Introduction
Many of the clinical parameters used for the initial
staging of patients with Hodgkin's lymphoma have a well-known,
partly independent prognostic value and are variably related to the
tumor burden (TB). This is the case of clinical stage, bulky mass,
number of involved regions, inguinal lymphadenopathy, number of
splenic nodules, and numerous serologic indicators, including
lactate dehydrogenase, β2-microglobulin, serum albumin, hemoglobin,
white blood cell count, peripheral lymphocyte count,
erythrosedimentation rate, and soluble CD30 and CD54 concentration
(1,2). All these factors can be individually
considered as vague and inaccurate surrogate indicators for TB.
However, in Hodgkin's lymphoma, as in several other tumors, a
direct measure of TB, even if only estimated, is invariably
prognostically superior to any other indicator. In some studies
conducted between 1986 and 1992 Specht et al (3,4)
devised and extensively applied an indirect technique for
estimating the TB. The method was semiquantitative, rather complex,
with some step of subjectivity, and relied partly on the evaluation
of abdominal lymphangiography, a radiologic examination that has
now been completely abandoned. Despite the potential inaccuracies,
TB measured in this way was demonstrated as the most important
prognostic factor and variably correlated with most of the clinical
parameters of the disease, which lost their independent predictive
power whenever TB was taken into account (4). The superiority of the TB over the
other single prognostic factors and composite prognostic scores was
confirmed by a more accurate assessment of TB through the
evaluation of the whole-body staging computed tomography (CT)
(5,6). This evaluation technique is direct,
quantitative, and more easily reproducible than that adopted by
Specht et al but it is not much simpler. It is probably for
this reason that in spite of its heuristic interest and clinical
advantages, with particular reference to the relationship
demonstrated with the efficacy of treatments (7), this technique has not been widely
applied.
Here we propose an easy, approximate estimate of TB,
which can be computed quickly with a pocket calculator from some
data of the patient's initial clinical evaluation, hoping that it
could be extensively applied in patients with Hodgkin's lymphoma,
whenever the direct measure seems to be unfeasible or too
complicated.
Patients and methods
Patients
Over the last 12 years we retrospectively measured
the relative tumor burden (rTB), the tumor burden normalized to
body surface area, of 507 patients with biopsy-proven, untreated
Hodgkin's lymphoma who entered some treatment protocols of the
Gruppo Italiano Studio Linfomi (GISL). Fifty-one patients with
early, favorable-stage disease were treated with combined VBM
chemotherapy (vinblastine, bleomycin and methotrexate) and
involved-field radiotherapy. One hundred and twenty-nine patients
with early unfavorable-stage disease were treated with a flexible
program including ABVD chemotherapy (doxorubicin, bleomycin,
vinblastine and dacarbazine) and involved-field radiotherapy. After
a block of three ABVD cycles these subjects underwent an early
restaging, then further received either one or three ABVD cycles
according to the recorded complete or partial response,
respectively. Another 327 patients with advanced–stage disease
entered two subsequent randomized trials in which an identical arm
with ABVD six cycles plus optional radiotherapy constituted the
reference standard treatment, which was administered to 117
patients. The experimental treatments included different
chemotherapy regimens, invariably administered in six cycles
followed by the same optional and limited radiotherapy as in the
ABVD arm. The regimens were six cycles of M(C)OPPEBVCAD
[mechlorethamine (or cyclophosphamide), vincristine, procarbazine,
prednisone, epidoxorubicin, bleomycin, vinblastine, lomustine,
melphalan and vindesine] in 103 patients and BEACOPP (bleomycin,
etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine
and prednisone) in 107. Details on staging, treatment and clinical
results of the trials that included the patients of the present
study were reported by Gobbi et al (8) for those with early favorable stages,
by Iannitto et al (9) for
the patients with early unfavorable stages, by Gobbi et al
(10) and Federico et al
(11) for patients with
advanced-stage disease.
