Introduction
Pancreatic cancer is a leading cause of
cancer-related deaths worldwide, with a 5-year survival rate of
<5% (1). For patients with
localized disease, radical surgery with blood vessel resection may
provide long-term benefits. However, even after curative resection,
patients with pancreatic cancer face a 25–50% rate of distant
metastases (2). The liver is the
most frequent site of metastasis from pancreatic cancer. During
hematogenous metastasis, tumor cells separate from the primary
site, travel through the blood stream, marginate and adhere to the
vascular endothelium, and transmigrate into extravascular sites
where colonization occurs. Diapedesis of tumor cells from the
circulation into secondary sites is believed to occur through a
mechanism similar to that of leukocyte extravasation, in which
cells must first contact and then roll along the endothelial cell
layer. Rolling requires interactions between selectin cell adhesion
molecules and their ligands (3,4).
Selectin-mediated tumor cell adhesion has been modeled in
vitro, with colorectal adenocarcinoma cells binding to
cytokine-activated vascular endothelium (5–9). The
fucosylated ligand components sialyl-LewisX
(sLeX) and sialyl-LewisA (sLeA)
found on the surface of circulating adenocarcinoma cells have been
shown to bind to endothelial selectin (6,9–14).
Studies have shown that anti-sLeX(10) and anti-sLeA(6,15)
antibodies block adhesion of epithelial cancer cell lines to human
umbilical vein endothelial cells (HUVEC), demonstrating the
specificity of this interaction.
Hepatic ischemia-reperfusion always occurs during
liver transplantation and liver resection using the Pringle method.
During pancreaticoduodenectomy (PD) with portal vein resection for
pancreatic cancer, portal vein clamping is used to reduce
intraoperative bleeding. However, this portal clamping causes
identical hepatic ischemia-reperfusion injury. Several studies have
shown that hepatic ischemia-reperfusion induces free radicals and
inflammatory cytokines (16–19);
therefore, hepatic ischemia-reperfusion may promote liver
metastasis (20).
The major objective of this study was to investigate
the early molecular events in liver colonization triggered by
hepatic ischemia-reperfusion that could subsequently determine the
course of metastasis.
Materials and methods
Animals
ICR nude mice weighing 20–40 g were purchased from
Charles River Japan Inc. (Kanagawa, Japan) and were maintained in
our Animal Care Center. All animals were given free access to food
and water. All animal experiments were performed according to
Guidelines for the Care and Use of Laboratory Animals of the
Kanazawa University.
Tumor cells
The Capan-1 human pancreatic cancer cell line, which
was isolated from a liver metastasis of a well-differentiated
pancreatic ductal adenocarcinoma in a 40-year-old Caucasian male,
was obtained from the American Type Culture Collection (Rockville,
MD, USA). This cell line was maintained in media supplemented with
2 mM L-glutamine, 10% fetal calf serum (FCS), 100 U/ml penicillin
and 100 μg/ml streptomycin. Cells were grown at 37°C in an
atmosphere of 95% air and 5% CO2. Tumor cells were
suspended in phosphate-buffered saline (PBS) at a density of
2×106 cells/ml. Each mouse received an intrasplenic
injection of 1×106 cells according to a previously
described method (21). Cell
viability was assessed with trypan blue prior to injection.
Treatment of animals
Mice were anesthetized with diehtylether, and the
abdomen was incised to expose the liver. Total hepatic ischemia was
induced by clamping the hepatic artery, portal vein, and bile duct.
Ischemia was detected visually by color changes in the liver. The
ischemic time was 20 min, which was well tolerated by the mice, and
the reperfusion time was 15 min. The mice were divided into 2
groups: the ischemia-reperfusion (I/R) group (n=20) and the control
group (no ischemia-perfusion, n=20). After hepatic
ischemia-reperfusion, 1×106 tumor cells in 0.5 ml of PBS
were injected into the spleen of the mice. The mice were then
closed with a 4.0 silk suture in a continuous running fashion. In
the control group, the tumor cells were injected immediately after
laparotomy without hepatic ischemia-reperfusion. The animals were
sacrificed 4 weeks later. The liver was removed and the number of
tumor nodules on the liver surface was counted.
In addition, to test the effect of anti-E-selectin
antibody on liver colonization, another 5 animals were inoculated
with 3 intraperitoneal injections of 100 μg of affinity-purified
anti-E-selectin antibody at 0, 3 and 6 h after hepatic
ischemia-reperfusion and tumor inoculation. The number of tumor
nodules was then counted (I/R+Ab group, n=5).
To determine whether hepatic ischemia-reperfusion
caused changes in local cytokine production and E-selectin mRNA
expression in the liver, hepatic ischemia-reperfusion was induced,
and livers were removed at different time intervals (0, 0.25, 0.5,
1, 3, 6, 9, 12 and 24 h) after ischemia-reperfusion. IL-1, TNF-α
and E-selectin mRNA levels were analyzed by reverse
transcription-polymerase chain reaction (RT-PCR) and southern
blotting. Liver fragments were frozen immediately in liquid
nitrogen and stored at −80°C until RNA extraction.
