Introduction
Colorectal cancer (CRC) is the second most common
cause of cancer related death in the Western societies (1). At the time of diagnosis 19% of
patients will present synchronous metastasis and 30–40% of patients
will develop distant metachronous metastasis (2). The liver is one of the most common
sites of metastasis and many molecular variables are still unclear.
Some studies have suggested that the accumulation of specific
alterations in cell growth genes are involved in cancer progression
(3) and tumorigenesis (4). Thus, different gene expression can
lead to unique transcriptional outcomes and consequently a multiple
signalling pathways can be simultaneously activated. NHERF1
(Na+/H+ exchanger regulatory factor 1, also
named EBP50) is an adaptor protein that links several cellular
receptors, ion transporters and other proteins to the plasma
membrane of different types of cells (5–9). The
association between specific growth factor receptors and NHERF1 has
already been analysed (8–10) and indicates a critical role for this
adaptor protein in growth factor signal transduction. Recently in
normal cells, the level of NHERF1 expression has been demonstrated
to have effects on the trafficking, expression and function of the
epidermal growth factor receptor (EGFR) (11). NHERF1 is a member of a family of
scaffold proteins with two homologous PSD-95/Disc-large/ZO-1 (PDZ)
domains, which mediate protein-protein interactions (12) and an ERM (Ezrin/Radixin/Moesin)
domain, with which it attaches to the cytoskeleton (13,14).
The PDZ1 domain interacts with the carboxyl-terminus of proteins
(15), while the ERM domain binds
to respective actin-associated ERM proteins (5). Proteins with PDZ domains are also
present at the brush border of mammalian, intestine and renal
proximal tubules (16,17). The loss of heterozygosity (LOH) at
the NHERF1 gene locus (17q25.1) is present in more
than 50% of human breast tumors (18); whereas the LOH is less frequent in
other tumor types, suggesting a pivotal role of NHERF1 during
breast carcinogenesis (18). The
NHERF1 protein is expressed in the luminal membrane of many
epithelia and has been studied in many tumor types such as
hepatocellular carcinoma (19)
schwannoma (20) and particularly
in breast cancer (21–23). Our previous studies showed that
NHERF1 plays a role in breast cancer progression and it is able to
induce an invasive phenotype in an ‘in vitro’ model of
breast cancer (24–26). Moreover, we showed that breast
carcinogenesis is characterized by increased cytoplasmic expression
of NHERF1 as the tumor progresses. The switch from apical membrane
to cytoplasmic expression is compatible with a dual role for NHERF1
as a tumor suppressor or tumor promoter dependent on its
subcellular localization (10).
Little is known about the involvement of NHERF1 in colorectal
cancer. A recent study reported that NHERF1 alterations correlate
with the progression and enhanced invasiveness of human colorectal
cancer and that its loss or cytoplasmic overexpression is a common
oncogenic event in carcinomas (26).
The aim of this study is to further clarify the role
of NHERF1 in colorectal carcinogenesis and progression, examining
the expression and the intracellular distribution of the protein in
non-neoplastic tissues, adenomas, primary tumors and metastatic
colorectal sites.
Materials and methods
Tissue samples
Formalin-fixed, paraffin-embedded sections from 51
metastatic colorectal carcinomas (mCRCs) were selected from the
files of the Department of Pathology of our Institute. For each
tumor the following histological sections were examined: primary
tumor (T) and surrounding non-neoplastic tissue (SNT) present on
the same section, the corresponding synchronous lymph node (LnM)
and liver metastases (LM). Distant non-neoplastic colorectal tissue
(DNT) and adenomas (ADNs) obtained from 20 of the 51 tumors were
also analyzed. The seventy five percent (15/20) of ADNs, measured
<1 cm, showed a high grade, while 41% (11/20) of ADNs showed
villousness >20%.
Histological sections were cut and stained with
hematoxylin and eosin for light microscopic examination. Ethical
approval of this study has been obtained from the Ethics Committee
of our Institute. The pathological features of patients with these
colorectal tumors were analysed by reviewing the histological
sections of the surgical specimen. The colorectal tumors were
graded and classified according to the World Health Organization
(WHO) criteria (27).
The clinicopathological characteristics of patients
are summarized in Table I.