Staging procedures were performed according to the
Cotswolds' Meeting (12)
recommendations. The criteria for inclusion of patients into the
present retrospective study were the following: availability of the
magnetic records of CT scans of the neck, thorax, abdomen and
pelvis performed before the start of treatment; availability of
information regarding presence of bulky mass, constitutional
symptoms, number of involved lymph nodes and extralymphatic
involvement; availability of a complete data set regarding
differential blood count [white blood cells (WBC), lymphocyte
(Ly)], hemoglobin concentration (Hb), eryhtrocyte sedimentation
rate (ESR), serum albumin (Alb), serum lactate dehydrogenase (LDH),
serum β2-microglobulin (β2-m); Karnofsky index; pattern of bone
marrow infiltration, if present (nodular or focal or diffuse);
patients' signed consent to the use of these radiologic and
clinical data. The International Prognostic Index (IPI) score
(13) was determined for each
patient. As indicated by Vassilakopoulos et al (14), the anatomical areas of involvement
that were considered for numbering were the following: Waldeyer's
ring; cervical and/or supraclavicular and/or occipital and/or
preauricular (right and left separately); axillary and/or
infraclavicular (right and left separately); epitrochlear (right
and left separately); mediastinal (one site); hilar (right and left
separately); paraortic (one site); coeliac and hepatic hilar nodes
(one site); mesenteric (one site); iliac and/or inguinal (right and
left separately); spleen and/or splenic hilar nodes (one site);
popliteal (right and left separately). Each extranodal site of
involvement was considered separately and added to nodal ones. Each
lung was considered as a separate site. Bone marrow was also
considered as one site of involvement even in the case of multiple
lesions. A mediastinal mass greater than one-third of the maximum
diameter of the chest at T5-T6 level and/or peripheral or
retroperitoneal lymphadenopathy >10 cm in the largest diameter
were considered bulky masses (12).
Tumor burden assessment
The technical procedures for assessing tumor burden
from CT scans have been detailed carefully elsewhere (5,6). The
CT imaging had to be performed before the start of the treatment,
had to cover the neck, thorax, abdomen and pelvis, and images had
to be taken with non-ionic contrast medium. The majority of CT
scans were saved on magnetic records and were re-evaluated by means
of either the software resources available in the CT equipment or
the Osirix® software for Apple computers. Radiologists,
who were always blind to any clinical information about the
patients, systematically outlined every lymphomatous lesion, nodal
or extranodal, in each scan slice. This allowed calculation of the
areas of the lesions which were present in any slice; from the
thickness of the slices the volume of the tumor per slice and,
finally, the sum of the volumes in all the slices. The volume of
bone marrow involved was calculated from the volume of
hemopoietically active tissue using the simple Wickramasinghe's
formula (15) (hemopoietic bone
marrow =20 ml/kg body weight) applied to the ideal body weight
according to Devine (16) [ideal
body weight = 50 kg (for males) or 43.5 kg (for females) + 2.3 kg
per inch (2.54 cm) of height >5 feet (152.4 cm)]. A variable
fraction of the so calculated total marrow volume was taken into
consideration for the addition to the whole tumor burden according
to the microscopic pattern of the lymphoid infiltration, either
diffuse (50%) focal (10%) or nodular (5%) (4). Finally, the obtained TB was normalized
to the body surface area and this relative tumor burden (rTB),
expressed in cm3/m2, was utilized throughout
the study with two aims. First, to relate the patient's metabolic
and immunologic functions and parameters to the proportion of TB
with the size of the host rather than to the absolute tumor load
and, second, to ensure comparability with the amount of
antineoplastic drugs (usually undergoing the same
normalization).
Statistics
A series of simple and multiple regression analyses
with the elementary staging parameters as predictive variables and
the measured rTB as the dependent variable were conducted to search
for a simple combination of clinical parameters that could estimate
the total tumor volume with enough accuracy. The R2, as
an index of predictive ability, and the inferential tests on the
partial regression coefficients, as a measure of correlation, were
the guides for selecting the best variables. The dummy values ‘0’
and ‘1’ were assigned according to the absence or presence,
respectively, of the nominal variables (bulky mass, ‘B’ symptoms,
extranodal involvement, bone marrow involvement, gender) (17). The final objective was to determine
the set of a few clinical variables with the highest predictive
value of the measured rTB.