Oligonucleotide primers and probes
The sequences of the oligonucleotides used for
RT-PCR, and southern blotting were as follows. IL-1 forward,
5′-CAGATTCACAACTGTTCG TGAGCG-3′; IL-1 and reverse primer,
5′-AAGTCTGTCA TAGAG GGCAGTCCC-3′ (product size, 232 bp); IL-1
probe, 5′-CACATCAGCTGCTTATCCAGAGCTG-3′ (22); TNF-α forward,
5′-GCAGGTCTACTTTGGAGTCATTGC-3′ and reverse primer,
5′-TCCCTTTGCAGAACTCAGGAATGG-3′ (product size, 323 bp); TNF-α probe,
5′-TGTGCTCAGAGC TTTCAACAACTAC-3′ (23); E-selectin forward, 5′-GTGCG
GTGTACGTCCCTCTGGAGAAGTG-3′; and reverse primer,
5′-TCCCTTTGCAGAACTCAGGAATGG-3′ (product size, 535 bp); E-selectin
probe, 5′-TCAGAATCTACAGTGTACCTCATCTG-3′; GAPDH forward,
5′-GGTGAAGGTCGGTGTGAACGGATTT-3′ and reverse primer,
5′-AATGCCAAAGTTGTCATGGATGACC-3′ (product size, 502 bp); and GAPDH
probe, 5′-GTGCTGAGTATGTCGTGGAGTCTAC-3′ (24).
RT-PCR southern blot analysis
Total cellular RNA was extracted from each frozen
liver fragment by the acid guanidinium
thiocyanate-phenol-chloroform method (25). The concentration of total RNA was
determined by measuring the OD260. The purity of the RNA
was assessed by determining the OD260/280 ratio, and all
samples were >1.8. Aliquots of each total RNA sample (1 μg) were
subjected to reverse transcription (RT) at 42°C for 60 min using
Moloney murine leukemia virus reverse transcriptase (Toyobo Inc.,
Osaka, Japan). Subsequently, an aliquot of each RT product was
amplified in a DNA thermal cycler (MJ Research, Inc., Watertown,
MA, USA), using Taq DNA polymerase (Takara Bio Inc., Shiga, Japan)
and a pair of oligonucleotide primers in a final volume of 100 μl.
Each amplification cycle consisted of 94°C for 1 min, 58°C for 2
min, and 72°C for 1 min and 30 sec. After 25 cycles of
amplification, each RT-PCR mixture was electrophoresed on a 1.5%
agarose gel and blotted onto a nylon membrane (Pall BioSupport,
East Hills, NY, USA) according to the southern blot procedure
(26).
An antisense oligonucleotide probe was labeled at
the 3′ end with fluorescein-11-dUTP using terminal transferase
(Roche Diagnostics, Basel, Switzerland) and was hybridized to the
membrane at 43°C for 2 h in a shaking water bath according to the
ECL 3′-oligolabelling and detection system protocol (Amersham
Pharmacia Biotech, Uppsala, Sweden). After washing, the membrane
was blocked with blocking solution at room temperature for 30 min,
and then the membrane was incubated with antibody using diluted
anti-fluorescein HRP conjugate solution containing 0.5% (w/v)
bovine serum albumin at room temperature for 30 min. After the
membrane was washed, the membrane was incubated with detection
solution at room temperature for 10 min, and then covered with
Saran wrap and after exposed to X-ray film (Kodak, Rochester, NY,
USA).
Immunocytochemical analysis of
sLeA antigen on Capan-1 cells
Capan-1 cells were suspended in RPMI-1640 medium on
a Lab-Tek Chamber Slide (Nunc, Naperville, IL, USA) and incubated
at 37°C overnight in an atmosphere of 95% air and 5%
CO2. After washing with PBS, acetone and methanol were
added and the chamber, was incubated at 4°C for 30 min. Capan-1
cells were attached to this slide, which was stored at −20°C until
use. Immunocytochemical staining for sLeA antigen was
performed according to the EnVision technique (Dako, Glostrup,
Denmark). The slide was incubated with normal goat serum in PBS for
30 min at room temperature, and then incubated with anti-human
sLeA monoclonal antibody (diluted 1:100) overnight at
4°C. After washing with PBS, the slide was incubated with
biotinylated anti-mouse antibody for 120 min, followed by the
application of peroxidase-labeled streptavidin. The reaction
product was visualized with diaminobenzidine, and was
counterstained with hematoxylin.
Statistical analysis
The Chi-square and Mann-Whitney U tests were used to
determine statistical significance. A P-value of <0.05 was
considered significant.
Results
Liver colonization by Capan-1 cells after
hepatic ischemia-reperfusion
All animals in both groups survived until sacrifice.