Thirty-one patients were male and 20 female with a mean age of 63
years (range, 44–85). Of all adenocarcinomas, 12 (24%) cases showed
low grade of differentiation and 39 (76%) showed high grade of
differentiation. Pathological staging was T3 in 67% (n=34) of cases
and T4 in 33% (n=17) of cases; lymph node status was N1 in 28%
(n=14) and N2/N3 in 72% (n=37) of cases.
 | Table IClinicopathological characteristics of
51 mCRC patients. |
Table I
Clinicopathological characteristics of
51 mCRC patients.
| Characteristics | No. of patients
(%) |
|---|
| Age (years), median
(range) | 63 (44–85) |
| ≤63 | 26 (51) |
| >63 | 25 (49) |
| Gender |
| Male | 31 (61) |
| Female | 20 (39) |
| Adenocarcinoma | 51 (100) |
| Grade of
differentiation |
| Low | 12 (24) |
| High | 39 (76) |
| Pathological
staging (pTNM) |
| Tumor |
| T3 | 34 (67) |
| T4 | 17 (33) |
| Node |
| N1 | 14 (28) |
| N2, N3 | 37 (72) |
| Metastasis |
| M1 | 51 (100) |
Immunohistochemistry
Tissue sections (4-μm thick) were deparaffinized
with xylene, rehydrated in graded ethanol solutions, and in order
to enhance antigen retrieval, the slides were then immersed in 10
mM sodium citrate buffer (pH 6.0), boiled for 30 min on a hot
plate, and then allowed to cool for 20 min. Sections were incubated
for 10 min in 3% hydrogen peroxide in distilled water, washed in
PBS three times for 5 min. After, the sections were incubated
overnight at 4°C with rabbit polyclonal anti-human EBP50 antibody
(PA1-090 Affinity Bioreagents, Golden, CO; dilution 1:150).
Sections were then washed with PBS, incubated with biotinylated
link for 30 min, peroxidase-labelled streptavidin for 30 min, and
3-amino-9-ethylcarbazole substrate-chromogen (LSAB2 System-HRP;
DakoCytomation) for 15 min at dark. Counterstaining was done with
hematoxylin. Immunohistochemical staining was evaluated as
subcellular localization and classified as prevalently cytoplasmic,
membrane or nuclear localization for each sample (10).
The extent and pattern of staining also varied among
different tissues. NHERF1 expression was quantified by two
independent observers by counting the positive cells in 3
representative areas for each section, recorded as percentage of
stained cells/section and, the median value was used to form a
final score. According to the median value cut-off, the cases were
considered positive for cytoplasmic NHERF1 when immunoreactivity
was present in >70% of tumor cells examined, and positive for
nuclear NHERF1 when completely and darkly nuclear staining was
observed in 18% of tumor cells analysed. EGFR immunohistochemistry
was performed according to the instructions included with the EGFR
PharmDx kit (Dako Corp., Milan, Italy). For our study, 4-μm
sections were deparaffinized in 2 sequential xylene baths, 100%
ethanol, and 95% ethanol followed by a wash in wash-buffer solution
(Dako). The rehydrated sections were pretreated in an enzyme
solution (Proteinase-k) at room temperature for 5 min. After the
block of endogenous peroxidase activity, the sections were
incubated with EGFR MoAb (IgG1, clone 2-18C9, Dako) for 30 min,
employing 3′3-diaminobenzidine as a chromogenic substrate. Sections
were slightly counterstained with Mayer’s hematoxylin and mounted
in aqueous mounting medium (Glicergel, Dako). In each staining run,
an external positive control section consisting of Dako control
slide was used, and the negative control was performed by omitting
the application of the primary antibody.
Immunoistochemical staining was evaluated by one of
the authors (A.M.) together with an experienced pathologist (G.S.),
and then discordant cases were reviewed in a joint evaluation. IHC
scoring was based on the membrane immunoreactivity, according to
the American Joint Committee (28):
score 0, no reactivity; score 1+, weak reactivity; score 2+,
moderate reactivity; score 3+, strong reactivity. Cytoplasmic
staining was considered non-specific and was not included in the
scoring. The tumors overexpressing EGFR were scored mainly 3+
(n=11), 2+ (n=20) and 1+ (n=6); those not overexpressing EGFR were
scored 0 (n=14). One of 2 LnM and 2/3 LM were scored 2+, while
those with negative EGFR were 1 LnM and 1 LM.
Immunofluorescence of NHERF1/EGFR
Colocalization of membrane NHERF1 and EGFR was
performed on 18 tumor specimens [13 showed moderate EGFR reactivity
(2+) and 5 showed strong reactivity (3+)], 1 metastatic lymph
nodes, and 2 liver metastases assessed immunohistochemically.