The study population was subdivided into two
independent subsets of patients. We explored first the training
sample of 254 subjects, and comparatively evaluated the predictive
ability of the elementary staging parameters and selected the best
combination. In the second subset involving 253 patients, we
cross-validated the predictive accuracy of the selection made in
the first group. The patients were subdivided by assigning
alternate patients to the two groups following the chronological
order of recruitment into the study.
The failure-free survival was computed from the
beginning of treatment up until one of the following events:
disease progression during treatment, incomplete remission at the
end of treatment, relapse or death from the disease.
Results
Table I illustrates
the clinical characteristics of the patients studied and displays
that both the training and test sample were well-balanced.
 | Table IMain clinical characteristics of the
whole patient population, of the training subgroup and of the test
subgroup. |
Table I
Main clinical characteristics of the
whole patient population, of the training subgroup and of the test
subgroup.
| Whole population | Training
subgroup | Test subgroup |
|---|
| Number of
patients | 507 | 254 | 253 |
| Gender (M/F) | 253/254 | 131/123 | 122/131 |
| Age (year)a | 34.3±15.4 | 34.4±15.2 | 34.3±15.7 |
| Stage
(I/II/III/IV) | 55/274/105/73 | 28/130/56/40 | 27/144/49/33 |
| Histological
type |
| LP/LRHL/NS | 20/8/361 | 11/3/177 | 9/5/184 |
|
MC/LD/unclassifiable | 94/17/7 | 48/10/5 | 46/7/2 |
| ‘B’ symptoms | 210 | 100 | 110 |
| Bulky mass | 169 | 84 | 85 |
| Bone marrow
involvement | 30 | 17 | 13 |
| Extranodal
involvement | 161 | 90 | 71 |
| No. of involved
sites |
| ≤2/3–5 | 180/236 | 84/131 | 96/105 |
| 6–10/>10 | 86/5 | 37/2 | 49/3 |
| IPI score
(≤2/≥3) | 379/128 | 191/63 | 188/65 |
| ESR (mm/first
hour)a | 49.9±34.8 | 49.2±34.4 | 50.7±35.2 |
| Hb (g/dl)a | 12.4±1.8 | 12.4±1.8 | 12.4±1.9 |
| Albumin
(g/dl)a | 3.89±0.60 | 3.90±0.60 | 3.89±0.59 |
| LDH (U/ml)a | 382±188 | 382±170 | 382±200 |
| β2-microglobulin
(U/l)a | 2.28±1.53 | 2.40±1.94 | 2.17±1.12 |
| WBC count
(109/l)a | 10.5±4.2 | 10.3±4.4 | 10.8±4.0 |
| Lymphocyte count
(109/l)a | 1.7±1.2 | 1.8±1.5 | 1.8±1.0 |
The evaluation of the simple regressions towards the
measured rTB of the 20 staging parameters listed in Table II demonstrated that most of the
variables were statistically correlated with the rTB at a highly
significant level whereas their individual R2 is
generally low, apart from that of the IPI score, due to its
multiparametric characteristic. The data presented in Table II indicated that the possibility of
improving the prediction of rTB made with a single parameter by
combining a certain number of parameters would be worth
exploring.
 | Table IISimple regressions of each clinical
variable vs. the relative tumor burden (rTB) in the training
subgroup of 254 patients. |
Table II
Simple regressions of each clinical
variable vs. the relative tumor burden (rTB) in the training
subgroup of 254 patients.