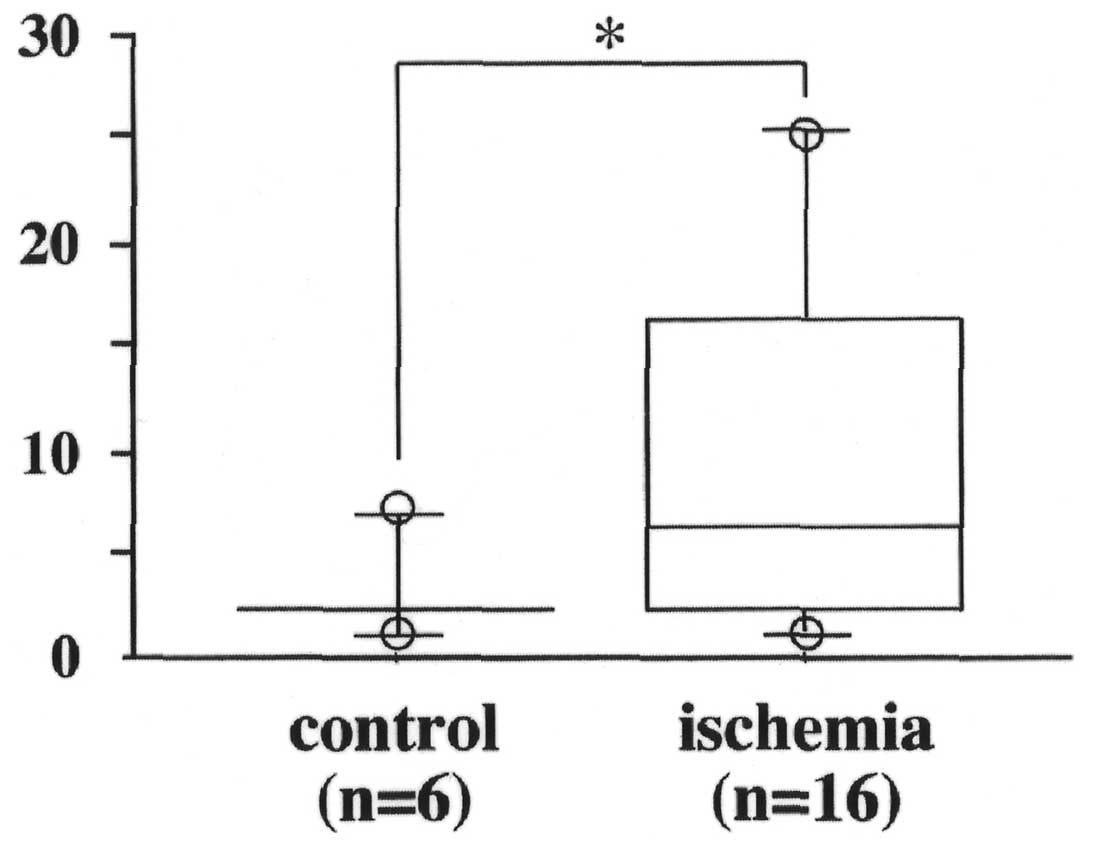
As shown in Table I, 16 of the 20
(80%) mice in the I/R group developed hepatic metastases, which was
significantly higher than the incidence of hepatic metastases in
the control group (6 of 20, 30%) (P<0.01). Moreover, mice in the
I/R group had more tumor nodules than in the control group
(P=0.06). The number of metastases per mouse in animals positive
for liver metastasis ranged from 1 to 7 (median, 2.7) in the
control group, and from 1 to 25 (median, 9.9) in the I/R groups
(Fig. 1).
 | Table IIncidence of liver metastasis in
control mice and in mice with hepatic ischemia-reperfusion injury
treated or not treated with the anti-E-selectin antibody. |
Table I
Incidence of liver metastasis in
control mice and in mice with hepatic ischemia-reperfusion injury
treated or not treated with the anti-E-selectin antibody.
| I/R group
(n=20) | Control group
(n=5) | I/R+Ab group
(n=20) |
|---|
| Liver metastasis
(+) | 16 | 6 | 2 |
| Incidence | 80% | 30% | 40% |
Effect of anti-E-selectin antibody on
liver colonization after hepatic ischemia-reperfusion
As shown in Table I,
injection of anti-E-selectin antibody reduced hepatic metastasis of
Capan-1 cells. Only 2 of the 5 (40%) mice injected with
anti-E-selectin antibody developed liver metastases and the
metastases in the positive animals were 6 and 9, respectively.