Immunofluorescence analyses were done as previously described
(25). Briefly, serial sections of
formalin-fixed tissue sections embedded in paraffin wax, 3-μm
thickness, were obtained from the same colorectal tissue blocks
used for immunohistochemistry. Rehydrated slides were immersed in a
10 mM citrated buffer, pH 6.0, at 95°C for 30 min for antigen
retrieval and, prior to incubation with primary antibody, were
treated for 10 min with 0.2% bovine serum albumin (BSA) to block
non-specific protein binding. Primary antibodies were: mouse IgG
anti-EGFR (clone 13/EGFR; 1:100 dilution) (BD Transduction
Laboratories™) and rabbit IgG NHERF1 (PA1-090; Affinity
Bioreagents) (1:150 dilution). After, sections were incubated for 1
h with the Alexa Fluor 488 goat anti-mouse IgG1 and Alexa Fluor 568
goat anti-rabbit IgG secondary antibody conjugates (Molecular
Probes, Eugene, OR, USA) (1:1000 dilution).
Images were acquired on a BX40 microscope (Olympus)
with a SenSys 1401E-Photometrics charge-coupled device camera. Each
fluorophore used was excited independently and sequential detection
was performed and co-localization analysis was performed using the
open source Image J version 1.38 (http://rsb.info.nih.gov/ij/).
Statistical analysis
The two-tailed non-parametric Kruskal-Wallis and
Mann-Whitney tests were used to compare the different expression
levels of NHERF1 among different colorectal tissues. The
association between NHERF1 and clinicopathological variables, and
the correlation between membrane NHERF1 and EGFR were examined
using χ2 test, and χ2 test for trend as
appropriate. Statistical significance was calculated for a 95%
confidence interval (P<0.05). Calculations were performed using
Prism version 5.00 software package (GraphPad Software, San Diego,
CA, USA).
Results
NHERF1 protein expression in metastatic
colorectal cancer tissues
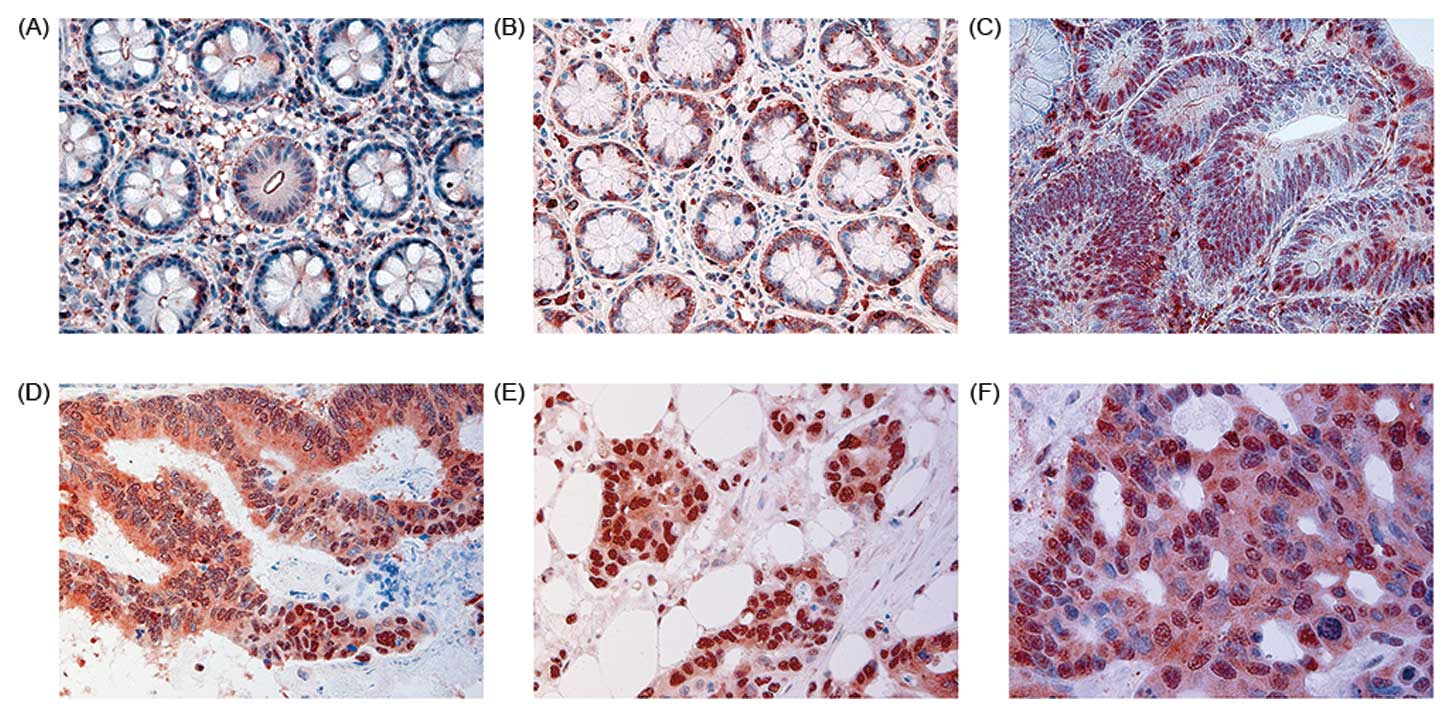
The DNT showed NHERF1 mostly expressed at the apical
membrane (Fig. 1A). In those
samples, median apical membrane expression was 15.0% (0–55 range of
positive cells), whereas the median cytoplasmic staining was 5%
(0–60). In SNT, present on the same slide of the tumor, decreased
apical membrane and increased cytoplasmic immunoreactivity of
NHERF1 were observed (Fig. 1B).