| Coefficient | P-value | R2 |
|---|
| IPI score | 58.167 | <0.0001 | 0.378 |
| Bulky mass | 113.507 | <0.0001 | 0.186 |
| Extranodal
involvement | 81.875 | <0.0001 | 0.113 |
| Hb (g/dl) | −22.168 | <0.0001 | 0.107 |
| ESR (mm/1 h) | 1.116 | <0.0001 | 0.097 |
| No. of involved
sites | 16.361 | <0.0001 | 0.089 |
| ‘B’ symptoms | −74.431 | <0.0001 | 0.086 |
| Stage | 41.592 | <0.0001 | 0.082 |
| Karnofsky index | −3.101 | <0.0001 | 0.076 |
| Serum LDH (U/l) | 0.175 | <0.0001 | 0.069 |
| Serum albumin
(g/dl) | −49.699 | <0.0001 | 0.059 |
| WBC
(109/l) | 0.005 | 0.0002 | 0.026 |
| Serum
β2-microglobulin (μg/l) | 0.013 | 0.0102 | 0.025 |
| Bone marrow
involvement | 80.314 | 0.0006 | 0.023 |
| Age (years) | −1.138 | 0.0015 | 0.020 |
| Gender (M/F) | 25.663 | 0.0207 | 0.011 |
| Lymphocytes
(109/l) | −0.010 | 0.0254 | 0.010 |
| Histological
type | 11.311 | 0.0543 | 0.007 |
| Lymphocytes
(%) | −0.001 | 0.9508 | 0.000 |
A series of multiple regressions selected only the
following three staging variables strictly correlated with rTB: IPI
score, bulky mass and number of involved sites (a multiple
regression with these three variables had an R2 of
0.496). A careful evaluation of the relationship between these
three variables and rTB (Table
III) showed that the best regression of the IPI score with rTB
is not linear, but quadratic (R2=0.464 vs. 0.378).
Moreover, the compared analysis of the regression coefficients
showed that the presence of a bulky mass corresponds, on average
and as far as the prediction of rTB is concerned, to about three
additional involved sites besides those actually recorded.
 | Table IIIBivariate regressions with rTB of the
IPI and the IPI2 score, then of the bulky mass and of
the number of involved sites. |
Table III
Bivariate regressions with rTB of the
IPI and the IPI2 score, then of the bulky mass and of
the number of involved sites.
| Coefficient | SE | T-value | P-value |
|---|
| Bivariate
regression with rTB of IPI and IPI2 |
| Intercept | 66.524 | 9.964 | 6.676 | <0.0001 |
| IPI | 18.129 | 8.868 | 2.044 | 0.0414 |
|
IPI2 | 8.508 | 1.753 | 4.853 | <0.0001 |
| Bivariate
regression with rTB of bulky mass and the no. of involved
sites |
| Intercept | 6.882 | 7.141 | 0.964 | 0.3356 |
| Bulky mass | 94.558 | 7.537 | 12.547 | <0.0001 |
| No. involved
sites | 28.147 | 1.584 | 17.764 | <0.0001 |
The multiple regression with these two transformed
variables, the squared IPI and the number of involved sites
augmented by three in case of bulky mass, reached an R2
of 0.673 in the training sample and 0.661 in the test sample. The
following equation, [−4.3 + 8.3 × IPI2 + 22.7 × no. of
involved sites (+3 if bulky mass is present)], drawn from the
multiple regressions, was used to calculate an estimated rTB
(Es-rTB) in all cases. This, in turn, showed a highly significant
correlation with the measured rTB, with an R2 of 0.878
in the training sample (95% confidence interval: 0.827–0.919) and
0.844 in the test sample (95% confidence interval: 0.790–0.898).