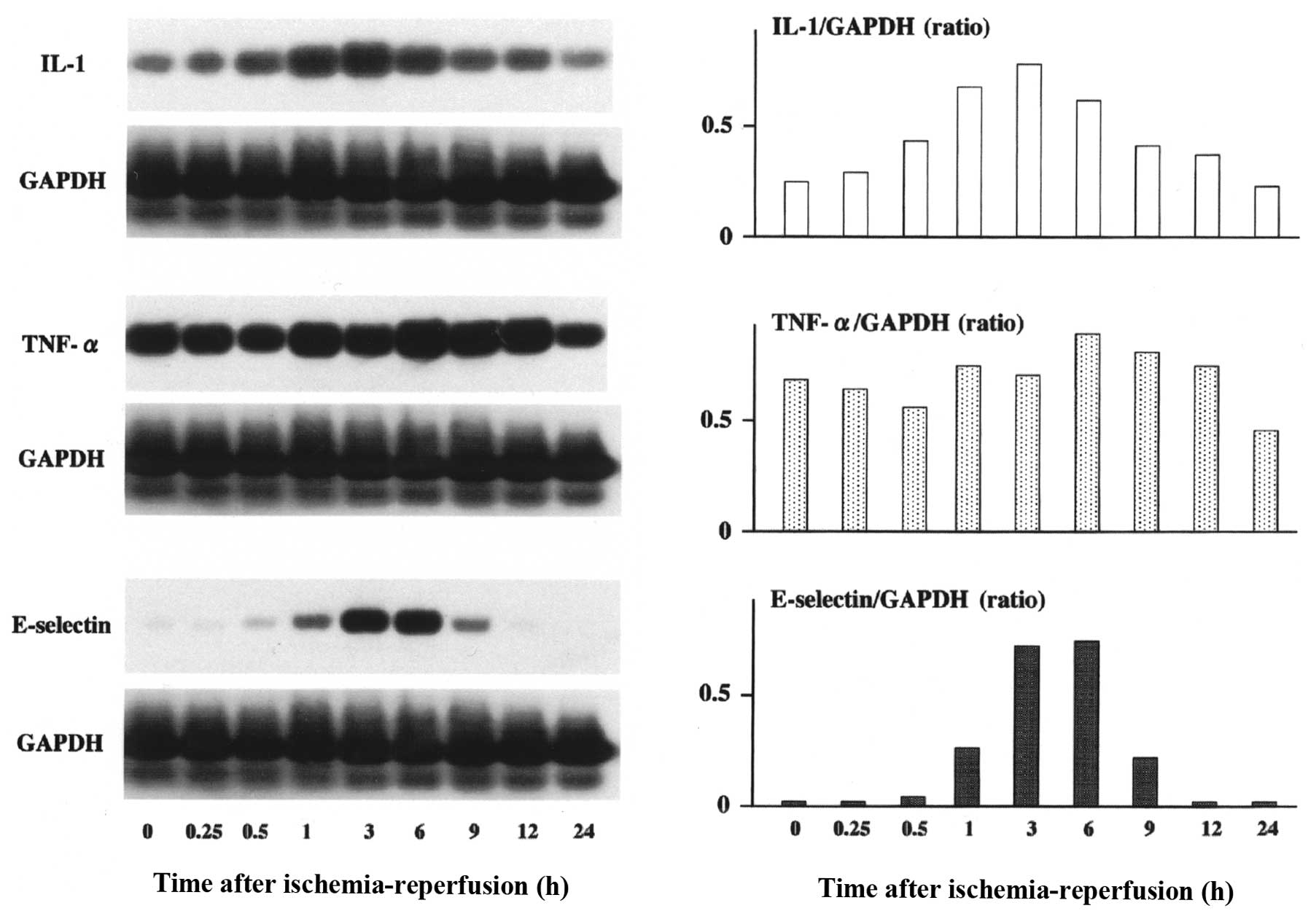
Expression of IL-1, TNF-α and E-selectin
m-RNA after hepatic ischemia-reperfusion
IL-1 mRNA began to increase 30 min after
ischemia-reperfusion, reached maximal levels 3 h after
ischemia-reperfusion and decreased to basal levels 24 h after
ischemia-reperfusion (Fig. 2).
E-selectin mRNA levels began to rise within 1 h after
ischemia-reperfusion, reached a maximum at 6 h, and then returned
to basal levels by 24 h after hepatic ischemia-reperfusion. Hepatic
ischemia-reperfusion had no significant effect on TNF-α expression
at any of the time intervals examined (Fig. 2).