Apical membrane staining observed in those samples was 11.0%
(0–48), while cytoplasmic staining was 10% (0–60).
In ADN, a different localization of NHERF1 was
observed in both high and low grade ADNs (Fig. 1C). Median value of apical membrane
expression was 2% (0–40 range of positive cells), while median
value of cytoplasmic staining was 57.5% (40–70). In T, NHERF1
expression showed a cytoplasmic trend similar to ADN (Fig. 1D). The median value of cytoplasmic
staining was 60% (20–80), whereas the median value of membrane
staining was 5% (0–60). In synchronous LnM (Fig. 1E) and LM (Fig. 1F), results similar to T were
observed. LnM showed 60% (5–80) of cytoplasmic staining and 0%
(0–10) of membrane staining. Likewise, LM exhibited 70% (20–80) of
cytoplasmic staining and 0% (0–20) membrane immunoreactivity.
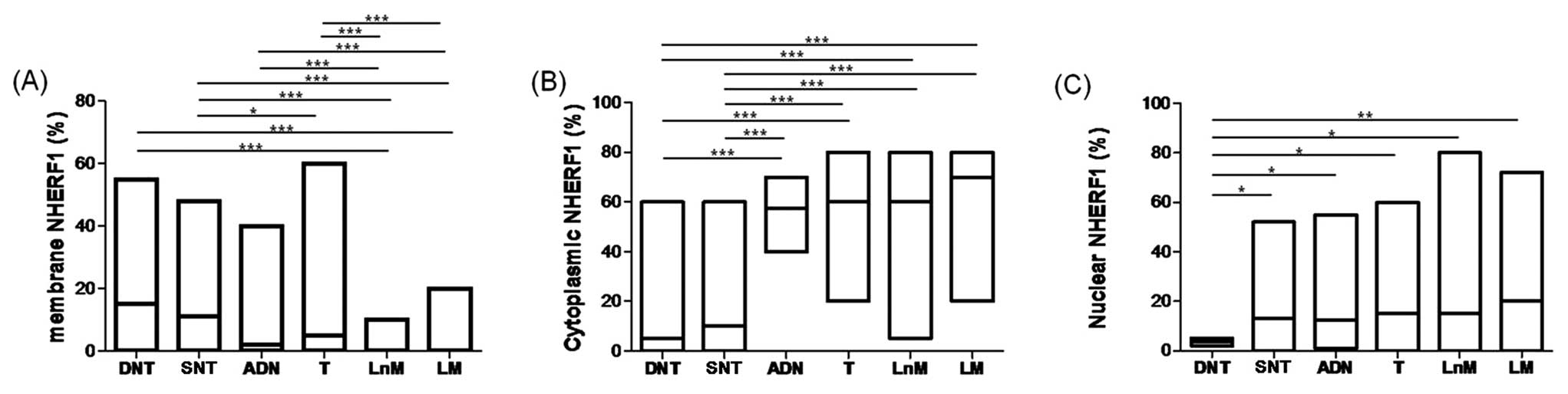
As showed in Fig.
2A, membrane NHERF1 expression detected in LnM and LM showed a
significant decrease with respect to DNT (P<0.0001). Moreover,
membrane NHERF1 in T, LnM and LM decreased significantly compared
to SNT (P<0.0001). Finally, LnM and LM showed a decrease in
membrane expression with respect to both ADN and T
(P<0.0001).
The cytoplasmic staining of NHERF1 increased
significantly comparing the DNT to ADN, T, LnM and LM,
(P<0.0001, for all). Moreover, in ADN, T, LnM and LM the
immunoreactivity was more intense than observed in SNT
(P<0.0001, for all) (Fig.
2B).
Nuclear NHERF1 expression was present in 80%
(154/193) of samples examined (Fig.