The accuracy of the Es-rTB derived in this way seemed to be
acceptable, since the error of the estimate is lower than 50% of
the mean value of rTB (143.4 cm3/m2) in 81%
of the cases of the training sample and in 77% of those of the test
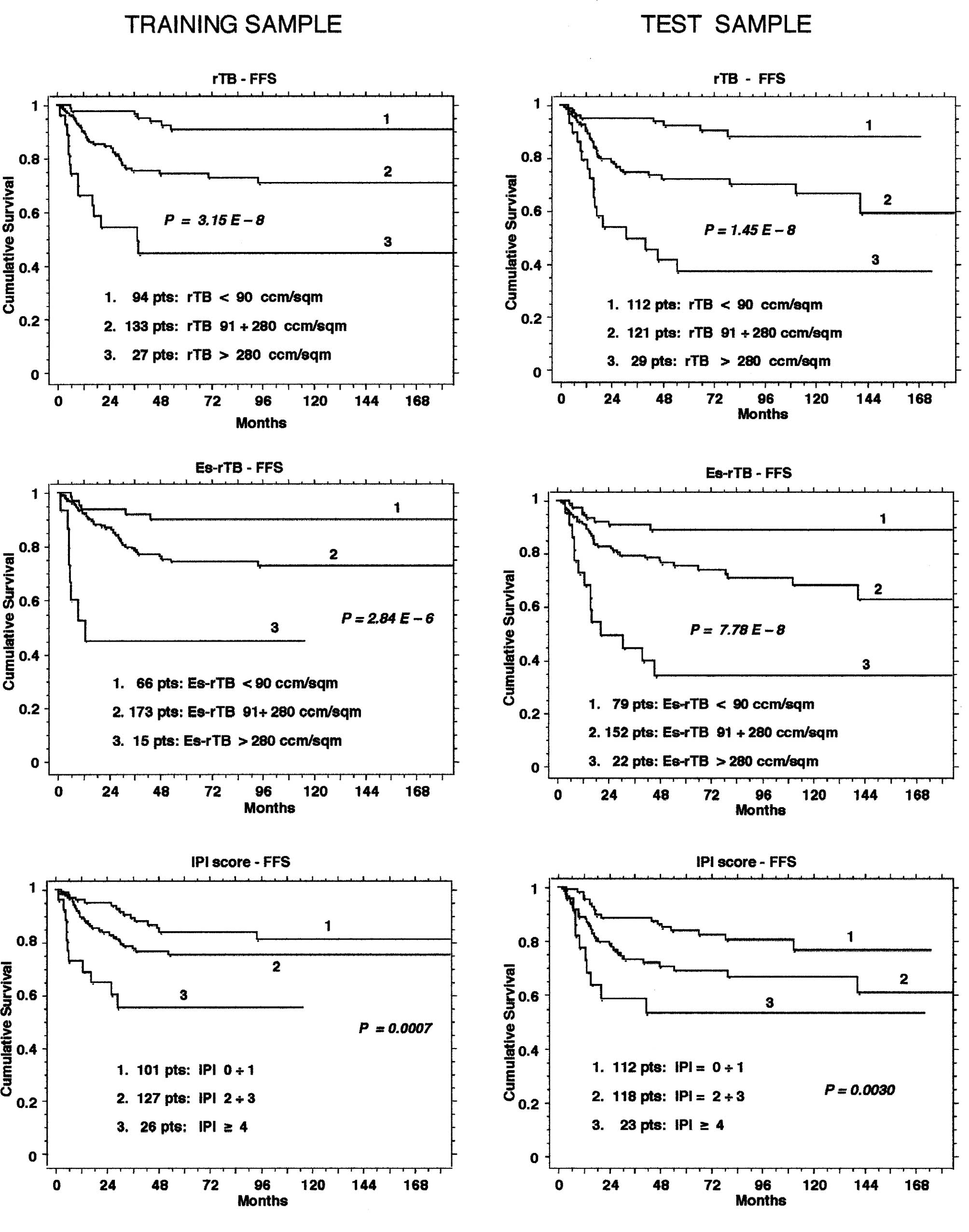
sample. Most convincingly, the Es-rTB retained a better prognostic
discrimination than the IPI score alone in terms of failure-free
survival both in the training sample and in the test sample
(Fig. 1). This result shows that
the Es-rTB can be an easy prognostic tool for Hodgkin's lymphoma,
one that is much simpler than the measured rTB, and sufficiently
accurate and reliable for clinical purposes.
Discussion
If the total tumor burden is considered as the whole
amount of the truly malignant cells (the Reed-Sternberg and the
Hodgkin cells) this should correspond to a small component of the
pathologic tissue of Hodgkin's lymphoma. This is mostly represented
by a variable mixture of reactive, non-neoplastic small
lymphocytes, eosinophils, neutrophils, histiocytes and plasma
cells, and precisely for this reason it would appear that the
quantification of the purely neoplastic component might be more
interesting and useful than evaluations including the reactive
component.