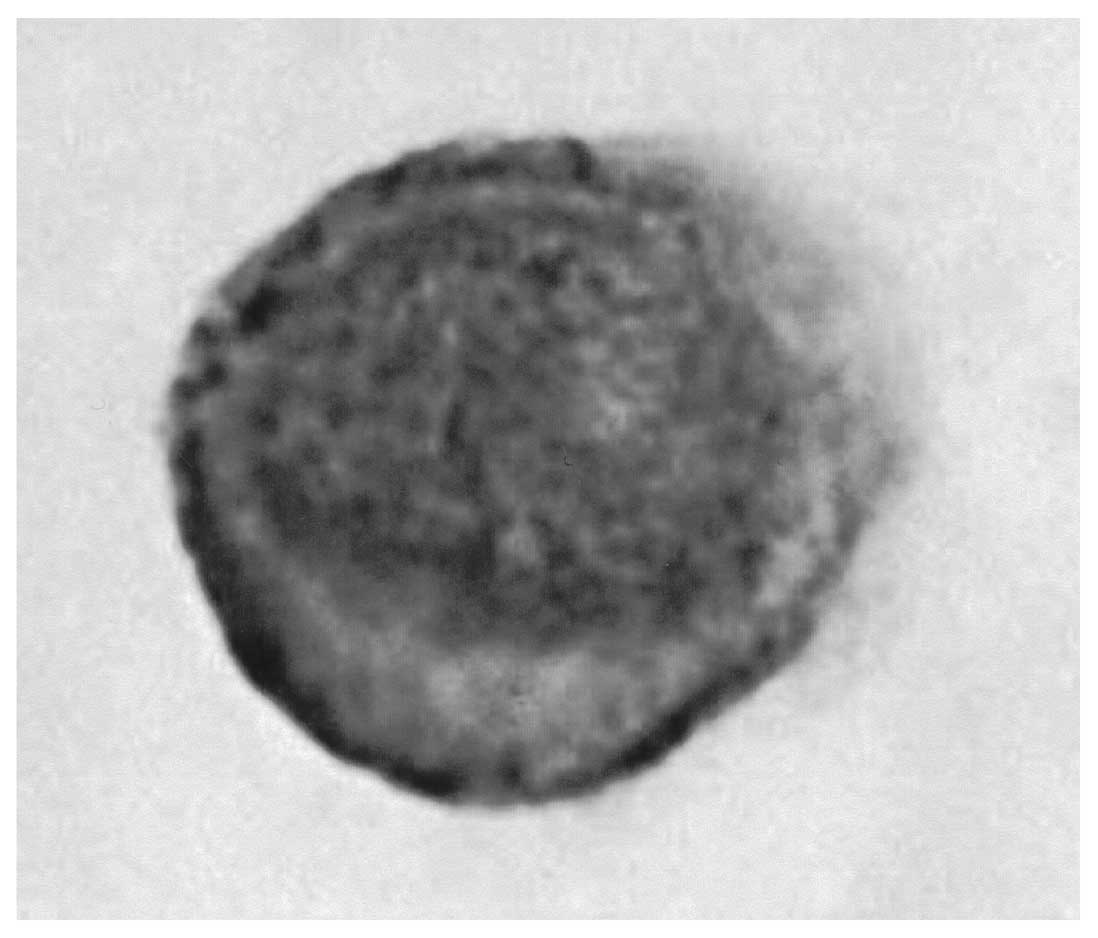
Expression of aLeA antigen on
Capan-1 cells
As shown in Fig. 3,
sLeA antigen was expressed on the surface of Capan-1
cells.
Discussion
Tumor metastasis is a multistep process requiring
detachment of malignant cells from the primary tumor mass,
penetration of blood vessels, evasion of immune surveillance,
attachment to the endothelium of distant organs, penetration of the
secondary tissue, and formation of new tumor colonies. The ability
of disseminated cancer cells to establish metastases in secondary
organs is regulated by a combination of factors including access to
the organ microvasculature and specific secondary tissue-tumor cell
interactions (27,28). Interaction between tumor cells and
the secondary tissue endothelium is believed to be a key step in
the metastatic cascade (29), and
is thought to be mediated by adhesion receptor-ligand pairs, some
of which are involved in physiological leukocyte-endothelial
interactions (30). The various
combinations of cell surface molecules expressed by tumor cells may
serve as ligands for endothelial cell surface receptors (5,10,11,31,32),
which are typically induced by mediators of inflammation (5,31,32).
Therefore, a local inflammatory response may facilitate circulating
tumor cell adhesion and arrest. Takada et al(9) showed that epithelial cancer cells have
the ability to adhere to endothelial cells, and that their adhesion
is enhanced by activation of endothelial cells with cytokines such
as IL-1. They also showed that E-selectin (endothelial leukocyte
adhesion molecule −1: ELAM-1), first introduced as an adhesion
molecule that mediates leukocyte adhesion to endothelial cells
(33), is of particular importance
in the adhesion of human epithelial cancer cells to vascular
endothelial cells.
E-selectin is an adhesion molecule that is not
expressed on normal endothelial cells. However, E-selectin is
transiently expressed on the surface of vascular endothelium after
stimulation with IL-1 and TNF-α, and has been implicated in the
initial events of neutrophil extravasation in the inflammatory
response. E-selectin also appears to be involved in tumor invasion
and metastasis, since E-selectin mediates adherence of leukemia and
colon cancer cells to activated endothelial cells. Several studies
have implicated E-selectin in the adhesion of cancer cells to
vascular endothelial cells (6,8,10,34,35).