1A-F). We observed high and homogeneous nuclear expression in
SNT, ADN, T, LnM and LM, with respect to DNT. Interestingly, DNT
(3.5%, range 2–5%) showed a significantly lower nuclear expression
than detected in NT (13.0%, range 0–52), ADN (12.5%, range (1–55),
T (15.0%, range (0–60), and LnM (15.0%, range 0–80) (P<0.05).
However, the nuclear staining was more intense in LM (20.0%, range
0–72) than in DNT (3.5%, range 2–5) (P<0.001) (Fig. 2C). On the basis of a contingency
analysis of 51 mCRC cases, any statistically significant
association between NHERF1 expression within the three compartments
and clinicopathological characteristics was revealed (Table I).
Subcellular colocalization of NHERF1 with
EGFR in mCRC tissues
Of the tumors 45% (23/51) retained a low amount of
NHERF1 in the membrane compartment, and 78% (18/23) of these cases
showed overexpression of EGFR (score 2+/3+). On univariate
analysis, positive membrane NHERF1 expression was statistically
significantly associated with moderate EGFR immunostaining (score
2+) (13/23 vs. 7/28, P=0.035). To analyze the interaction between
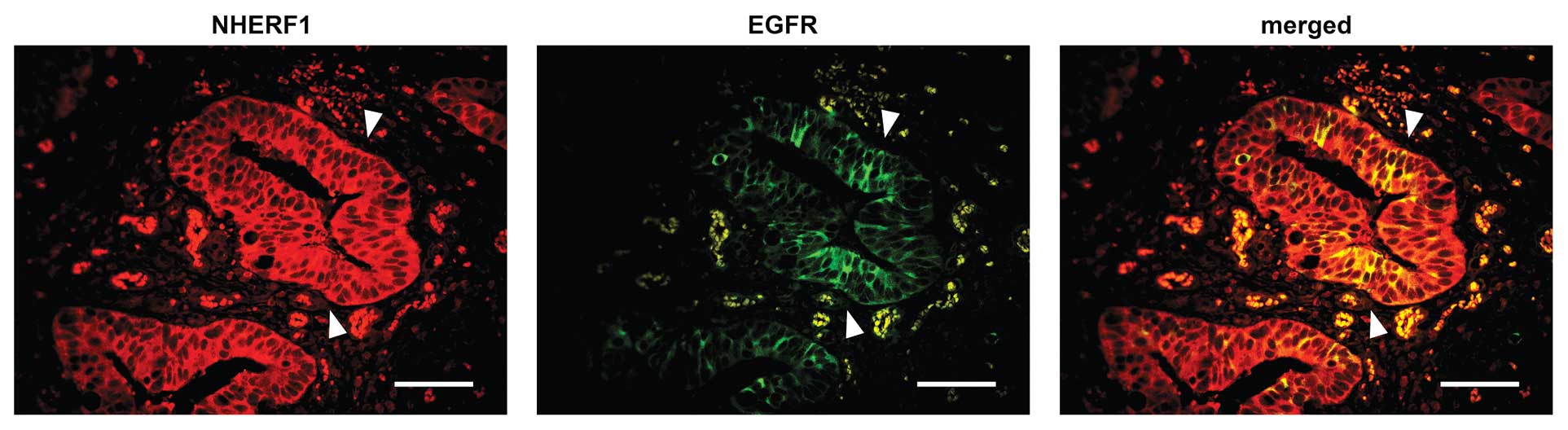
NHERF1 and EGFR, we examined their localization by
immunofluorescence on tumor specimens, lymph node and liver
metastatic tissues. In primary tumor and metastatic cells, NHERF1
showed its mostly cytoplasmic localization, together with large
areas of NHERF1 nuclear localization, especially where cells became
no longer polarized (Fig. 3).
Noteworthy, in 45% (23/51) of tumor cases, 4% (2/51) of lymph node
and 5% (3/51) of liver metastases, a low amount of NHERF1 was still
localized in the membrane. At membrane compartment, NHERF1
colocalized with EGFR only when this receptor was overexpressed and
showed strong or moderate reactivity (score 2+/3+) (Fig. 3); in the cases with no EGFR
reactivity (score 0), no membrane NHERF1 expression was
observed.
Discussion
NHERF1 is an adaptor protein frequently expressed in
different human cancers. It is associated with β-catenin through
its PDZ2 domain (19) and
consequently involved in cancer progression.
In this study, we focused on the observation of
expression and subcellular localization of NHERF1 to evaluate its
involvement in different sites of metastatic colorectal cancer.