However, although it seems unquestionable that the
neoplastic cells are the primary determinants of the biological and
clinical alterations, both local and systemic, ultimately
conditioning the prognosis, quantification of the purely neoplastic
cells, when performed, did not clearly improve predictive ability
(2) over computation of the whole
cell population. This might mean that the variable intensity of the
biological activity of the tumor cells is more important than their
absolute number, and that their cytokine production and consequent
immunologic interactions are crucial for the recruitment of a
variably abundant mass of reactive non-neoplastic cells. These
constitute a larger proportion of the volume of the clinically
evident lesions compared to the neoplastic elements by which they
are directly or indirectly stimulated, and the biological activity
of which they reflect. Thus, it seems reasonable that, for clinical
and prognostic purposes, it may be less important to assess the
neoplastic component separately from the reactive one. In fact, in
clinical practice therapeutic decisions are based on the whole size
of the lesions, as evaluated by palpation, ultrasonography, CT and
positron emission tomography. These techniques do not discriminate
between neoplastic and reactive cell populations.
Some comments are needed to clarify the aspects of
the equation used to estimate the rTB. It is essentially based on
two parameters, the number of involved sites, which can also
incorporate information on the presence of bulky mass, and the IPI
score. The number of involved sites was pointed out as a partial
surrogate parameter of tumor burden by Vassilakopoulos et al
(14) and its importance was
partially confirmed by our results. Specifically, in the prognostic
comparison with the measured rTB performed in a previous analysis
(4), the number of involved sites
was found to be the second best factor related to the
time-to-treatment failure and to disease-free survival and the
third best factor related to less than complete response or relapse
within 12 months of the end of the treatment. The definition of the
anatomic regions for the number of involved sites as suggested by
Vassilakopoulos et al (14)
has been demonstrated to be prognostically valid. In this study the
average prognostic correspondence of bulky mass to about three
further involved sites besides those actually recorded is an
indirect confirmation of its value.
The paramount predictive power of the IPI score
related to the rTB is likely due to the score's integration of
seven elementary prognostic parameter (serum albumin <4 g/dl,
hemoglobin <10.5 g/dl, male gender, stage IV, age ≥45 yr, white
cell count ≥15,000/μl and lymphocyte count <600/μl or <8%),
nearly all of which are independently related to the rTB (see
Table II). The inclusion of the
IPI score in the equation was fully expected. However, its
quadratic regression with the measured rTB was unexpected and
indicates that the presence of any additional parameter within the
IPI score is supported by an additional, rather than constant,
increase of the amount of tumor. This provides further evidence of
the relationship between the activity of the neoplastic component
and the effects of its cytokine production, both locally, with
recruitment of reactive cells, and systemically, with functional
depression of liver and bone marrow (as reflected by many of the
IPI variables).
Despite the elevated R2 the equation has
a notable estimate error, which cannot be further reduced by other
available pre-therapeutic parameters. Nevertheless, the estimated
rTB retained a significant prognostic advantage over the IPI score.
Moreover, as recently pointed out, the estimated rTB could help to
identify the possibility of response to a given treatment in
relation to the initial rTB (5).
Thus, the indirect estimate of rTB can be proposed for
investigational and clinical use when a direct measurement cannot
be performed, at least until a new, simplified technique for the
direct assessment of rTB becomes available.
Acknowledgements
This study was supported in part by grants from the
Fondazione IRCCS Policlinico S. Matteo, Pavia, and from the
‘Ferrata-Storti Foundation’, Pavia. We are indebted to Dr Rachel
Stenner for her careful assistance with English language editing of
this manuscript.
References
|
1
|
Specht L: Prognostic factors in Hodgkin's
disease. Dan Med Bull. 39:409–422. 1992.
|
|
2
|
Josting A: Prognostic factors in Hodgkin
lymphoma. Expert Rev Hematol. 3:583–592. 2010. View Article : Google Scholar
|
|
3
|
Specht L and Nissen NI: Prognostic
significance of tumor burden in Hodgkin's disease PS I and II.
Scand J Haematol. 36:367–375. 1986. View Article : Google Scholar
|
|
4
|
Specht L: Tumor burden as the main
indicator of prognosis in Hodgkin's disease. Eur J Cancer.