It has been suggested that E-selectin is involved in the
preferential homing of metastasizing cells to the liver (36), and that E-selectin mediates the
metastasis of certain tumor types (36,37).
Uotani et al reported that induction of E-selectin after
partial hepatectomy promotes liver metastasis in mice (38).
We examined the expression of cytokines, such as
IL-1 and TNF-α, and E-selectin by RT-PCR and southern blotting
after hepatic ischemia-reperfusion. Our results showed maximum
expression of E-selectin mRNA 6 h after hepatic
ischemia-reperfusion, with a return to baseline levels by 24 h
after ischemia-reperfusion. This finding is consistent with
previous reports (38,39).
In liver surgery for pancreatic cancer with portal
resection, temporary hepatic pedicle clamping has been used to
reduce intraoperative bleeding since the report by Pringle
(40). However, this clamping
causes ischemia-reperfusion injury (19,41),
and ischemia-reperfusion injury elicits endothelial cell injury
that can manifest as swelling, detachment from the underlying
basement membrane, and compromised barrier function. These events
might be accompanied by leukocyte-endothelial cell adhesion, which
manifest as rolling, firm adhesion, and emigration of leukocytes in
post-capillary venules of the microvasculature (42). Several studies have reported that
hepatic ischemic-reperfusion induces free radicals and inflammatory
cytokines (16–19). Free radicals and cytokines, such as
TNF-α and IL-1 are implicated in the acceleration of hepatic
metastasis, and therefore it is possible that hepatic
ischemia-reperfusion promotes liver metastasis (20). Our study verified that hepatic
ischemia-reperfusion promotes liver metastasis of Capan-1 cells;
the number of tumor nodules in the I/R group was significantly
greater than that in the control group. We also found that
administration of an anti-E-selectin monoclonal antibody to mice in
the I/R group after tumor inoculation inhibited liver metastasis.
This finding is supported by previous reports which demonstrated
that a neutralizing murine E-selectin monoclonal antibody abrogated
hepatic metastasis in vivo(36,38).
The sialylated, fucosylated tetrasaccharides
LeX and sLeA and related carbohydrate
structures, have been identified as selectin ligands (6,9–14,34).
Interestingly, sLeX and sLeA have also been
identified as markers of progression in several types of carcinoma,
particularly carcinomas of the gastrointestinal tract, which
commonly metastasize to the liver (43,44).
In vitro adhesion studies have shown that carbohydrate
determinants adhere to TNF-α-inducible E-selectin on HUVECs
(6–9,13,36).
Our study showed that sLeA was expressed
on the surface of Capan-1 cells. It has been reported that
sLeA on pancreatic carcinoma cells is an important
ligand for E-selectin on activated endothelial cells (15,45).
These results suggested that sLeA expression on
pancreatic carcinoma cell lines might modulate events related to
carcinoma cell-to-endothelial cell attachment, thereby contributing
to hematogenous metastasis in vivo(46). A recent study reported that
intermittent hepatic ischemia-reperfusion preconditioning minimizes
liver metastasis (47). The
usefulness of intermittent hepatic ischemia-reperfusion
preconditioning in Pringle’s method for hepatectomy is well known
(16–19,40).
During PD with portal vein resection and reconstruction, portal
vein clamping is necessary to reduce intraoperative bleeding.
However, portal vein clamping-unclamping causes
ischemia-reperfusion of the liver, but congestion-re-outflow in the
small intestine. To avoid congestion of the small intestine, we
have been using small intestinal ischemic preconditioning by
clamping the superior mesenteric artery (SMA) during portal vein
clamping in PD with portal vein resection. We have reported that
small intestinal ischemia-reperfusion injury was reduced by
ischemic preconditioning induced by clamping the SMA (48). Small intestinal ischemic
preconditioning by clamping the SMA could suppress the increase in
cytokines and reduce undesirable liver metastasis from pancreatic
cancer due to surgical procedures such as intraoperative portal
vein clamping.
In conclusion, hepatic ischemia-reperfusion causes
increased liver metastases as well as increased expression of
E-selectin, which has been reported to mediate metastasis of cancer
cells that express sLeA. Agents that interfere with
hepatic E-selectin induction and/or function might have
therapeutic, anti-metastatic effects during the early stages of
liver colonization.
References
|
1
|
International Agency for Research on
Cancer, World Health Organization. Globocan 2008. World Health
Organization. Web site. http://globocan.iarc.fr/.
Accessed February 17, 2012
|
|
2
|
Evans DB, Abbruzzese JL and Willett CG:
Cancer of the pancreas. Cancer, Principles and Practice of
Oncology. De Vita VT, Hellman S and Rosenberg SA: 6th edition.
Lippincott Williams and Wilkins; Philadelphia: pp. 1126–1161.
2001
|
|
3
|
Lasly LA: Selectins: interpreters of
cell-specific carbohydrate information during inflammation.