Differently to a previous study (26), here we assessed a more wide and
homogeneous series of human CRC and metastatic sites composed of
non-neoplastic tissue, adenoma, primary tumor, and synchronous
lymph node and liver metastases. Our results confirm the presence
of NHERF1 at the apical membrane of epithelial cells in DNTs
(10,23). However, biological difference of
NHERF1 expression among different tissues is present and can be
associated at the adenoma-carcinoma sequence (26). The SNT, present on the same section
of the tumor area, showed a persistence of apical membrane NHERF1
expression, more intense than in tumor and metastatic sites.
However, here we observed the increasing of cytoplasmic and nuclear
staining, demonstrating the rapid biochemical changes respect to
morphological evidence during the tumor development. This would
suggest a strong influence of the tumor on its SNT, and the loss of
membrane NHERF1 expression could be compatible with onset of an
aggressive phenotype (26). Results
obtained by this study indicate that NHERF1 represents an early
marker of pre-morphological triggering of carcinogenesis.
Even if the analyzed ADNs were few and not evenly
distributed, in both low and high grade adenomas, we already
observed a shift from apical membrane to cytoplasmic and nuclear
expression, suggesting the involvement of NHERF1 in first steps of
the pre-malignant lesions. In fact, the increase of cytoplasmic and
nuclear NHERF1 expression suggests a possible direct interaction
between NHERF1 and β-catenin, setting up Wnt/β-catenin pathway and
consequently the cancer progression (19). Comparing the SNT with other tumor
sites we observed a greater percentage of cytoplasmic staining in
the T, and in both LnM and LM. This change in cytoplasmic protein
expression has been described previously by Hayashi et al
regarding adenomas and ‘in vitro’ models (26). In NHERF1-depleted cells, the
ectopically expressed wt-NHERF1 was distributed in the cytoplasm
with a concomitant reduction in epithelial morphology and increased
cell proliferation. These results support our previous study that
described a significant change in subcellular distribution of
NHERF1 moving from breast non-neoplastic tissue to carcinoma
(10). Consequently, the elevated
cell proliferation in tumor area could be a consequence of higher
cytoplasmic NHERF1 expression in tumor compared to SNT. This would
rely on the direct interaction between NHERF1 and β-catenin, with
further activation of the Wnt/β-catenin pathway, as previously
demonstrated (26).
Furthermore, nuclear NHERF1 expression was reported
previously in hepatocellular carcinoma (19) and in breast cancer (10). Shibata et al underlined that
NHERF1 interacts with β-catenin through its PDZ2 domain,
establishing a complex formation in hepatocellular carcinoma model
(19). In the current study we
found nuclear NHERF1 expression in 80% of the colon samples
analyzed and surprisingly, nuclear expression appeared already in
SNT and ADN lesions, but not in DNT samples. Our findings indicated
that there are significant changes in the expression of the nuclear
NHERF1 in colorectal adenocarcinoma and adenoma in comparison to
non-neoplastic tissue. This implies that nuclear NHERF1 is involved
in the pathogenesis of colorectal tumors, opening a new
undiscovered and challenging role for NHERF1 as a player in
carcinoma progression. Nuclear NHERF1 could collaborate at
initiation and maintaining the tumor phenotype, possibly by
associating with β-catenin. Based on these findings and those of
other studies, NHERF1 would act as a tumor suppressor when
localized at the apical level of the membrane and as an oncogenic
protein when localized in the cytoplasm and nucleus (10,29,30).
Moreover, as a scaffolding protein, NHERF1 is
associated with a number of growth factor TK receptors, such as the
Her2/neu (10) and the EGFR
(31), promoting dimerization and
activation of mitogenic signals. The downregulation in metastases
has recently been indicated also in other previously published
studies (32–34). EGFR overexpression has been found to
be associated with tumor progression and poor survival in several
tumor types, such as in CRC (35),
and importantly, our results underlined the involvement of NHERF1
along with EGFR in the CRC progression.
Previously, it has been reported that NHERF1 and
EGFR colocalized at the cell membrane of colon cancer tissues
(30). In this study, we observed
that in a small percentage of tumors NHERF1 maintained a membrane
localization and a positive direct correlation between membrane
NHERF1 and EGFR expression was evidenced by their colocalization.
The CRC cell architecture is completely overturned and cells are
able to receive different extracellular signals from the tumor
microenvironment; in this pathological state NHERF1, having lost
its exclusively apical domain function, is overexpressed in the
cytoplasm and nucleus and starts to coordinate intracellular cancer
pathways. These data reiterate that NHERF1 alterations correlate
with progression and enhanced invasiveness of human colorectal
cancer.