28:1982–1985. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gobbi PG, Ghirardelli ML, Solcia M, Di
Giulio G, Merli F, Tavecchia L, Bertè R, Davini O, Levis A, Broglia
C, et al: Image-aided estimate of tumor burden in Hodgkin's
disease: evidence of its primary prognostic importance. J Clin
Oncol. 19:1388–1394. 2001.PubMed/NCBI
|
|
6
|
Gobbi PG, Broglia C, Di Giulio G, Mantelli
M, Anselmo P, Merli F, Zinzani PL, Rossi G, Callea V, Iannitto E,
et al: The clinical value of tumor burden at diagnosis in Hodgkin
lymphoma. Cancer. 101:1824–1834. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gobbi PG, Valentino F, Bassi E, Coriani C,
Merli F, Bonfante V, Marchianò A, Gallamini A, Bolis S, Stelitano
C, et al: Chemoresistance as a function of the pretherapy tumor
burden and the chemotherapy regimen administered: differences
observed with two current chemotherapy regimens for advanced
Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 5:396–402. 2011.
View Article : Google Scholar
|
|
8
|
Gobbi PG, Broglia C, Merli F, Dell’Olio M,
Stelitano C, Iannitto E, Federico M, Bertè R, Luisi D, Molica S,
Cavalli C, Dezza L and Ascari E: Vinblastine, bleomycin and
methotrexate chemotherapy plus irradiation for patients with
early-stage, favorable Hodgkin's lymphoma: the experience of the
Gruppo Italiano Studio Linfomi (GISL). Cancer. 98:2393–2401. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iannitto E, Minardi V, Gobbi PG, Calvaruso
G, Tripodo C, Marcheselli L, Luminari S, Merli F, Baldini L,
Stelitano C, et al: Response-guided ABVD chemotherapy plus
involved-field radiation therapy for intermediate-stage Hodgkin
lymphoma in the pre-positron emission tomography era: a Gruppo
Italiano Studio Linfomi (GISL) prospective trial. Clin Lymphoma
Myeloma. 9:138–144. 2009. View Article : Google Scholar
|
|
10
|
Gobbi PG, Levis A, Chisesi T, Broglia C,
Vitolo U, Stelitano C, Pavone V, Cavanna L, Santini G, Merli F, et
al: ABVD versus modified Stanford V versus MOPPEBVCAD with optional
and limited radiotherapy in intermediate- and advanced-stage
Hodgkin's lymphoma: final results of a multicenter randomized trial
by the Intergruppo Italiano Linfomi. J Clin Oncol. 23:9198–9207.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Federico M, Luminari S, Iannitto E,
Polimeno G, Marcheselli L, Montanini A, La Sala A, Merli F,
Stelitano C, Pozzi S, et al: ABVD compared with BEACOPP compared
with CEC for the initial treatment of patients with advanced
Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo
Studio dei Linfomi Trial. J Clin Oncol. 27:805–811. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lister TA, Crowther D, Sutcliffe SB,
Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA and
Tubiana M: Report of a committee convened to discuss the evaluation
and staging of patients with Hodgkin's disease: Cotswolds meeting.
J Clin Oncol. 7:1630–1636. 1989.PubMed/NCBI
|
|
13
|
Hasenclever D and Diehl V: A prognostic
score for advanced Hodgkin's disease. International Prognostic
Factors Project on Advanced Hodgkin's Disease. N Engl J Med.
339:1506–1514. 1998. View Article : Google Scholar
|
|
14
|
Vassilakopoulos TP, Angelopoulou MK,
Siakantaris MP, Kontopidou FN, Dimopoulou MN, Barbounis A,
Grigorakis V, Karkantaris C, Anargyrou K, Chatziioannou M, et al:
Prognostic factors in advanced stage Hodgkin’s lymphoma: the
significance of the number of involved anatomic sites. Eur J
Haematol. 67:279–288. 2001.
|
|
15
|
Wickramasinghe SN: Human Bone Marrow.
Blackwell Scientific Publications; Oxford: pp. 211–216. 1976
|
|
16
|
Devine BJ: Gentamicin therapy. Drug Intell
Clin Pharm. 8:650–655. 1974.
|
|
17
|
Armitage P and Berry G: Statistical
Methods in Medical Research. 2nd edition. Blackwell Scientific
Publication; Oxford: pp. 369–396. 1987
|