Science. 258:964–969. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sass PM: The involvement of selectins in
cell adhesion, tumor progression, and metastasis. Cancer Invest.
16:322–328. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lauri D, Needham L, Martin-Padura I and
Dejana E: Tumor cell adhesion to endothelial cells: endothelial
leukocyte adhesion molecule-1 as an inducible adhesive receptor
specific for colon carcinoma cells. J Natl Cancer Inst.
83:1321–1324. 1991. View Article : Google Scholar
|
|
6
|
Takada A, Ohmori K, Takahashi N, et al:
Adhesion of human cancer cells to vascular endothelium mediated by
a carbohydrate antigen, sialyl Lewis A. Biochem Biophys Res Commun.
179:713–719. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dejana E, Martin-Padura I, Lauri D, et al:
Endothelial leukocyte adhesion molecule-1-dependent adhesion of
colon carcinoma cells to vascular endothelium is inhibited by an
antibody to Lewis fucosylated type I carbohydrate chain. Lab
Invest. 66:324–330. 1992.
|
|
8
|
Tozeren A, Kleinman HK, Gfant DS, Morales
D, Mercurio AM and Byers SW: E-selectin-mediated dynamic
interactions of breast- and colon-cancer cells with
endothelial-cell monolayers. Int J Cancer. 60:426–431. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takada A, Ohmori K, Yoneda T, et al:
Contribution of carbohydrate antigens sialyl Lewis A and sialyl
Lewis X to adhesion of human cancer cells to vascular endothelium.
Cancer Res. 53:354–361. 1993.PubMed/NCBI
|
|
10
|
Walz G, Aruffo A, Kolanus W, Bevilaqua M
and Seed B: Recognition by ELAM-1 of the sialyl-Lex determinant on
myeloid and tumor cells. Science. 250:1132–1135. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Majuri ML, Mattila P and Renkonen R:
Recombinant E-selectin-protein mediates tumor cell adhesion via
sialyl-Lea and sialyl-Lx. Biochem Biohys Res
Commun. 182:1376–1382. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foxall C, Watson SR, Dowbenko D, et al:
The three members of the selectin receptor family recognize a
common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J
Cell Biol. 117:895–902. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Izumi Y, Taniuchi Y, Tsuji T, et al:
Characterization of human colon carcinoma variant cells selected
for sialyl Lex carbohydrate antigen: liver colonization and
adhesion to vascular endothelial cells. Exp Cell Res. 216:215–221.
1995. View Article : Google Scholar
|
|
14
|
Srinivas U, Påhlsson P and Lundblad A:
E-selectin: sialyl Lewis a dependent adhesion of colon cancer
cells, is inhibited differently by antibodies against E-selectin
ligands. Scand J Immunol. 44:197–203. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwai K, Ishikura H, Kaji M, et al:
Importance of E-selectin (ELAM-1) and sialyl Lewis(a) in the
adhesion of pancreatic carcinoma cells to activated endothelium.
Int J Cancer. 54:972–977. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura N, Muraoka R, Horiuchi T, et al:
Intermittent hepatic pedicle clamping reduces liver and lung
injury. J Surg Res. 78:11–17. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uchinami M, Muraoka R, Horiuchi T, et al:
Effect of intermittent hepatic pedicle clamping on free radical
generation in the rat liver. Surgery. 124:49–56. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horiuchi T, Muraoka R, Tabo T, Uchinami M,
Kimura N and Tanigawa N: Optimal cycles of hepatic ischemia and
reperfusion for intermittent pedicle clamping during liver surgery.
Arch Surg. 130:754–758. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colletti LM, Remick DG, Burtch GD, Kunkel
SL, Strieter RM and Campbell DA Jr: Role of tumor necrosis
factor-alpha in the pathophysiologic alterations after hepatic
ischemia/reperfusion injury in the rat. J Clin Invest.
85:1936–1943. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doi K, Horiuchi T, Uchinami M, et al:
Hepatic ischemia-reperfusion promotes liver metastasis of colon
cancer. J Surg Res. 105:243–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohta T, Futabgami F, Tajima H, et al:
Inhibitory effect of a serine protease inhibitor, FOY-305 on the
invasion and metastasis of human pancreatic cancer. Int J Oncol.
11:813–817. 1997.PubMed/NCBI
|
|
22
|
Lomedico PT, Gubler U, Hellmann CP, et al:
Cloning and expression of murine interleukin-1 cDNA in
Escherichia coli. Nature. 312:458–462. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fransen L, Müller R, Marmenout A, et al:
Molecular cloning of mouse tumour necrosis factor cDNA and its
eukaryotic expression. Nucleic Acids Res. 13:4417–4429. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sabath DE, Broome HE and Prystowsky MB:
Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major
interleukin 2-induced transcript in a cloned T-helper lymphocyte.