In conclusion, nuclear NHERF1 expression showed a
heterogeneous distribution in the different colon sites, from
non-neoplastic tissue to carcinoma and metastases. It confirms a
dynamic role of the protein in colorectal cancer, not only as
physiological scaffolding protein. In particular, the nuclear
NHERF1 expression, present in the early stages of carcinogenesis,
probably contributes to onset of the malignant phenotype. Thus, we
hypothesize the direct involvement of nuclear NHERF1 in both
carcinogenesis and progression of colorectal cancer.
Acknowledgements
A.A. would like to thank Rossana Daprile for
technical help. S.J.R. would like to thank the Italian Association
for Cancer Research (AIRC) grant no. 5167 and the PIO grant no. 3
for supporting this study.
Abbreviations:
|
NHERF1
|
Na+/H+ exchanger
regulatory factor 1
|
|
mCRC
|
metastatic colorectal cancer
|
|
SNT
|
surrounding non- neoplastic tissue
|
|
DNT
|
distant non-neoplastic tissue
|
|
ADN
|
adenoma
|
|
T
|
primary tumor
|
|
LnM
|
synchronous lymph node metastasis
|
|
LM
|
liver metastasis
|
|
EGFR
|
epidermal growth factor receptor
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics. CA Cancer J Clin.
58:71–96. 2008.
|
|
2
|
Horner MJ, Ries LAG, Krapcho M, et al:
SEER cancer statistics review, 1975–2006. Bethesda, MD: National
Cancer Institute. http://seercancergov/csr/1975-2006/.
2009
|
|
3
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 1:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sjöblom T, Jones S, Wood LD, et al: The
consensus coding sequences of human breast and colorectal cancers.
Science. 13:268–274. 2006.
|
|
5
|
Bretscher A, Chambers D, Nguyen R and
Reczek D: ERM-Merlin and EBP50 protein families in plasma membrane
organization and function. Annu Rev Cell Dev Biol. 16:113–143.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voltz JW, Weinman EJ and Shenolikar S:
Expanding the role of NHERF, a PDZ-domain containing protein
adapter, to growth regulation. Oncogene. 20:6309–6314. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahon MJ, Donowitz M, Yun CC and Segre GV:
Na(+)/H(+) exchanger regulatory factor 2 directs parathyroid
hormone 1 receptor signalling. Nature. 20:858–861. 2002.
|
|
8
|
Shenolikar S, Voltz JW, Cunningham R and
Weinman EJ: Regulation of ion transport by the NHERF family of PDZ
proteins. Physiology. 19:362–369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weinman EJ, Hall RA, Friedman PA, Liu-Chen
LY and Shenolikar S: The association of NHERF adaptor proteins with
g protein-coupled receptors and receptor tyrosine kinases. Annu Rev
Physiol. 68:491–505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mangia A, Chiriatti A, Bellizzi A,
Malfettone A, Stea B, Zito FA, Reshkin SJ, Simone G and Paradiso A:
Biological role of NHERF1 protein expression in breast cancer.
Histopathology. 55:600–608. 2009. View Article : Google Scholar
|
|
11
|
Curto M, Cole BK, Lallemand D, Liu CH and
McClatchey AI: Contact-dependent inhibition of EGFR signaling by
Nf2/Merlin. J Cell Biol. 5:893–903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fanning AS and Anderson JM: PDZ domains:
fundamental building blocks in the organization of protein
complexes at the plasma membrane. J Clin Invest. 103:767–772. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reczek D, Berryman M and Bretscher A:
Identification of EBP50: A PDZ-containing phosphoprotein that
associates with members of the ezrin-radixin-moesin family. J Cell
Biol. 6:169–179. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louvet-Vallée S: ERM proteins: from
cellular architecture to cell signalling. Biol Cell. 92:305–316.
2000.PubMed/NCBI
|
|
15
|
Mahon MJ and Segre GV: Stimulation by
parathyroid hormone of a NHERF-1-assembled complex consisting of
the parathyroid hormone I receptor, phospholipase Cbeta, and actin
increases intracellular calcium in opossum kidney cells. J Biol
Chem. 28:23550–23558. 2004. View Article : Google Scholar
|
|
16
|
Fouassier L, Duan CY, Feranchak AP, et al:
Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the
apical membrane of rat liver epithelia. Hepatology. 33:166–176.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donowitz M, Cha B, Zachos NC, Brett CL,
Sharma A, Tse CM and Li X: NHERF family and NHE3 regulation. J
Physiol. 567:3–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan Y, Wang L and Dai JL: Suppression of
breast cancer cell growth by Na+/H+ exchanger
regulatory factor 1 (NHERF1). Breast Cancer Res. 8:R632006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata T, Chuma M, Kokubu A, Sakamoto M
and Hirohashi S: EBP50, a beta-catenin associating protein,
enhances Wnt signaling and is overexpressed in hepatocellular
carcinoma. Hepatology. 38:178–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fraenzer JT, Pan H, Minimo L Jr, Smith GM,
Knauer D and Hung G: Overexpression of the NF2 gene inhibits
schwannoma cell proliferation through promoting PDGFR degradation.
