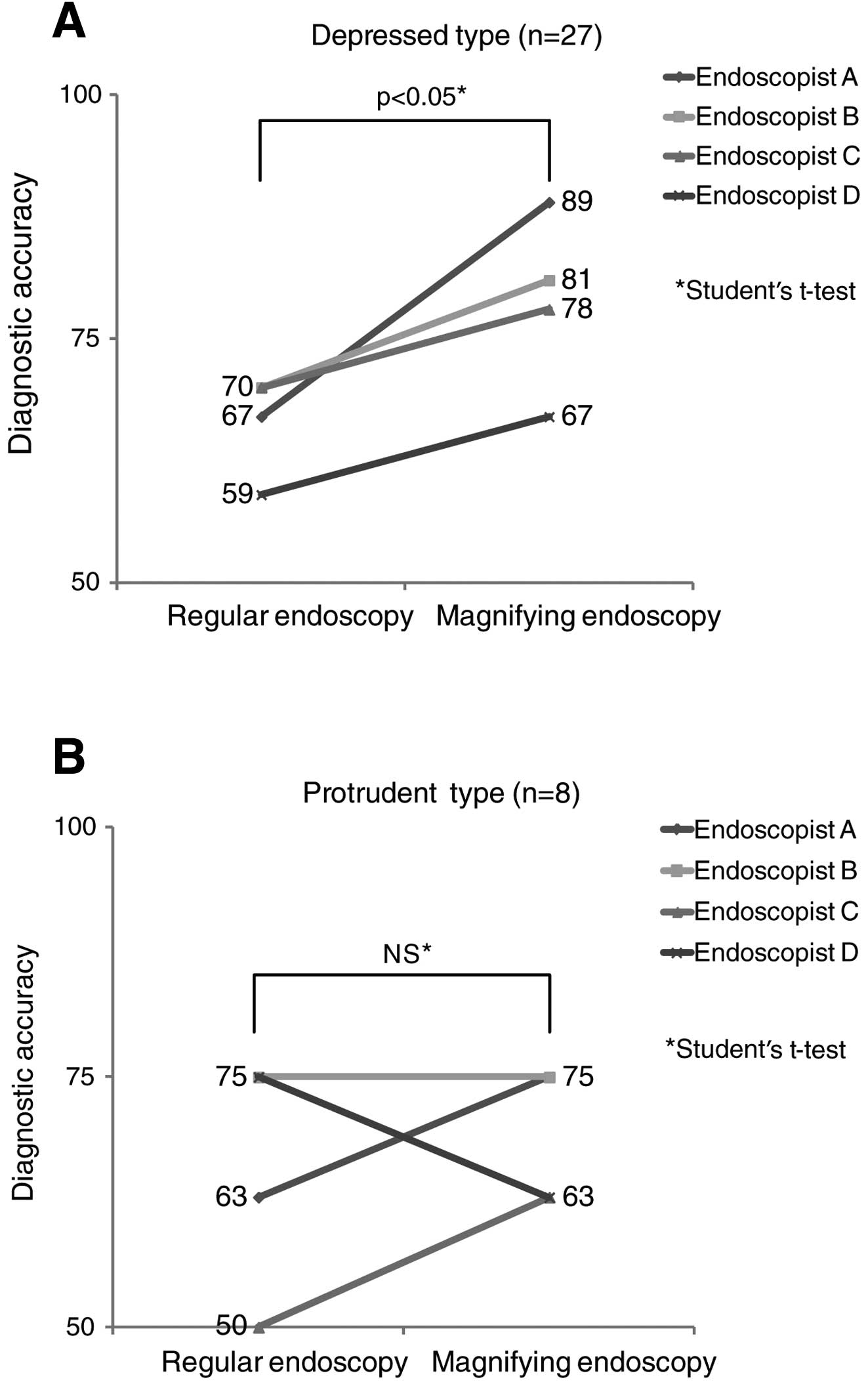
Assessment II: comparison of diagnostic
accuracy for invasion depth by regular endoscopy and that by
magnifying endoscopy
For depressed-type gastric cancer, diagnostic
accuracy for invasion depth by regular and magnifying endoscopy was
67 and 89% by endoscopist A, 70 and 81% by endoscopist B, 70 and
78% by endoscopist C and 59 and 67% by endoscopist D, respectively.
Assuming that all patients with a score of ≥2 points had SM2
cancer, diagnostic accuracy by magnifying endoscopy was higher than
that by regular endoscopy for all 4 endoscopists, indicating a
significantly higher diagnostic accuracy of magnifying endoscopy
(p=0.044) (Fig. 6A).
For protruding-type gastric cancer, diagnostic
accuracy by regular and magnifying endoscopy was 63 and 75% by
endoscopist A, 75 and 75% by endoscopist B, 50 and 63% by
endoscopist C and 75 and 63% by endoscopist D, respectively, with
no significant difference in diagnostic accuracy between the two
endoscopic procedures (p=0.170) (Fig.
6B).