Gene. 91:185–191. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Southern EM: Detection of specific
sequences among DNA fragments separated by gel electrophoresis. J
Mol Biol. 98:503–517. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brodt P: Adhesion receptors and
proteolytic mechanisms in cancer invasion and metastasis. Cell
Adhesion and Invasion in Cancer Metastasis. Brodt P: Landes,
Austin; pp. 167–242. 1996
|
|
28
|
Radinsky R and Fidler IJ: Regulation of
tumor cell growth at organ-specific metastases. In Vivo. 6:325–331.
1992.PubMed/NCBI
|
|
29
|
Zetter BR: The cellular basis of
site-specific tumor metastasis. N Eng J Med. 322:605–612. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zetter BR: Adhesion molecules in tumor
metastasis. Semin Cancer Biol. 4:219–229. 1993.PubMed/NCBI
|
|
31
|
Rice GE and Bevilacqua MP: An inducible
endothelial cell surface glycoprotein mediates melanoma adhesion.
Science. 246:1303–1306. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bevilacqua MP and Nelson RM: Selectins. J
Clin Invest. 91:379–387. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bevilacqua MP, Stengelin S, Gimbrone MA Jr
and Seed B: Endothelial leukocyte adhesion molecule 1: an inducible
receptor for neutrophils related to complement regulatory proteins
and lectins. Science. 243:1160–1165. 1989. View Article : Google Scholar
|
|
34
|
Phillips ML, Nudelman E and Gaeta FC:
ELAM-1 mediates cell adhesion by recognition of a carbohydrate
ligand, sialyl-Lex. Science. 250:1130–1132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang K, Baeckström D and Hansson GC: A
secreted mucin carrying sialyl-Lewis a from colon carcinoma cells
binds to E-selectin and inhibits HL-60 cell adhesion. Int J Cancer.
59:823–829. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brodt P, Fallavollita L, Bresalier RS,
Meterissian S, Norton CR and Wolitzky BA: Liver endothelial
E-selectin mediates carcinoma cell adhesion and promotes liver
metastasis. Int J Cancer. 71:612–619. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khatib AM, Kontogiannea M, Fallavollita L,
Jamison B, Meterissian S and Brodt P: Rapid induction of cytokine
and E-selectin expression in the liver in response to metastatic
tumor cells. Cancer Res. 59:1356–1361. 1999.PubMed/NCBI
|
|
38
|
Uotani H, Yamashita I, Nagata T, Kishimoto
H, Kashii Y and Tsukada K: Induction of E-selectin after partial
hepatectomy promotes metastases to liver in mice. J Surg Res.
96:197–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Burke J, Zibari GB, Brown MF, et al:
Hepatic ischemia-reperfusion injury causes E-selectin upregulation.
Transplant Proc. 30:2321–2323. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pringle JH: Notes on the arrest of hepatic
hemorrhage due to trauma. Ann Surg. 48:541–549. 1908. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Colletti LM, Kunkel SL and Walz A: The
role of cytokine networks in the local liver injury following
hepatic ischemia/reperfusion in the rat. Hepatology. 23:506–514.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brown MF, Zibari G, Burney D, Granger DN
and McDonald JC: Hepatic ischemia/reperfusion affects leukocyte
rolling and velocity. Clin Transplant. 11:511–515. 1997.PubMed/NCBI
|
|
43
|
Hanisch FG, Hanski C and Hasegawa A:
Sialyl Lewis(x) antigen as defined by monoclonal antibody AM-3 is a
marker of dysplasia in the colonic adenoma-carcinoma sequence.
Cancer Res. 52:3138–3144. 1992.
|
|
44
|
Nakamori S, Kameyama M and Imaoka S:
Increased expression of sialyl Lewisx antigen correlates with poor
survival in patients with colorectal carcinoma: clinicopathological
and immunohistochemical study. Cancer Res. 53:3632–3637. 1993.
|
|
45
|
Kaji M, Ishikura H, Kishimoto T, et al:
E-selectin expression induced by pancreas-carcinoma-derived
interleukin-1 alpha results in enhanced adhesion of
pancreas-carcinoma cells to endothelial cells. Int J Cancer.
60:712–717. 1995. View Article : Google Scholar
|
|
46
|
Kishimoto T, Ishikura H, Kimura C,
Takahashi T, Kato H and Yoshiki T: Phenotypes correlating to
metastatic properties of pancreas adenocarcinoma in vivo: the
importance of surface sialyl Lewis(a) antigen. Int J Cancer.
69:290–294. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoshida M, Horiuchi T, Uchinami M, et al:
Intermittent hepatic ischemia-reperfusion minimizes liver
metastasis in rats. J Surg Res. 111:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takeshita M, Tani T, Harada S, et al: Role
of transcription factors in small intestinal ischemia-reperfusion
injury and tolerance induced by ischemic preconditioning.
Transplant Proc. 42:3406–3413. 2010. View Article : Google Scholar
|