Int J Oncol. 24:1493–1500. 2003.PubMed/NCBI
|
|
21
|
Stemmer-Rachamimov AO, Wiederhold T,
Nielsen GP, et al: NHE-RF, a merlin-interacting protein, is
primarily expressed in luminal epithelia, proliferative
endometrium, and estrogen receptor-positive breast carcinomas. Am J
Pathol. 158:57–62. 2001. View Article : Google Scholar
|
|
22
|
Song J, Bai J, Yang W, Gabrielson EW, Chan
DW and Zhang Z: Expression and clinicopathological significance of
oestrogen-responsive ezrin-radixin-moesin-binding phosphoprotein 50
in breast cancer. Histopathology. 51:40–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georgescu MM, Morales FC, Molina JR and
Hayashi Y: Roles of NHERF1/EBP50 in cancer. Curr Mol Med.
8:459–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cardone RA, Bagorda A, Bellizzi A, Busco
G, Guerra L, Paradiso A, Casavola V, Zaccolo M and Reshkin SJ:
Protein kinase A gating of a pseudopodial-located
RhoA/ROCK/p38/NHE1 signal module regulates invasion in breast
cancer cell lines. Mol Biol Cell. 16:3117–3127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cardone RA, Bellizzi A, Busco G, Weinman
EJ, Dell’Aquila ME, Casavola V, Azzariti A, Mangia A, Paradiso A
and Reshkin SJ: The NHERF1 PDZ2 domain regulates
PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor
cells. Mol Biol Cell. 18:1768–1480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi Y, Molina JR, Hamilton SR and
Georgescu MM: NHERF1/EBP50 is a new marker in colorectal cancer.
Neoplasia. 12:1013–1022. 2010.PubMed/NCBI
|
|
27
|
Hamilton SR, Vogelstein B, Kudo S, Riboli
E, Nakamura S and Hainaut P: Carcinoma of the colon and rectum. WHO
Classification of Tumors: Pathology and Genetics of Tumors of
Digestive System. Hamilton SR and Aaltonen LA: IARC Press; Lyon,
France: pp. 105–119. 2000
|
|
28
|
Goldstein NS and Armin M: Epidermal growth
factor receptor immunohistochemical reactivity in patients with
American Joint Committee on Cancer Stage IV colon adenocarcinoma:
implications for a standardized scoring system. Cancer.
92:1331–1346. 2001. View Article : Google Scholar
|
|
29
|
Takahashi Y, Morales FC, Kreimann EL and
Georgescu MM: PTEN tumor suppressor associates with NHERF proteins
to attenuate PDGF receptor signalling. EMBO J. 5:910–920. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kreimann EL, Morales FC, de Orbeta-Cruz J,
Takahashi Y, Adams H, Liu TJ, McCrea PD and Georgescu MM: Cortical
stabilization of beta-catenin contributes to NHERF1/EBP50 tumor
suppressor function. Oncogene. 26:5290–5299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lazar CS, Cresson CM, Lauffenburger DA and
Gill GN: The Na+/H+ exchanger regulatory
factor stabilizes epidermal growth factor receptors at the cell
surface. Mol Biol Cell. 15:5470–5480. 2004.
|
|
32
|
Wei Q, Shui Y, Zheng S, Wester K, Nordgren
H, Nygren P, Glimelius B and Carlsson J: EGFR, HER2 and HER3
expression in primary colorectal carcinomas and corresponding
metastases: implications for targeted radionuclide therapy. Oncol
Rep. 25:3–11. 2011.PubMed/NCBI
|
|
33
|
Ljuslinder I, Malmer B,
Isaksson-Mettävainio M, Oberg A, Henriksson R, Stenling R and
Palmqvist R: ErbB 1–4 expression alterations in primary colorectal
cancers and their corresponding metastases. Anticancer Res.
29:1489–1494. 2009.
|
|
34
|
Scartozzi M, Bearzi I, Berardi R,
Mandolesi A, Pierantoni C and Cascinu S: Epidermal growth factor
receptor (EGFR) downstream signalling pathway in primary colorectal
tumours and related metastatic sites: optimising EGFR-targeted
treatment options. Br J Cancer. 97:92–97. 2007. View Article : Google Scholar
|
|
35
|
Krasinskas AM: EGFR signaling in
colorectal carcinoma. Patholog Res Int. 9329322011.PubMed/NCBI
|