Discussion
Accurately assessing invasion depth is very
important for determining the treatment strategy for gastric
cancer. This is usually done using a combination of regular
endoscopy, gastric fluoroscopy and endoscopic ultrasound techniques
which provide a certain degree of diagnostic accuracy.
Recently, a consensus has been reached regarding the
effectiveness of magnifying endoscopy for determining invasion
depth for IPCL classification of esophageal cancer (7) and pit-pattern classification of colon
cancer (8). Although magnifying
endoscopy has been used for observation of gastric mucosa for many
years (9,10), modification of gastric mucosa by
acid and chronic inflammation caused by Helicobacter pylori
infection complicates evaluation of the mucosal fine structure on
magnified images and have delayed the development of this
technique. The recent development of narrow-band imaging (NBI), a
procedure of image-enhanced endoscopic imaging, has improved the
visibility of fine mucosal and vascular structures of gastric
cancer and is being established as a new diagnostic tool.
Magnifying endoscopy has been shown to be effective
for differentiating benign from malignant tumors (11), determining tissue type (12) and determining the extent of gastric
cancer (13). Acetic acid spraying
is also an effective strategy for determining the extent of cancer
in which the cancerous portion is observed as a red area against a
white background of normal mucosa. A recent study suggests that the
use of acetic acid spraying combined with endoscopy enables
detailed observation of surface structures (14). In the present study, acetic acid
spraying in patients with obscure fine mucosal structure also
enabled confirmation of the presence or absence of normal
structure. With a limited number of studies available, no consensus
has been reached regarding the usefulness of NBI-ME for determining
invasion depth in gastric cancer. A review of preoperative
magnified images of SM1 and SM2 invasion sites revealed a higher
frequency of ‘non-structure’, ‘scattery vessel’ and ‘multi-caliber
vessel’ in differentiated SM2 cancer. We thus evaluated the
usefulness of these findings as indicators of SM2 cancer. Results
revealed a significant difference in the frequency of the
indicators between SM1 and SM2 cases of depressed-type gastric
cancer, demonstrating their potential as indicators of SM2
invasion. In assessment II, patients with depressed-type gastric
cancer with a score of ≥2 points (2 or more indicators) had SM2
cancer significantly more frequently than SM1 cancer, suggesting
the usefulness of scoring the indicators.
When assuming that all patients with depressed-type
gastric cancer with a score of ≥2 points had SM2 cancer, diagnostic
accuracy for invasion depth by magnifying endoscopy was
significantly higher than that by regular endoscopy, objectively
demonstrating the usefulness of the scoring system itself.
In patients with protruding gastric cancer,
indicator ‘scattery vessel’ was more frequently observed in SM2
cases (3/4, 75%) than in SM1 cases (1/4, 25%). Of the 4 patients
with SM2 cancer, 3 patients (75%) had a score of 2. Although no
statistically significant difference was observed due to a small
number of patients, a certain trend was observed in score
distribution among patients with protruding-type cancer, as was
among those with depressed-type cancer. At the same time, of the 4
patients with SM1 cancer, 2 (50%) patients had indicator
‘non-structure’, 1 (25%) had indicator ‘scattery vessel’ and 1
(25%) had a score of 2 points, suggesting that some patients have
magnified endoscopic findings that do not correspond to any of the
indicators. Regular endoscopic findings of SM deep invasion sites
in patients with protruding-type gastric cancer are characterized
by a gradual rise appearing like a submucosal tumor, tense feeling
and double protrusion as a result of the formation of a cancerous
mass in the submucosa. More case reports are needed to verify the
usefulness of magnifying endoscopy compared with diagnosis on the
basis of characteristic findings by regular endoscopy.
Several investigators have reported that SM cancer
is associated with the appearance of non-structure areas in fine
mucosal structure, irregularly-shaped, dilated or extended
microvessels and evidence of a hypovascular tumor (15,16). A
decreased degree of tumor differentiation has been associated with
the appearance of open loop, tortuous, and bizarre-type abnormal
microvessels (17). The fact that
similar magnifying endoscopic findings were obtained in the present
study may suggest a certain trend.
Only a limited number of studies, including the
present study, have statistically examined the frequency of
magnifying endoscopic findings between SM1 and SM2 cases. None of
the previous studies have scored indicators or compared diagnostic
accuracy, as was performed in assessments II and III. Thus, the
present study is notable in that it examined the determination of
invasion depth in SM2 cancer cases from an unprecedented point of
view.
One of the remaining issues is to obtain
pathological proof of the histological characteristics of tumors by
using the indicators to assess a deep invasion site. Because
magnifying endoscopy shows only the structure and vascular
architecture of the mucosal surface, histological findings in a
deep invasion site can only be estimated from differences in
surface structure. We currently consider that the ‘non-structure’
aspect indirectly reflects a decreased density of glandular ducts
and desmoplastic reaction due to deep invasion of cancer. With
regard to microvascular changes, indicator ‘scattery vessel’
appears to represent a decreased vascular density due to an
increase or expansion of stromal components associated with deep
invasion of cancer. Indicator ‘multi-caliber vessel’ appears to
reflect the presence of severely dilated and tortuous capillaries
with various diameters in the stroma between glandular ducts.
Further studies of a prospective design with
one-to-one comparisons of magnified endoscopic images with
corresponding histological images of deep invasion sites are thus
needed to obtain histological evidence supporting the present
findings, with special attention paid to the amount of stroma and
vascular architecture.
Finally, if the surface of a tumor consists of
non-cancerous epithelium, it is not necessarily undifferentiated
cancer with an extended middle mucosal layer, but may be a special
type of differentiated cancer. This is one of the limitations of
magnifying endoscopy as it only observes the mucosal surface; thus,
this diagnostic strategy is not necessarily applicable to all kinds
of lesions. Nevertheless, the present study has suggested the
usefulness of magnifying endoscopy for determining invasion depth
in differentiated, depressed-type gastric cancer, the most common
type of gastric cancer lesion.
In conclusion, magnifying endoscopic findings of
‘non-structure’, ‘scattery vessel’ and ‘multi-caliber vessel’ can
possibly serve as indicators of SM2 invasion when determining the
invasion depth of depressed-type gastric cancer. Patients with
depressed-type gastric cancer and a score of ≥2 points (2 or more
indicators) had SM2 cancer significantly more frequently than SM1
cancer, suggesting the usefulness of scoring the indicators.
References
|
1
|
Gotoda T, Yanagisawa A, Sasako M, et al:
Incidence of lymph node metastasis from early gastric cancer:
estimation with a large number of cases at two large centers.
Gastric Cancer. 3:219–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanaoka N, Tanabe S, Mikami T, et al:
Mixed-histologic-type submucosal invasive gastric cancer as a risk
factor for lymph node metastasis: feasibility of endoscopic
submucosal dissection. Endoscopy. 41:427–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tada M, Murakami A, Karita M, et al:
Endoscopic dissection of early gastric cancer. Endoscopy.
25:445–450. 1993. View Article : Google Scholar
|
|
4
|
Yahagi N, Fujishiro M, Kakushima N, et al:
Endoscopic submucosal dissection for early gastric cancer using the
tip of an electro-surgical snare (thin type). Dig Endosc. 16:34–38.
2004. View Article : Google Scholar
|
|
5
|
Ono H, Kondo H, Gotoda T, et al:
Endoscopic mucosal dissection for treatment of early gastric
cancer. Gut. 48:225–229. 2001. View Article : Google Scholar
|
|
6
|
Japanese Gastric Cancer Association.
Gastric Cancer Treatment Guidelines. 3rd edition. Kanehara; Tokyo:
2010
|
|
7
|
Inoue H: Magnification endoscopy in the
esophagus and stomach. Digestive Endoscopy. 13:S40–S41. 2001.
View Article : Google Scholar
|
|
8
|
Kudo S, Tamura S, Nakajima T, et al:
Diagnosis of colorectal tumorous lesions by magnifying endoscopy.
Gastrointest Endosc. 44:8–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salem SN and Truelove SC: Dissecting
microscope appearance of the gastric mucosa. Br Med J. 2:1503–1504.
1964. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakaki N, Iida Y, Okazaki Y, et al:
Magnifying endoscopic observation of the gastric mucosa,
particularly in patients with atrophic gastritis. Endoscopy.
10:269–274. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao K, Oishi T, Matsui T, et al: Novel
magnified endoscopic findings of microvascular architecture in
intramucosal gastric cancer. Gastrointest Endoscopy. 56:279–284.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakayoshi T, Tajiri H, Matsuda K, et al:
Magnifying endoscopy combined with narrow band imaging system for
early gastric cancer: correlation of vascular pattern with
histopathology (including video). Endoscopy. 36:1080–1084. 2004.
View Article : Google Scholar
|
|
13
|
Nagahama T, Yao K, Maki S, et al:
Advantage of magnifying endoscopy with narrow-band imaging (NBI)
over standard endoscopy for determining the margins of lateral
extent of early gastric cancer. Endoscopy. 42:A992010.
|
|
14
|
Oyama T, Tomori A, Ishii E, et al:
Histopathological diagnosis of gastric cancers by magnifying
endoscopy with NBI. Stomach Intestine. 46:943–955. 2010.
|
|
15
|
Otsuka Y, Niwa Y, Ohmiya N, et al:
Usefulness of magnifying endoscopy in the diagnosis of early
gastric cancer. Endoscopy. 36:165–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ono S, Kato M, Itoh T, et al: Diagnosis of
intestinal type gastric cancer with SM1 invasion by magnifying
endoscopic findings. Stomach Intestine. 42:99–109. 2007.
|
|
17
|
Takeuchi Y, Ishii H, Yao K, et al:
Histopathological diagnosis of gastric cancer using magnifying
endoscopy combined with the narrow band imaging - a proposal of
classification for morphological findings according to VS
classification. Stomach Intestine. 46:943–955. 2011.
|